Abstract
SLX4, the newly identified Fanconi anemia protein, FANCP, is implicated in repairing DNA damage induced by DNA interstrand cross-linking (ICL) agents, topoisomerase I (TOP1) inhibitors, and in Holliday junction resolution. It interacts with and enhances the activity of XPF-ERCC1, MUS81-EME1, and SLX1 nucleases, but the requirement for the specific nucleases in SLX4 function is unclear. Here, by complementing a null FA-P Fanconi anemia cell line with SLX4 mutants that specifically lack the interaction with each of the nucleases, we show that the SLX4-dependent XPF-ERCC1 activity is essential for ICL repair but is dispensable for repairing TOP1 inhibitor-induced DNA lesions. Conversely, MUS81-SLX4 interaction is critical for resistance to TOP1 inhibitors but is less important for ICL repair. Mutation of SLX4 that abrogates interaction with SLX1 results in partial resistance to both cross-linking agents and TOP1 inhibitors. These results demonstrate that SLX4 modulates multiple DNA repair pathways by regulating appropriate nucleases.
Key Points
Mutational analysis of the Fanconi anemia nuclease scaffold SLX4/FANCP reveals lesion-dependent functional requirements for XPF, MUS81, and SLX1 in DNA repair.
The UBZ domain and SLX4-XPF complex are critical for interstrand cross-link repair and the SLX4-MUS81 complex repairs CPT and PARP inhibitor-induced damage.
Introduction
Repair of DNA damage during S phase of the cell cycle is extremely challenging, as suggested by the plethora of proteins that participate in signaling and repair of lesions that block replisome progression.1-3 Although cells have evolved to repair the endogenous damage that causes replication stalling or collapse, these pathways have been most successfully probed using chemotherapeutic agents, such as interstrand cross-linking (ICL) agents like mitomycin C (MMC) and topoisomerase 1 (TOP1) inhibitors, including camptothecin (CPT). MMC covalently links the Watson and Crick DNA strands, preventing progression of replication forks.4 CPT forms a complex with TOP1, trapping the enzyme on the nicked DNA resulting in DNA double-strand break (DSB) formation during DNA replication and in the collapse of replication forks.5
Depletion of SLX4 from human cells leads to enhanced sensitivity to both ICL agents and to CPT.6,7 Consistent with this observation, biallelic mutations of the SLX4 gene have been identified in patients with Fanconi anemia (FA), a rare recessive genetic disorder characterized by genome instability, bone marrow failure, cancer predisposition, and hypersensitivity to ICL agents.8,9 To date, 14 FA complementation groups have been identified in FA patients, and the 15th gene (RAD51C) is mutated in an FA-like syndrome.10 The exact mechanism underlying ICL repair remains poorly understood, although recent studies using Xenopus egg extract have shown that the repair proceeds through multiple distinct steps requiring nucleases, translesion DNA polymerases, and homologous recombination proteins.11-13 The FA proteins are essential for this process as the nuclease and translesion synthesis steps depend on FANCD2 and its ubiquitination.11
A number of nucleases, including XPF, MUS81, SLX1, FAN1, and SNM1A, have been previously implicated in ICL repair.2,6,7,14-19 Three of them, XPF, MUS81, and SLX1, are found to interact with SLX4. Only a portion of cellular XPF interacts with SLX4,6,7 with the non-SLX4 bound XPF participating in nucleotide excision repair.20
Human cells with low levels of XPF or ERCC1, an obligate XPF partner, are sensitive to UV and to DNA cross-linking agents.18,21 FA-P cells, which have truncation mutations in SLX4, are not sensitive to UV, indicating that the XPF bound to SLX4 is not necessary for nucleotide excision repair.8 MUS81 has not yet been reported to be mutated in any human disorder; however, the knockout mice and cells derived from them are sensitive to cross-linking agents.22,23 Mus81 knockout mouse embryonic fibroblasts are not significantly sensitive to CPT,23 although depletion of MUS81 from human cells leads to CPT sensitivity.7 Slx1 knockout mice have not yet been reported, and the depletion of SLX1 resulted in conflicting conclusions about the importance of this nuclease in repairing CPT and ICL damage.6,7,24
Here, using patient-derived SLX4 null cell lines in combination with a panel of exogenously expressed SLX4 mutants, we have been able to dissect the role of SLX4 as a context-dependent nuclease scaffold. We show that, depending on the lesion, different modules of SLX4 activity are required, with the XPF interaction being essential for cross-link repair and MUS81 interaction being essential for repair of CPT and poly(ADP-ribose) polymerase (PARP) inhibitor-induced DNA damage.
Methods
FA cell lines
Cell lines were derived from persons with FA registered in the International Fanconi Anemia Registry after obtaining informed written consent in accordance with the Declaration of Helsinki. The Institutional Review Board of Rockefeller University approved these studies.
Cell culture
U2OS and 293T cells were grown in DMEM supplemented with 10% (volume/volume) FBS, 100 units of penicillin per milliliter and 0.1 mg of streptomycin per milliliter (all from Invitrogen). Fibroblasts were grown in DMEM supplemented with 15% (volume/volume) FBS, 100 units of penicillin per milliliter and 0.1 mg of streptomycin per milliliter, nonessential amino acids, and 1 times GlutaMAX (Invitrogen). Fibroblasts were incubated at 3% oxygen. BJ cells are normal foreskin fibroblasts obtained from ATCC. Cell lines were immortalized with a catalytic subunit of human telomerase (hTERT) and/or were transformed by HPV E6 and E7 proteins as indicated in the text.
Plasmids
The wild-type (WT) SLX4 cDNA was a kind gift from the Harper Lab (Harvard Medical School, Boston, MA). N- and C-terminal mutants of SLX4 were amplified by PCR and were cloned in pDONR233. Primers used in cloning are shown in supplemental Table 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). SLX4 (1-671) expression vector produces the truncated SLX4 mutant, p.Leu672ValfsX119, which is described previously.8 Internal deletion and point mutation SLX4 mutants were generated with the QuikChange II XL Site-Directed Mutagenesis kit (Agilent Technologies) using a template of WT SLX4 cDNA cloned in pENTR plasmid. Primers used in mutagenesis are shown in supplemental Table 3. The mutants were recombined into pHAGE vector using LR clonase (Invitrogen). HPV E6 and E7 and hTERT expression plasmids were described previously.8
Antibodies and immunoprecipitations
The following antibodies were used: HA (Covance, MMS-101R), ERCC1 (Santa Cruz Biotechnology, FL297), XPF/ERCC4 (Bethyl Laboratory, A301-315A), MUS81 (Sigma-Aldrich, M1445), GFP (Roche Applied Science, 11814460001), FANCD2 (Novus, NB 100-182), and α-tubulin (Sigma-Aldrich, T9026). For immunoprecipitations, cells were lysed in MCLB (50mM Tris-HCl, pH 7.5, 150mM NaCl, and 0.5% Nonidet P-40) supplemented with protease inhibitors (Roche Diagnostics) and phosphatase inhibitors (Calbiochem). A total of 1 or 2 mg of protein extract was incubated with 5 μL of anti-HA agarose (Sigma-Aldrich, A 2095). After 5 washes in lysis buffer, the immunoprecipitates were eluted in Tris-glycine SDS sample buffer and size fractionated on Novex 3%-8% Tris-Acetate gel (Invitrogen).
RNAi and quantitative RT-PCR
For siRNA depletion experiments, U2OS or fibroblast cells were transfected with a pool of 3 siRNAs against SLX4, XPF, or MUS81 (sequences listed in supplemental Table 4) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instruction. For quantitative RT-PCR, total RNA was prepared from U2OS cells with RNeasy kit (QIAGEN). Superscript III reverse transcriptase followed by Platinum cybergreen super mix (Invitrogen) was used according to the manufacturer's instruction. β-actin was used as a control. Primers for quantitative RT-PCR of SLX4 and β-actin are shown in supplemental Table 5.
Immunofluorescence
Immunofluorescence was performed on U2OS and human fibroblasts as described previously.25 Cells were grown on coverslips and washed twice with PBS. Cells were pre-extracted with 0.1% Triton X-100 in PBS, fixed in 3.7% formaldehyde, and permeablized with NP-40. Cells were washed with PBS twice and blocked with PBG (0.2% [weight/volume] cold fish gelatin, 0.5% [weight/volume] BSA in PBS) for 20 minutes. Coverslips were incubated with relevant antibodies overnight at 4°C. Cells were washed 3 times with PBG and incubated with the appropriate secondary antibody. After 3 additional washes in PBG, the coverslips were embedded in Vectashield (Vector Laboratories) supplemented with 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescence images were examined and taken at room temperature by the Axio Observer.A1 fluorescence microscope (Carl Zeiss), equipped with a Plan- Apochromat 63× NA-1.4 oil objective, the AxioCam CCD camera, and the AxioVision Rel Version 4.7 software.
MMC, CPT, and PARP inhibitor sensitivity assay
A total of 2.5 × 104 cells were plated in each well of a 6-well plate in triplicate. At 24 hours later, MMC (Sigma-Aldrich, M4287), CPT (Sigma-Aldrich, C9911), or PARP inhibitor obtained from KuDOS (KU005894826 ) was added at final concentrations from 0-100nM for MMC, 0-16nM for CPT, or 0-10μM for PARP inhibitor. For PARP inhibitor sensitivity assay, drug-containing medium was replaced every 48 hours. After 8 days in culture, cell numbers were counted with a Z2 Coulter counter (Beckman Coulter). The cell numbers at each dose of drug were divided by the cell number in the untreated sample to calculate the percent survival. For the MMC and CPT sensitivity assays with depletion of XPF and MUS81, 500 cells were plated in each well of a 96-well Opaque White Microtest plate (BD Biosciences, 353296) in triplicate. At 24 hours later, MMC or CPT was added. After 5 days in culture, cell survival was determined using the Cell Titer-Glo reagent (Promega) according to the manufacturer's instruction. Commercially available PARP inhibitor, Olaparib (O-9210, LC Laboratories) has been used for sensitivity assays and showed similar effectiveness to the PARP inhibitor used in this study (data not shown).
Results
Human FA-P fibroblast cell line, RA3331, is null for SLX4
To study the SLX4-dependent nuclease requirements for ICL and CPT-induced DNA repair, we have used human FA complementation group P (FA-P) cell line, RA3331,8 which has been transformed using HPV E6 and E7 proteins and immortalized with a catalytic subunit of human telomerase hTERT (RA3331/E6E7/hTERT). We have chosen the RA3331 cell line for functional study of SLX4 as we could detect neither the full-length or truncated SLX4 by immunoprecipitation with antibodies raised against the N- or C- terminus of SLX4 in this cell line8 (data not shown). The mutations in this person are predicted to give rise to frameshift and premature stop codons in both alleles8 (protein effect p.Leu672ValfsX119 and pLeu172PhefsX22 from the maternal and paternal allele, respectively), most likely resulting in activation of nonsense mediated decay pathway.
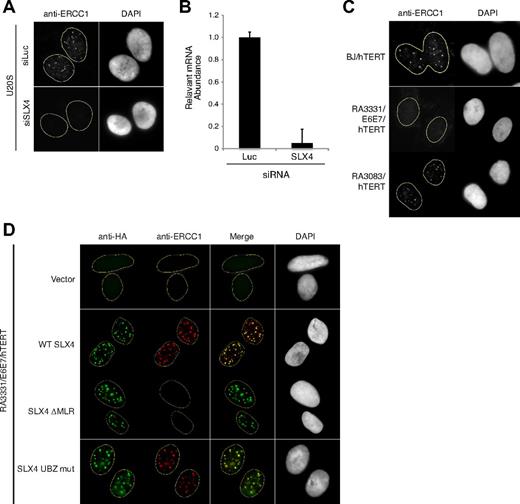
ERCC1 focus formation is entirely dependent on the presence of SLX4, as depletion of SLX4 in U2OS cell line abrogates all ERCC1 foci as visualized by indirect immunofluorescence (Figure 1A-B; supplemental Figure 1A). As expected, we could not detect any ERCC1 foci in the RA3331/E6E7/hTERT cell line, whereas normal ERCC1 foci were detected in BJ fibroblasts from a healthy donor and another FA-P cell line, RA3083/hTERT, which expresses a mutant form of SLX4 internally deleted for the UBZ domains of SLX4 (Figure 1C; supplemental Figure 1B).8 In addition, the RA3331/E6E7/hTERT cell line regains the ability to form ERCC1 foci when it is complemented with WT SLX4 cDNA (Figure 1D; supplemental Figure 1C). These results indicate that the RA3331 cell line is null for SLX4, resulting in an ideal setting for study of SLX4 mutants in DNA repair.
RA3331 FA-P cell line is null for SLX4. (A) Indirect immunofluorescence with an antibody against ERCC1 in U2OS cells transfected with a combination of 3 siRNAs against SLX4 or against Luciferase (Luc) as a control. The U2OS cells were pre-extracted with Triton-X100 before fixation. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). (B) Efficiency of SLX4 knockdown. The bar graph represents the decrease in relative SLX4 mRNA in U2OS cell line transfected with Luc or SLX4 siRNA. The error bars represent SD in triplicate experiments. (C) Indirect immunofluorescence with an antibody against ERCC1 in BJ/hTERT, RA3083/hTERT, and RA3331/E6E7/hTERT cell lines. The cell lines were prepared as in panel A. (D) Indirect immunofluorescence staining of RA3331/E6E7/hTERT expressing indicated SLX4 mutants using anti-HA and anti-ERCC1 antibodies. Nuclei were stained with DAPI. The cell lines were prepared as in panel A.
RA3331 FA-P cell line is null for SLX4. (A) Indirect immunofluorescence with an antibody against ERCC1 in U2OS cells transfected with a combination of 3 siRNAs against SLX4 or against Luciferase (Luc) as a control. The U2OS cells were pre-extracted with Triton-X100 before fixation. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). (B) Efficiency of SLX4 knockdown. The bar graph represents the decrease in relative SLX4 mRNA in U2OS cell line transfected with Luc or SLX4 siRNA. The error bars represent SD in triplicate experiments. (C) Indirect immunofluorescence with an antibody against ERCC1 in BJ/hTERT, RA3083/hTERT, and RA3331/E6E7/hTERT cell lines. The cell lines were prepared as in panel A. (D) Indirect immunofluorescence staining of RA3331/E6E7/hTERT expressing indicated SLX4 mutants using anti-HA and anti-ERCC1 antibodies. Nuclei were stained with DAPI. The cell lines were prepared as in panel A.
SLX4 interacts with XPF-ERCC1, MUS81-EME1, and SLX1 through MLR, SAP, and SBD domains, respectively
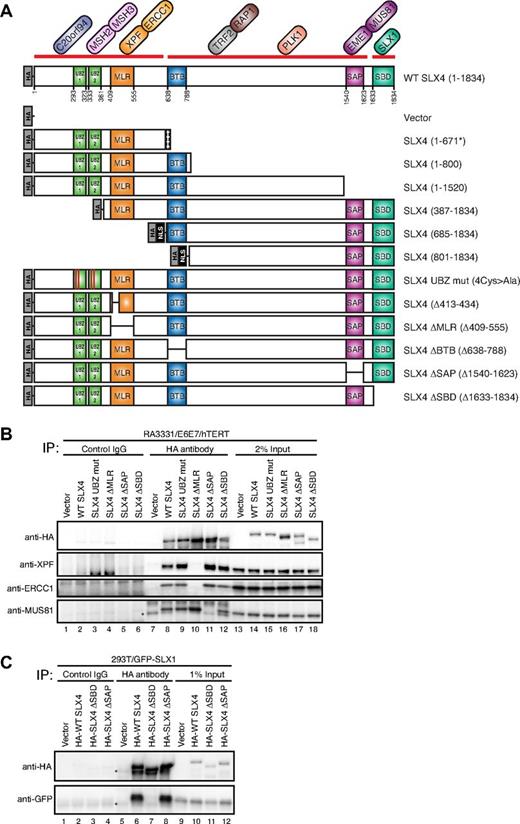
Human SLX4 contains multiple conserved domains (Figure 2A). The N-terminal segment of SLX4 was shown to interact with XPF-ERCC1 heterodimer,7,27 and it contains MUS312-MEI9 interaction-like region (MLR),28 which has been previously implicated in the interaction between Drosophila melanogaster orthologs of SLX4 and XPF. MUS81 has been shown to interact with SLX4 between amino acids 1043 and 1664,7,27 which contains a well-conserved SAP domain. SLX1 interacts with the extreme C terminus of SLX4 (residues 1632-1834) through SLX1 binding domain (SBD).7,27
SLX4 interacts with XPF/ERCC1, MUS81/EME1, and SLX1 through the MLR, SAP, and SBD domains, respectively. (A) Schematic illustration of the domain structure of SLX4 and its interacting partners, along with N-terminally HA-tagged SLX4 mutants used in this study. The broad interaction domains indicated by a thick red line were reported before.7 SV40 nuclear localization signal (NLS) was used for SLX4 (685-1834) and SLX4 (801-1834) to target them into the nucleus. SLX4 (1-671*) indicates the predicted mutant protein, p.Leu672ValfsX1198 in the cell line RA3331. (B) Analysis of the interacting partners of SLX4 mutants. The cell extracts from RA3331/E6E7/hTERT cell lines expressing the indicated SLX4 mutants were subjected to immunoprecipitation using anti-HA antibody or a control IgG. SLX4, XPF, ERCC1, and MUS81 were identified by immunoblotting with appropriate antibodies. A total of 2% of cell lysate used for immunoprecipitation was analyzed as a control (2% Input). Immunoprecipitation of the other SLX4 mutants used in this study are shown in supplemental Figure 2. (C) Cell extracts from 293T cells stably expressing GFP-SLX1 and HA-tagged SLX4 mutants were immunoprecipitated with anti-HA antibody or control IgG. SLX4 and SLX1 were identified by immunoblotting with anti-HA and anti-GFP antibodies, respectively. Asterisks indicate cross-reacting band. A total of 1% of cell lysate used for immunoprecipitation was analyzed as a control (1% Input).
SLX4 interacts with XPF/ERCC1, MUS81/EME1, and SLX1 through the MLR, SAP, and SBD domains, respectively. (A) Schematic illustration of the domain structure of SLX4 and its interacting partners, along with N-terminally HA-tagged SLX4 mutants used in this study. The broad interaction domains indicated by a thick red line were reported before.7 SV40 nuclear localization signal (NLS) was used for SLX4 (685-1834) and SLX4 (801-1834) to target them into the nucleus. SLX4 (1-671*) indicates the predicted mutant protein, p.Leu672ValfsX1198 in the cell line RA3331. (B) Analysis of the interacting partners of SLX4 mutants. The cell extracts from RA3331/E6E7/hTERT cell lines expressing the indicated SLX4 mutants were subjected to immunoprecipitation using anti-HA antibody or a control IgG. SLX4, XPF, ERCC1, and MUS81 were identified by immunoblotting with appropriate antibodies. A total of 2% of cell lysate used for immunoprecipitation was analyzed as a control (2% Input). Immunoprecipitation of the other SLX4 mutants used in this study are shown in supplemental Figure 2. (C) Cell extracts from 293T cells stably expressing GFP-SLX1 and HA-tagged SLX4 mutants were immunoprecipitated with anti-HA antibody or control IgG. SLX4 and SLX1 were identified by immunoblotting with anti-HA and anti-GFP antibodies, respectively. Asterisks indicate cross-reacting band. A total of 1% of cell lysate used for immunoprecipitation was analyzed as a control (1% Input).
To further delineate the interaction domains of SLX4 with XPF-ERCC1, MUS81-EME1 and SLX1, and to create SLX4 mutants unable to interact with each of these nucleases, we generated a series of N-, C-, and internal-deletion mutants of SLX4, all of which were HA-tagged (Figure 2A). In addition, the 2 conserved cysteines of each UBZ domain (Cys296, Cys299, Cys336, and Cys339) were mutated to alanines to create the SLX4 UBZ mut (Figure 2A). The UBZ domain has been shown to bind to ubiquitin chains in vitro; however, its exact function is unknown.8 All mutant cDNAs were stably expressed in the RA3331/E6E7/hTERT cell line using lentiviral transduction.
Cell lysates from the RA3331/E6E7/hTERT cell line expressing each of the SLX4 mutants were subjected to immunoprecipitation with anti-HA antibody, and the eluates were immunoblotted with anti-XPF, anti-ERCC1, and anti-MUS81 antibodies. It was previously proposed that a conserved motif within the region responsible for the interaction between D melanogaster MUS312SLX4 and MEI9XPF (residues 413-434)27 or BTB domain (broad-complex, tramtrack, and bric à brac, 684-788)29 of SLX4 is required for the XPF interaction. However, immunoprecipitation (supplemental Figure 2B) showed that XPF-ERCC1 is still able to interact with SLX4 (Δ413-434) or SLX4 ΔBTB (Δ638-788). In an efforts to fine map the interaction domain, we found that XPF-ERCC1 was absent in the immunoprecipitates from SLX4 ΔMLR (Δ409-555; Figure 2B lane 10), SLX4 (685-1834), and SLX4 (801-1834), not one of which contains the MLR domain (supplemental Figure 2A lanes 15 and 16). Hereafter we named the region (residues 409-555) as MLR domain. Other SLX4 mutants containing the MLR domain interacted with XPF-ERCC1 (Figure 2B; supplemental Figure 2A). In addition, SLX4 ΔMLR-expressing cells lacked ERCC1 foci by indirect immunofluorescence although SLX4 ΔMLR could itself be recruited to foci (Figure 1D; supplemental Figure 1C). These data show that the MLR domain is responsible for SLX4-XPF interaction.
Next, we turned our attention to the MUS81-SLX4 interaction and could demonstrate that MUS81 interacts with SLX4 through the SAP domain because the SLX4 mutant with an internal SAP domain deletion failed to interact with MUS81 (Figure 2B lane 11). All other SLX4 mutants lacking the SAP domain, such as SLX4 (1-671) SLX4 (1-800), and SLX4 (1-1520), failed to interact with MUS81 (supplemental Figure 2A lanes 11-13), and the SLX4 mutants in which the SAP domain was retained were able to interact with MUS81 (Figure 2B; supplemental Figure 2).
To confirm that SLX1 interacts with SLX4 through the SBD domain, we generated a 293T cell line expressing GFP-SLX1 and HA-tagged WT SLX4, SLX4 ΔSAP or SLX4 ΔSBD. After immunoprecipitations using anti-HA antibody, the eluates were probed for GFP-SLX1. SLX1 failed to interact with SLX4 ΔSBD but interacted with WT SLX4 and SLX4 ΔSAP (Figure 2C) as shown in previous studies.7,27 Lastly, SLX4 UBZ mut was able to interact with XPF-ERCC1 and MUS81 and also colocalized with ERCC1, indicating that the ubiquitin-binding functions of SLX4's UBZ domains are dispensable for both XPF and MUS81 interactions (Figure 2B lane 9; Figure 1D; supplemental Figure 1C).
SLX4-XPF interaction plays an essential role in ICL repair but is dispensable for repairing CPT and PARP inhibitor-induced damage
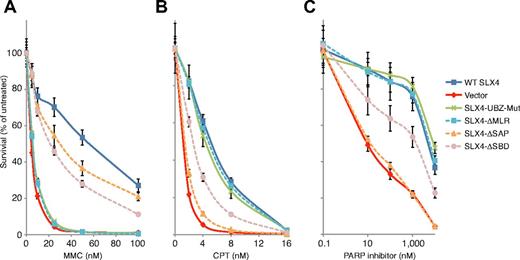
To study the role of XPF in the repair of MMC, CPT, and PARP inhibitor-induced damage, we assessed drug sensitivity of the RA3331/E6E7/hTERT cell line expressing various mutant cDNAs depicted in Figure 2A. The cell line transduced with empty vector was sensitive to MMC and CPT, and the resistance to both drugs was restored by expression of WT SLX4 through lentiviral transduction (Figure 3A-B). The RA3331/E6E7/hTERT cell line expressing SLX4 ΔMLR completely failed to rescue MMC sensitivity but displayed resistance to CPT at the same level as WT SLX4-expressing cells (Figure 3A-B). Consistently, the SLX4 mutants lacking the MLR domain, and thus unable to interact with XPF, did not rescue MMC sensitivity but rescued CPT sensitivity (supplemental Figure 3A-B).
Analysis of the sensitivity of RA3331/E6E7/hTERT cell lines expressing SLX4 mutants to MMC, CPT, and PARP inhibitor. (A) MMC sensitivity in RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. (B) CPT sensitivity in RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. (C) PARP inhibitor sensitivity assay of RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. RA3331/E6E7/hTERT fibroblast cell lines expressing WT SLX4, empty vector, SLX4 UBZ mut, SLX4 ΔMLR, SLX4 ΔSAP, and SLX4 ΔSBD were treated in triplicate with increasing concentrations of MMC (0-100nM), CPT (0-16nM), and PARP inhibitor (0-10μM). After 8 days in culture, the cell number was determined using a Coulter counter. The number of cells at each drug concentration was divided by the number of cells in the untreated sample to calculate the percentage of cell survival. The error bars represent SD from 3 replicates. Sensitivity assays in cells expressing the other SLX4 mutants used in this study are shown in supplemental Figure 3.
Analysis of the sensitivity of RA3331/E6E7/hTERT cell lines expressing SLX4 mutants to MMC, CPT, and PARP inhibitor. (A) MMC sensitivity in RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. (B) CPT sensitivity in RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. (C) PARP inhibitor sensitivity assay of RA3331/E6E7/hTERT cell lines expressing indicated SLX4 mutants. RA3331/E6E7/hTERT fibroblast cell lines expressing WT SLX4, empty vector, SLX4 UBZ mut, SLX4 ΔMLR, SLX4 ΔSAP, and SLX4 ΔSBD were treated in triplicate with increasing concentrations of MMC (0-100nM), CPT (0-16nM), and PARP inhibitor (0-10μM). After 8 days in culture, the cell number was determined using a Coulter counter. The number of cells at each drug concentration was divided by the number of cells in the untreated sample to calculate the percentage of cell survival. The error bars represent SD from 3 replicates. Sensitivity assays in cells expressing the other SLX4 mutants used in this study are shown in supplemental Figure 3.
Next, we addressed the function of XPF-ERCC1 in ICL repair. It has been previously proposed that XPF-ERCC1 might play a role either in the incision step, in processing of ICL-induced DSBs facilitating their repair by homologous recombination during ICL repair, or even in the homologous recombination repair itself.16,30-34 To shed light on the in vivo function of XPF in the process of ICL repair, we used PARP inhibitor sensitivity assay to test the functional relevance of SLX4 and XPF interaction in homologous recombination. It is known that homologous recombination defect is synthetically lethal with inhibition of PARP.35,36 Therefore, we have used sensitivity to PARP inhibitor as a surrogate marker for homologous recombination competence of the SLX4 null cells expressing SLX4 mutants. The parental SLX4 null RA3331 cell line was sensitive to a PARP inhibitor and complementation with WT SLX4 restored resistance to it (Figure 3C). SLX4 ΔMLR and all other mutants, which we have determined to be null for an XPF-SLX4 interaction, complemented PARP sensitivity to the same degree as WT SLX4, indicating that the XPF-SLX4 interaction is dispensable for homologous recombination. Therefore, these findings favor the model that XPF-ERCC1 is involved in the incision step during ICL repair.
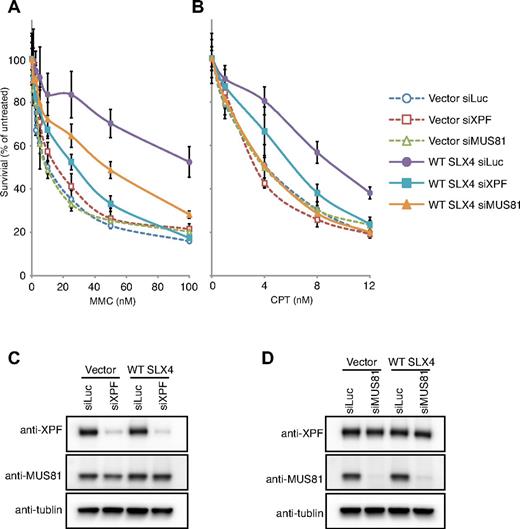
To test the SLX4-independent functions of XPF-ERCC1 in ICL repair, we performed the MMC sensitivity assays after depletion of XPF in the RA3331/E6E7/hTERT cell lines transduced with empty vector or WT SLX4 (Figure 4C). As, expected, depletion of XPF in the presence of the SLX4 (Figure 4A WT SLX4 siXPF) led to enhanced sensitivity of complemented cells to MMC. In contrast, depletion of XPF failed to potentiate the sensitivity to MMC in the absence of the SLX4 (Figure 4A Vector siXPF). These observations demonstrate that the function of XPF-ERCC1 in ICL repair is fully dependent on SLX4 and that free XPF is unable to participate in ICL repair as measured by the sensitivity assay. XPF depletion in SLX4 null cell line also had no further effect on CPT sensitivity (Figure 4B Vector siXPF). Although the interaction between SLX4 and XPF was not required for rescuing CPT sensitivity (Figure 3B SLX4ΔMLR), depletion of XPF in the presence of the SLX4 displayed enhanced sensitivity to CPT (Figure 4B WT SLX4 siXPF), suggesting that XPF-ERCC1 might possess an SLX4-independent function in the repair of CPT-induced DNA lesions.
Genetic interaction between SLX4 and nucleases in the response of MMC and CPT. (A) MMC sensitivity in RA3331/E6E7/hTERT cell lines expressing the empty vector and WT SLX4 after knocking-down indicated nucleases. (B) CPT sensitivity in RA3331/E6E7/hTERT cell lines expressing the empty vector and WT SLX4 after knocking down indicated nucleases. (C-D) The efficiency of knockdown of XPF and MUS81 was measured in whole cell extracts by Western blotting. The siRNA against Luciferase (siLuc) was used as a negative control. (A-B) The error bars represent SD from 3 replicates.
Genetic interaction between SLX4 and nucleases in the response of MMC and CPT. (A) MMC sensitivity in RA3331/E6E7/hTERT cell lines expressing the empty vector and WT SLX4 after knocking-down indicated nucleases. (B) CPT sensitivity in RA3331/E6E7/hTERT cell lines expressing the empty vector and WT SLX4 after knocking down indicated nucleases. (C-D) The efficiency of knockdown of XPF and MUS81 was measured in whole cell extracts by Western blotting. The siRNA against Luciferase (siLuc) was used as a negative control. (A-B) The error bars represent SD from 3 replicates.
The UBZ domain is critical for SLX4 to repair ICLs but is not required for CPT or PARP inhibitor-induced DNA damage repair
We have previously shown that the SLX4 patient cell line with an in-frame deletion of part of the first and the whole second UBZ domain was sensitive to MMC but not to CPT.8 To extend those findings, we tested SLX4 (387-1834), which lacks the UBZ domain but interacts with XPF (supplemental Figure 2A lane 6), and found that it failed to rescue the MMC sensitivity (supplemental Figure 3A). However, it conferred resistance to CPT and PARP inhibitor to the same level as WT SLX4 (supplemental Figure 3B-C). To further characterize the UBZ domain, we mutated 4 cysteines (Cys 296, 299, 336, and 339), which are necessary for proper folding of the UBZ domains, to alanines in full-length SLX4 (SLX4 UBZ mut in Figure 2A) and tested MMC, CPT, and PARP inhibitor sensitivity of the RA3331/E6E7/hTERT cell line expressing this mutant. SLX4 UBZ mut cells were as sensitive to MMC as SLX4 null cells but were fully resistant to CPT and PARP inhibitor (Figure 3A-C), implying that UBZ domains are critical only for SLX4-dependent repair of ICLs. The exact function of the domain needs to be further explored. We have previously implicated the UBZ domain in binding to K63-linked chains in vitro and speculated that that interaction might be able to bring the XPF-ERCC1 complex to the site of the ICL damage.8 However, ERCC1 is able to form foci, even in the cells that express SLX4 UBZ mut (Figure 1D), suggesting that the UBZ domains are not important for localizing the XPF-ERCC1 complex to sites of damage but instead have additional uncharacterized function in vivo.
MUS81-SLX4 interaction is critical for repairing CPT and PARP inhibitor-induced DNA repair
We and others have reported that SLX4 plays a role in resolving CPT-induced DNA lesions.6-8 To determine the issue of which SLX4-recruited nucleases are necessary for CPT resistance, we investigated CPT sensitivity of RA3331/E6E7/hTERT cell lines expressing the SLX4 mutants depicted in Figure 2A. SLX4 ΔSAP, and other SLX4 mutants lacking the SAP domain, including SLX4, (1-671) SLX4 (1-800), and SLX4 (1-1520), completely failed to rescue the CPT sensitivity (Figure 3B; supplemental Figure 3B), implying that MUS81 bound to SLX4 plays a critical role in CPT-induced DNA damage repair. Interestingly, SLX4 ΔSAP was able to rescue the MMC sensitivity, although not to the same extent as the WT SLX4 (Figure 3A), suggesting that MUS81 has a minor role in repairing the ICL lesions. We also assessed the PARP inhibitor sensitivity of the SLX4 mutants that were unable to bind to MUS81 and identified this interaction to be essential for resistance to PARP inhibitor (Figure 3C; supplemental Figure 3C). These data demonstrate that the interaction between SLX4 and MUS81 plays an important role in processing of stalled replication forks because of PARP inhibition and/or in homologous recombination itself.
Next, we asked whether the non–SLX4-associated MUS81-EME1 plays a role in the repair of MMC- and CPT-induced DNA lesions. To this end, MUS81 was depleted in RA3331/E6E7/hTERT cell lines expressing empty vector and WT SLX4 (Figure 4D). As expected, depletion of MUS81 in the presence of the SLX4 increased the sensitivity to MMC, but not to the level of SLX4 null cells (Figure 4A WT SLX4 siMUS81). Further depletion of MUS81 in SLX4 null cells resulted in the same MMC sensitivity as in the parental SLX4 null cells (Figure 4A Vector siMUS81), implying that the function of MUS81 in ICL repair depends on the SLX4. Depletion of MUS81 from both the vector and SLX4-transduced SLX4 null cell lines resulted in sensitivity to CPT equal to that found in the SLX4 null cells (Figure 4B WT SLX4 siMUS81 and Vector siMUS81). These observations demonstrate that the functions of MUS81 in the repair of CPT-induced damage are fully governed by SLX4.
SLX1-SLX4 interaction plays some role in MMC, CPT, and PARP inhibitor-induced DNA repair
Although SLX1 displays an in vitro Holliday junction (HJ) resolvase activity, the in vivo function of SLX1 in repair at a stalled fork is not clear. Experiments using siRNA depletion of SLX1 gave conflicting results on the importance of this nuclease in repairing CPT damage but showed that it was required for ICL repair.6,7 However, using a mouse model, it has been shown that the SLX4 lacking the interaction with SLX1 was able to fully complement MMC resistance in mouse embryonic fibroblasts deficient in SLX4.24 In our system, expression of the SLX4 ΔSBD mutant that cannot interact with SLX1 conferred partial resistance to MMC, CPT, and PARP inhibitor (Figure 3). It is unclear whether it is the HJ resolvase activity of SLX1-SLX4 that is necessary in these repair pathways, but it is interesting that the cell line expressing SLX4 (1-1520), which lacks both the MUS81 and SLX1 interaction domains, results in higher MMC sensitivity than the cell lines expressing mutants that cannot interact with either MUS81 or SLX1 alone (supplemental Figure 3A), suggesting that the functions of MUS81 and SLX1 are nonoverlapping at the stalled replication fork.
BTB domain is important for resistance to MMC but not to other damaging agents
The BTB domain is responsible for protein dimerization and interaction with non-BTB proteins.37 We found that the BTB domain of human SLX4 was not responsible for the interaction with XPF, MUS81, or SLX1 (supplemental Figure 2B). However, RA3331/E6E7/hTERT cell line expressing SLX4 ΔBTB only partially rescued MMC sensitivity, although it conferred full resistance to CPT and PARP inhibitor (supplemental Figure 3). The role of the BTB domain of SLX4 in ICL repair represents an important question for the future.
The upstream components of the FA pathway are not involved in resistance to CPT or PARP inhibitor-induced DNA damage repair
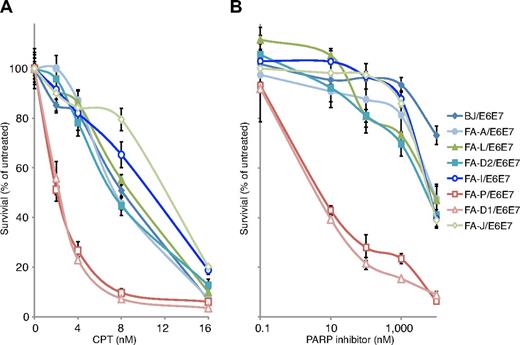
Our findings opened a question as to which of the functions of SLX4 were the result of defects in the FA pathway and which might be FA-independent. Acute knockdown of FANCI and FANCD2 in U2OS cells leads to CPT sensitivity,2 although the human lymphoblasts from FA patients are not sensitive to CPT, with the exception of FANCM, which has functions outside of the FA pathway.38 Mouse embryonic fibroblasts lacking FANCA, FANCC, or FANCD2 were sensitive to PARP inhibition, although the extent of their sensitivity compared with true homologous recombination null cell lines was unclear.36 We chose 7 FA patient fibroblast cell lines, which are sensitive to MMC (supplemental Figure 4), to test the CPT and PARP inhibitor sensitivity: FA-A (RA3087), FA-L (RA3100), FA-D2 (RA2645), FA-I (RA2480), FA-J (RA2374), FA-D1 (RA3226), and FA-P (RA3331; supplemental Table 1) and a BJ (normal fibroblast) cell line as a WT control. Neither FA core complex nor FANCI or FANCD2 defective cell lines were sensitive to CPT or PARP inhibitor. This was in contrast to FA-P (FANCP/SLX4) and FA-D1 (FANCD1/BRCA2) deficient cell lines, which were both sensitive to CPT as well as PARP inhibitor (Figure 5A-B). These findings suggest that, in the setting of patient fibroblasts and lymphoblasts,38 the upstream components of the FA pathway are not involved in repairing CPT- and PARP inhibitor-induced DNA lesions, but the downstream effectors of the FA pathway, such as SLX4 and BRCA2, play an FA-pathway independent role in these repair processes.
Analysis of CPT and PARP inhibitor sensitivity of FA fibroblast cell lines. (A) CPT sensitivity assay of the indicated FA fibroblast cell lines. (B) PARP inhibitor sensitivity assay of the indicated FA fibroblast cell lines. All cell lines have been transduced with HPV E6E7. Indicated FA cell lines were treated in triplicate with increasing concentrations of CPT (0-16nM) and PARP inhibitor (0-10μM). After 8 days in culture, the cell number was determined with Coulter counter. The number of cells at each drug concentration was divided by the number of cells in the untreated sample to calculate the percentage of cell survival. The error bars represent SD from 3 replicates.
Analysis of CPT and PARP inhibitor sensitivity of FA fibroblast cell lines. (A) CPT sensitivity assay of the indicated FA fibroblast cell lines. (B) PARP inhibitor sensitivity assay of the indicated FA fibroblast cell lines. All cell lines have been transduced with HPV E6E7. Indicated FA cell lines were treated in triplicate with increasing concentrations of CPT (0-16nM) and PARP inhibitor (0-10μM). After 8 days in culture, the cell number was determined with Coulter counter. The number of cells at each drug concentration was divided by the number of cells in the untreated sample to calculate the percentage of cell survival. The error bars represent SD from 3 replicates.
Discussion
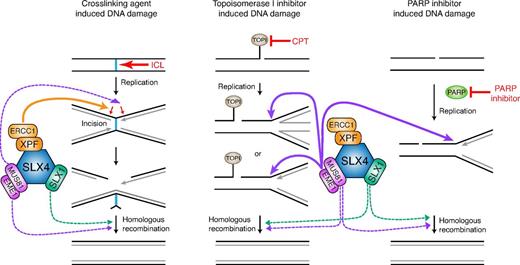
Using a FA patient cell line null for SLX4, we have dissected the nuclease requirements for repairing specific DNA lesions induced by MMC, CPT, and PARP inhibitor. We demonstrate that the repair of ICL damage in human cells is dependent on the XPF-SLX4 complex and to a much lesser extent on SLX1-SLX4 or MUS81-SLX4. Repair of CPT and PARP inhibitor-induced DNA damage requires MUS81 with some contribution of SLX1 but no involvement of XPF associated with SLX4. We further provide evidence that there is no additional function of the non-SLX4–associated pool of XPF-ERCC1 and MUS81-EME1 in the repair of MMC- and CPT-induced DNA lesions, respectively. In combination with previous data, these findings put XPF as the major nuclease that makes incisions during ICL repair and MUS81 as the nuclease necessary for processing stalled replication because of TOP1 frozen on the DNA (Figure 6). Surprisingly, in the absence of PARP activity, MUS81 is also required to process the damage that presumably occurs at the site of a single-strand break (SSB). Based on the parallel requirements for MUS81 in CPT- and PARP inhibitor-induced damage repair, we speculate that the MUS81 activity is required in PARP inhibitor-treated cells during S phase at the stalled fork, when there is a risk that a persistent SSB would become a DSB. It is also possible that MUS81 participates in the later stages of homologous recombination, although if that were the case, we would expect a greater contribution of MUS81 to ICL resistance. It should be noted that our experiments were done using low levels of CPT, which in recent experiments39 were shown to cause no DSBs. Instead, Chaudhuri et al observed PARP1-dependent replication fork reversal under low CPT treatment.39 Based on our data, we propose that such reversed forks need MUS81 for their processing. Further studies will be crucial to address how these reversed forks are resolved and how MUS81-EME1 acts on these substrates.
A proposed model of the SLX4-associated activities during the repair of MMC, CPT, and PARP inhibitor-induced DNA damage. MMC induces DNA ICLs. CPT traps a TOP1 cleavage complex, composed of TOP1 and nicked DNA, and prevents religation of the nicks. Unrepaired nicked DNA can result in reversed forks or turn into DSB when it encounters replication machinery in S phase. Inhibition of PARP prohibits SSB repair. Unrepaired SSB forms DSB during DNA replication, although we speculate that they might also cause reverse forks as seen for the CPT-induced damage. Straight lines and dotted lines represent proposed major and minor activity of the indicated nucleases. XPF-SLX4 complex plays a major role in the ICL repair but is dispensable for repairing CPT and PARP inhibitor-induced DNA lesions. MUS81-SLX4 interaction is critical for conferring resistance to CPT and PARP inhibitor through its activity on stalled/reversed forks, and plays a minor role in ICL repair, possibly during incision or in the homologous recombination step. SLX1-SLX4 resolvase participates in all 3 DNA repair processes, although its function is minor possibly because of existence of parallel/redundant processing pathways.
A proposed model of the SLX4-associated activities during the repair of MMC, CPT, and PARP inhibitor-induced DNA damage. MMC induces DNA ICLs. CPT traps a TOP1 cleavage complex, composed of TOP1 and nicked DNA, and prevents religation of the nicks. Unrepaired nicked DNA can result in reversed forks or turn into DSB when it encounters replication machinery in S phase. Inhibition of PARP prohibits SSB repair. Unrepaired SSB forms DSB during DNA replication, although we speculate that they might also cause reverse forks as seen for the CPT-induced damage. Straight lines and dotted lines represent proposed major and minor activity of the indicated nucleases. XPF-SLX4 complex plays a major role in the ICL repair but is dispensable for repairing CPT and PARP inhibitor-induced DNA lesions. MUS81-SLX4 interaction is critical for conferring resistance to CPT and PARP inhibitor through its activity on stalled/reversed forks, and plays a minor role in ICL repair, possibly during incision or in the homologous recombination step. SLX1-SLX4 resolvase participates in all 3 DNA repair processes, although its function is minor possibly because of existence of parallel/redundant processing pathways.
Our data indicate that multiple pathways, which are necessary for the DNA repair at the stalled replication fork, can use SLX4, a single but modular scaffold. It is not surprising that various lesions necessitate different nucleases for their processing, but it is interesting that they are coordinated by the same scaffold. One advantage of having one scaffold would be the efficiency of dealing with a multitude of lesions at the replication fork when a priori it might not be clear which activity might be necessary for proper repair or where sequential activity might be required. Another very interesting feature of the SLX4-interacting nucleases is that, in the absence of SLX4, they cannot act by themselves. The in vitro data suggested that SLX4 enhances the activity of the associated nucleases,6 but in vivo, the activity at the lesion site is completely missing if SLX4 is absent. The simplest explanation might be that the nucleases cannot be properly localized at the fork unless they are associated with SLX4. Another possibility is that the activation of the activity occurs by post-translational modifications of SLX4 or of the nucleases themselves and can occur only in the setting of the complex. Either of those possibilities allows for the proper regulation of the activities, which, if unregulated, would lead to genomic instability.40
We have identified the essential activities of SLX4-XPF and SLX4-MUS81 at the sites of DNA damage induced by MMC, CPT, and PARP inhibitor. Our studies implicate SLX1 in some of those activities as well, but the SLX4 null cells complemented with SLX4 that did not interact with SLX1 were only partially defective in resistance to MMC, CTP, or PARP inhibitor. There are a few possibilities that might explain why we have not identified the essential function of SLX1. One is that we have not probed the system with the right lesion, repair of which might be completely dependent on SLX4-SLX1 interaction. It is possible that the requirement might be only in the meiotic compartment but not in the mitotic cells, which we are studying. The other possibility is that there is a redundancy in the system and other proteins, such as GEN1 nuclease or BLM helicase, might be able to step in when SLX1 is not present. Indeed, in the absence of BLM, codepletion of GEN1 and SLX4 leads to chromosome segmentation, which has been suggested to indicate abnormal chromosome condensation at the sites of unresolved Holliday junctions.41
Besides the MLR, SAP, and SBD domains, which interact with XPF, MUS81, and SLX1, respectively, we have also deleted or mutated 2 other domains that are important for ICL repair, the UBZ and the BTB domains. The UBZ domain, as anticipated from our previous work,8 is essential for ICL repair, but its exact function in the repair process is unclear because the UBZ mutant form of SLX4 can interact with all the nucleases, and it appears that it also forms foci with XPF, implying that it can localize to damage sites. Because UBZ domains are known to interact with ubiquitinated proteins and SLX4 is an effector of the FA pathway, the obvious candidate for the UBZ interaction would be ubiquitinated FANCD2. Indeed, it was recently reported that the colocalization of SLX4 with FANCD2 is dependent on the presence of the UBZ domain in chicken B-cell line DT40.42 However, so far, we have been unable to show FANCD2-SLX4 interaction or colocalization of the 2 proteins in human cells. Unlike the mutation of the UBZ domain, the deletion of the BTB domain led to mild sensitivity of cells to MMC but no sensitivity to the other drugs tested, which indicates that the BTB domain might be important for interaction with another factor that participates, although it is not essential in ICL repair.
The cellular phenotype of the SLX4 null cells is complex, showing sensitivity to cross-linking agents, TOP1, and PARP inhibitors. However, as we show for fibroblasts (Figure 5) and as was previously shown for lymphoblasts,38 FA cell lines from the majority of the other FA complementation groups are only sensitive to the cross-linking agents and not to CPT or PARP inhibitor. This suggests that SLX4, like BRCA2, PALB2, and FANCM, has functions outside of the FA pathway proper. How these different functions affect the hematopoietic stem cell function in the FA-P persons has not yet been explored. However, it is clear that FA-P persons, whose cells are sensitive to MMC in vitro but have intact response to CPT, can display bone marrow failure. Four persons from FA-P group have been reported to have in-frame deletions of the UBZ domains. Cell lines from these persons were sensitive to MMC but not to CPT.8,9 Three of these persons had bone marrow failure with 2 of them requiring bone marrow transplantation at ages of 8.5 and 9.5 years. In light of our findings that the UBZ domain and the XPF-SLX4 interaction are specifically necessary for the ICL repair, we speculate that those are the key activities missing in the FA patients. Two of the other reported FA-P persons appear to be null for SLX4, and they both had more severe developmental phenotype than the 4 persons who were only missing the UBZ domain. One of them (EUFA1354) also had bone marrow failure, needing a bone marrow transplantation at the age of 10.8,9 The other person (IFAR414/1) had a milder hematologic phenotype, which can be explained by the finding that this person's lymphoblasts, unlike his fibroblasts, were not sensitive to MMC. This is indicative of mosaicism in the hematopoietic system, which is common in FA patients.43 This person, however, developed a squamous cell carcinoma of the tongue at 21 years of age and died of the disease a year later.8,9 In the 2 persons, who completely lack SLX4, we do not know whether the more severe phenotype is the result of stochastic events or whether it could be attributed to additional functions of SLX4 in DNA repair as described here. This question could be addressed in the mouse where SLX4 deficiency leads to brain and eye development defects, hydrocephalus, blood cytopenias and infertility.24 Knockin of some of the SLX4 mutants lacking the interaction with each of nucleases, as well as study of the SLX1 knockout mouse might shed further light on the in vivo function of the SLX4-associated nucleases. The advancing technologies of induced pluripotent stem cell development and genetic manipulation of human embryonic stem cells will also permit the further understanding of SLX4 in the hematopoietic system.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their participation in the International Fanconi Anemia Registry and members of the laboratory of A.S. for insightful comments.
This work was supported in part by the Anderson Cancer Center at Rockefeller University, Burroughs Wellcome Fund Career Award for Medical Scientists (A.S.), Starr Center Consortium grant, and the National Center for Research Resources (grant UL1RR024143). A.S. is a Rita Allen Foundation scholar, an Irma T. Hirschl scholar, the Alexandrine and Alexander Sinsheimer Foundation scholar, and a recipient of a Doris Duke Clinical Scientist Development Award.
National Institutes of Health
Authorship
Contribution: Y.K. and A.S. designed the study and wrote the manuscript; Y.K., G.S.S., U.V., and A.S. performed mutation analysis and functional studies; and F.P.L., A.D.A., and A.S. recruited subjects and collected samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Agata Smogorzewska, Laboratory of Genome Maintenance, Rockefeller University, 1230 York Ave, New York, NY 10065-6399; e-mail: asmogorzewska@rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal