Abstract
Treatment of mantle cell lymphoma (MCL) in younger patients remains a challenge. We report results of a phase 2 trial using cytarabine and rituximab as induction regimen before autologous stem cell transplantation. Patients younger than 66 years with stage 3 or 4 MCL were included. Treatment consisted of 3 courses of CHOP21 with rituximab at the third one and 3 of R-DHAP. Responding patients were eligible for autologous stem cell transplantation with TAM6 or BEAM. Sixty patients were included. Median age was 57 years. Characteristics of patients were: BM involvement 85%, leukemic disease 48%, gastrointestinal involvement 52%, Performance Status > 16%, lactate dehydrogenase > 1N 38%, Mantle Cell Lymphoma International Prognostic Index (low 55%, intermediate 38%, high 13%). The overall response rate was 93% after (R)-CHOP and 95% after R-DHAP. Although uncommon after (R)-CHOP (12%), 57% of patients were in complete response after R-DHAP. With median follow-up of 67 months, median event-free survival is 83 months, and median overall survival is not reached. Five-year overall survival is 75%. Comparison with a previous study without rituximab shows improvement of outcome (median event-free survival, 51 vs 83 months). No toxic death or unexpected toxicities were observed. This study confirms that induction with rituximab and cytarabine-based regimens is safe and effective in MCL patients. This regimen is currently compared with R-CHOP21 induction in a multicentric European protocol.
Key Points
Treatment of young patients with mantle cell lymphoma requires induction chemotherapy followed by autologous stem cell transplantation.
Higher efficacy without excess toxicity is obtained with high-dose cytarabine and rituximab before stem cell transplantation.
Introduction
Mantle cell lymphoma (MCL) is a distinct entity within the World Health Organization (WHO) classification of lymphoid neoplasm1 and represents approximately 8% of lymphoma. Typical clinical presentation includes male predominance, age of 50 years or older, and advanced stage with high frequency of BM, blood, and/or gastrointestinal involvement.2 Biologically, MCL cells are characterized by CD20+, CD5+, CD23−, and CCND1+ expression and, at a molecular level, by the chromosomal translocation t(11;14)(q13;q32), leading to overexpression of CCND1, which can be detected in virtually all cases by quantitative RT-PCR.3 At the therapeutic level, MCL is characterized by initial indolent features contrasting with usual resistance to first-line anthracycline-based therapy.4 At the beginning of this study, the outcome was virtually always fatal with a median overall survival (OS) of 3 or 4 years with classical treatment.
Despite recent advances, there is currently no standard therapy for newly diagnosed young patients (< 65 years) with MCL. In a randomized phase 3 trial, the European MCL network has demonstrated, for the first time, an OS benefit for younger patients with MCL with early consolidation with high-dose myeloablative radiochemotherapy followed by autologous stem cell transplantation (ASCT) over consolidation treatment with interferon.5 To improve complete response (CR) rate before ASCT, promising approaches were offered by modified induction therapy with cytarabine-based regimens.
More recently, the anti-CD20 monoclonal antibody rituximab has been extensively studied. Its efficacy in association with chemotherapy was still debated. On one hand, single-agent rituximab in MCL has only limited activity.6 On the other hand, Forstpointner et al have demonstrated, in a phase 3 randomized prospective study, a prolonged OS when rituximab is given with a combination of fludarabine, cyclophosphamide, and mitoxantrone compared with chemotherapy alone.7 It was confirmed recently in a meta-analysis,8 reporting improvement in OS for patients receiving R-chemotherapy compared with chemotherapy alone in MCL.
In an oligocentric study reported in 2002, we demonstrated that a higher response rate could be obtained by a sequential induction therapy with 3 cycles of CHOP21 followed by 3 cycles of DHAP (dexamethasone 40 mg days 1-4, cytarabine 2000 mg/m2/12 hours day 2, cisplatin 100 mg/m2 by continuous infusion over 24 hours day 1), and with an early consolidation with ASCT preceded by a conditioning regimen with cytarabine, melphalan, and total body irradiation (TBI).9 A prolonged event-free survival (EFS) was obtained for approximately half of the patients.
Here, we report a prospective phase 2 study in which we incorporated 4 infusions of rituximab to our standard regimen to confirm our previous results and to assess whether or not rituximab could improve response rate, EFS, and OS in association with high-dose cytarabine containing regimen.
Methods
Inclusion criteria
From 2000 to 2003, patients with newly diagnosed previously untreated Ann Arbor stage 3 or 4 MCL were enrolled in a prospective phase 2 multicentric clinical trial. MCL was confirmed by local pathologists and required histologic diagnosis according to the WHO classification. Either cyclin D1 hyperexpression assessed by quantitative RT-PCR or identification of a t(11;14) translocation on cytogenetic examination was required for diagnosis. Patients with blastoid variants, as defined by morphologic features and high mitotic rate following WHO criteria,1 were not included in the study. Patients must have at least 1 bidimensionally measurable lesion (lymph node > 1.5 cm in its largest dimension). Other inclusion criteria were Performance Status less than or equal to 2 and age of 18 years or older. Main exclusion criteria were other histologic types of lymphoma, pregnant or breastfeeding women, positivity for HIV serology, CNS or meningeal involvement, contraindication to any drug contained in the chemotherapy regimens, poor renal function (creatinine level > 150μM), poor hepatic function (total bilirubin level > 30μM, transaminases > 2.5 top normal level) unless related to liver involvement by MCL, poor BM reserve defined by neutrophils less than 1.5 G/L or platelets less than 100 G/L, unless related to BM infiltration, and any history of cancer during the past 5 years, with the exception of nonmelanoma skin tumors or in situ cervical carcinoma. Initial staging included complete physical examination; blood cell count with examination of blood smear; laboratory tests, including lactate dehydrogenase, β-2 microglobulin, and albumin; thoracic and abdominopelvic CT scan, BM biopsy; and endoscopy of digestive tract if indicated. Protocol was approved by the scientific committee of the Groupe d'Etude des Lymphomes de l'Adulte (GELA) and local ethical committee, and patients gave their informed consent before treatment according to the Declaration of Helsinki.
Treatment schedule
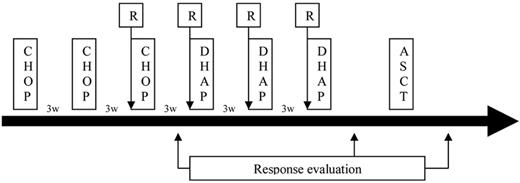
Patients received 3 cycles of CHOP21 (cyclophosphamide 750 mg/m2 day 1, doxorubicin 50 mg/m2 day 1, vincristine 1.4 mg/m2 day 1, and prednisone 40 mg/m2 days 1-5) every 21 days (Figure 1). At the time of the third cycle, patients received their first infusion of rituximab at a dose of 375 mg/m2. Whatever response, or earlier if patients progressed during CHOP therapy, patients received 3 cycles of DHAP every 21 days, associated with rituximab (375 mg/m2). Intrathecal prophylaxis (methotrexate 15 mg, cytarabine 40 mg, and corticosteroids) was administered according to physician decision.
Complete evaluation, including CT scan and BM biopsy, was performed. Responding patients were eligible for intensification. Peripheral blood stem cell harvest was performed after the third cycle of R-DHAP, requiring at least 3 × 106 CD34/kg. In case of harvest failure, a second procedure was allowed with high-dose G-CSF (10 μg/kg during 5 days). Then, patients received intensification with radio-chemotherapy with TBI (10 Gy over 3 days and twice-daily fractions), high-dose cytarabine (1500 mg/m2, 4 infusions every 12 hours), and high-dose melphalan (140 mg/m2). Peripheral stem cells were reinfused at day 0. Antimicrobial prophylaxis and use of G-CSF or erythropoietin were permitted according to physician decision.
Assessment of response and toxicity
Evaluation of disease control was performed after the 3 cycles of (R)-CHOP21, after the 3 cycles of R-DHAP, and 3 months after the end of treatment. Then, patients were followed every 3 months during the first year and thereafter every 6 months until progression or death. The staging workup included physical examination, complete blood cell count, and laboratory test, including lactate dehydrogenase level. Thoracic and abdominopelvic CT scan was performed at each follow-up point during treatment, at 6 and 12 months after treatment, and then every year. BM biopsy was performed at each follow-up point until normalization. Endoscopy of the digestive tract was performed before ASCT in case of initial gastrointestinal involvement. Response was evaluated according to Cheson criteria 1999.10 CR was defined by a reduction of the sum of diameter product of nodal and extranodal measurable lesions greater of 75% without (CR) or with (CRu) residual mass more than 15 mm and a normal BM examination. Partial response (PR) was defined by a reduction of initial tumor mass greater than 50% but less than 75% and/or persistence of BM involvement.
The primary objective was EFS, event as defined by relapse after CR or CRu, progression after PR, treatment modification, and death from any cause. Secondary objectives were OS, disease-free survival (DFS), progression-free survival (PFS), and analysis of treatment toxicity.
Toxicity was evaluated according to NCI Common Terminology Criteria for Adverse Events Version 3.0. All extra-hematologic toxicities were recorded during treatment and hematologic toxicity during transplantation phase.
Statistical methods
Patients were analyzed on an intent-to-treat basis.
EFS was defined as the time from study entry to first event. PFS was defined as the time from study entry to primary treatment failure, relapse, and death from any cause or last follow-up. OS was defined as the time from study entry to last follow-up or death from any cause.
Categorical data were compared using χ2 or Fisher exact tests. Survival functions were estimated by the Kaplan-Meier method and compared by log-rank test for each prognostic factor in univariate analysis. The Cox proportional hazards regression model was used to assess the effect of multiple variables.
Differences were considered significant if the 2-sided P value was less than .05. All statistical analyses were performed using SAS Version 9.13 software (SAS Institute).
Results
Patients
Sixty patients were enrolled in the study from 2000 to 2003. Patient characteristics are listed in Table 1. They were typical of MCL showing male predominance and median age of 57 years, without patients younger than 40 years. The majority of patients presented with Ann Arbor stage 4, with 85% of patients with BM involvement and half of them with leukemic phase (> 5 G/L) and gastrointestinal involvement proven by endoscopy, respectively. Other extra-nodal involvements were uncommon. Elevated lactate dehydrogenase was found in 38% of patients, and 18 of 38 patients had abnormal β2-microglobulin level. Mantle Cell Lymphoma International Prognostic Index score repartition for the population was similar to that reported in previous studies for a population of patients with MCL younger than 65 years.
Patient characteristics
| . | Present study (N = 60) . | Previous study9 (N = 28) . |
|---|---|---|
| Median age, y (range) | 57.5 (40-66) | 56 (33-64) |
| Sex ratio | 49 male/11 female | 23 male/5 female |
| Stage 3/4, no. (%) | 60/60 (100) | 28/28 (100) |
| B symptoms, no. (%) | 15/60 (25) | 14/28 (50) |
| PS > 1, no. (%) | 4/60 (7) | 0/28 (0) |
| LDH > N, no. (%) | 23/60 (38) | 12/28 (41) |
| β2-microglobulin > N, no. (%) | 18/38 (47) | 14/28 (50) |
| Median albumin, g/L (range) | 41 (26-60) | NA |
| Bone marrow involvement, no. (%) | 51/60 (85) | 25/28 (89) |
| Leukemic phase, no. (%) | 29/60 (48) | 14/28 (50) |
| Gastrointestinal involvement, no. (%) | 15/29 (52) | 11/23 (48) |
| MIPI, no. (%) | ||
| Low | 33/60 (55) | NA |
| Intermediate | 19/60 (32) | NA |
| High | 8/60 (13) | NA |
| . | Present study (N = 60) . | Previous study9 (N = 28) . |
|---|---|---|
| Median age, y (range) | 57.5 (40-66) | 56 (33-64) |
| Sex ratio | 49 male/11 female | 23 male/5 female |
| Stage 3/4, no. (%) | 60/60 (100) | 28/28 (100) |
| B symptoms, no. (%) | 15/60 (25) | 14/28 (50) |
| PS > 1, no. (%) | 4/60 (7) | 0/28 (0) |
| LDH > N, no. (%) | 23/60 (38) | 12/28 (41) |
| β2-microglobulin > N, no. (%) | 18/38 (47) | 14/28 (50) |
| Median albumin, g/L (range) | 41 (26-60) | NA |
| Bone marrow involvement, no. (%) | 51/60 (85) | 25/28 (89) |
| Leukemic phase, no. (%) | 29/60 (48) | 14/28 (50) |
| Gastrointestinal involvement, no. (%) | 15/29 (52) | 11/23 (48) |
| MIPI, no. (%) | ||
| Low | 33/60 (55) | NA |
| Intermediate | 19/60 (32) | NA |
| High | 8/60 (13) | NA |
NA indicates not applicable.
Response
All patients but 1 received the 3 cycles of (R)-CHOP21. One patient died of unknown cause 10 days after the first cycle. CR was uncommon (12%), and the majority of patients were in PR (Table 2). One patient was in progression after (R)-CHOP. Seven patients did not receive the 3 cycles of R-DHAP because of progressive disease (n = 3) or treatment toxicity (n = 4, see “Toxicity”). A high proportion of patients converted PR into CR after R-DHAP treatment; and finally, 57% of patients were in CR after complete induction treatment. Thirty percent were still in PR after induction treatment. Progressive disease was diagnosed for 7 of 60 patients; thus, they were not eligible for ASCT and were censored as failure for the EFS analysis.
Response rate
| . | After 3 (R)-CHOP, no. (%) . | After 3 R-DHAP, no. (%) . |
|---|---|---|
| CR/Cru | 7/60 (12) | 34/60 (57) |
| PR | 48/60 (80) | 18/60 (30) |
| SD | 3/60 (5) | 0/60 (0) |
| Progression | 1/60 (2) | 4/60 (7) |
| NE | 1/60 (2) | 4/60 (7) |
| . | After 3 (R)-CHOP, no. (%) . | After 3 R-DHAP, no. (%) . |
|---|---|---|
| CR/Cru | 7/60 (12) | 34/60 (57) |
| PR | 48/60 (80) | 18/60 (30) |
| SD | 3/60 (5) | 0/60 (0) |
| Progression | 1/60 (2) | 4/60 (7) |
| NE | 1/60 (2) | 4/60 (7) |
Finally, 49 of 53 remaining patients were autografted. Causes of nontransplantation included death during induction (1 patient), peripheral blood stem cell harvest failure (1 patient), progressive disease (4 patients), and treatment toxicity during induction (4 patients). One additional patient received allogeneic stem cell transplantation while he was in CR. Conditioning regimen was planned TAM6 protocol for 41 patients, and TBI plus high-dose cyclophosphamide for 1 patient. Because of induction treatment toxicity or unavailability of TBI, conditioning regimen with chemotherapy only was permitted and 7 patients received BEAM.
Response rate for patients who received the entire treatment, including ASCT, was 96% for CR (47 of 49 patients) and 4% for PR (2 of 49 patients). In an intent-to treat analysis for the entire cohort, CR was 78% with an overall response rate of 82%.
EFS and OS
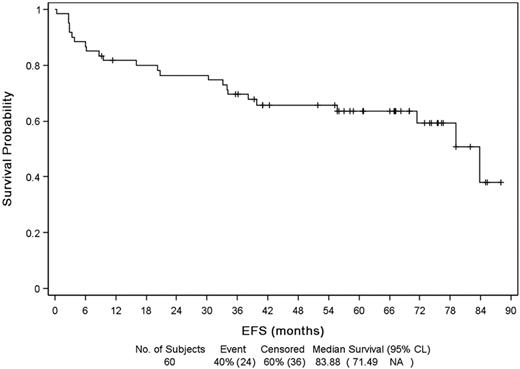
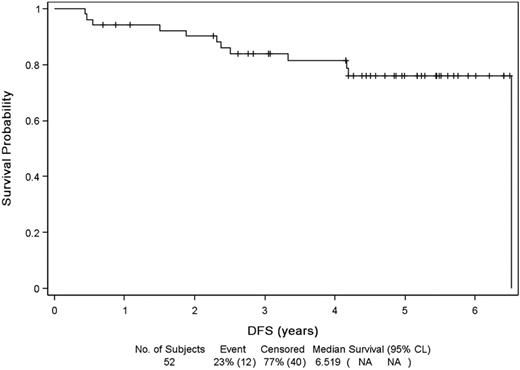
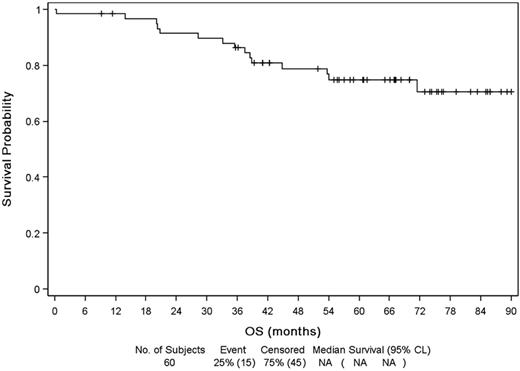
Median follow-up from diagnosis and initial therapy was 67 months (range, 0-90 months). Median EFS (Figure 2) was 83.9 months (range, 71.5 months to NR) with a probability of 5-year EFS of 64%. Relapse or progression was the principal cause of event (16 patients). Event was unrelated to lymphoma for 9 patients, mostly because of introduction of a new treatment for lymphoma out of progression (4 patients: because of toxicity for 3, allogeneic SCT while in CR for 1) or a death unrelated to disease progression (unknown cause, 1 patient; sepsis after surgery, 1 patient; other cancer, 3 patients). Median DFS was 78 months (Figure 3) and PFS 84 months. Median OS (Figure 4) was not reached, and OS rate at 5 years was 75%.
In univariate analysis, Performance Status more than 1, blood involvement, presence of B-symptoms, and elevated β2-microglobulin are associated with shorter OS, whereas gastrointestinal involvement and elevated β2-microglobulin are associated with shorter PFS and EFS. Response after (R)-CHOP or R-DHAP (CR vs PR) is not predictive of survival
The median EFS of patients receiving BEAM was 55 months, whereas it was not reached for patients receiving a TBI-containing regimen (P = .05).
Because the only difference between this study and results already published by our previous work (Lefrere et al9 ) was the introduction of 4 infusions of rituximab in induction phase, we compared results of these 2 cohorts. Baseline patients characteristics (with the exception of Mantle Cell Lymphoma International Prognostic Index, which was not available at this time) and treatments received with the exception of rituximab were similar.9 Despite a trend for a lower response rate (89% vs 82%) after rituximab, median EFS increased from 51 months to 83 months.
Toxicity
Renal toxicity was the main complication during induction phase, secondary to cisplatin in all cases. Five patients exhibited renal insufficiency, with 3 of 5 with grade 3 or 4 toxicity. This event led to treatment modification in these 5 patients with diminution of the administered cisplatin dose (50 mg/m2) or alternatively introduction of oxaliplatin (100 mg/m2 day 1) instead of cisplatin. One additional patient exhibited a grade 4 neurologic toxicity with transient paraplegia but with slight urologic dysfunction after intrathecal administration of prophylactic chemotherapy. There was neither unexpected infectious morbidity during the induction phase nor high-grade adverse event related to rituximab infusion. Despite the lack of past cardiovascular medical history, 1 patient had sudden death 10 days after the first infusion of CHOP.
Hematologic recovery after ASCT was as expected with median recovery at day 12 for neutrophils more than 1 g/L and sustained platelets more than 20 g/L. No patient died during the ASCT phase. Infectious toxicities were as expected, with only 1 case of Pneumocystis jirovecii infection. Three patients developed pulmonary symptoms requiring oxygenotherapy, with nonspecific interstitial syndrome on chest x-ray. Investigations did not confirm infectious complications, and idiopathic interstitial pneumonia, perhaps related to conditioning regimen, was finally diagnosed. All 3 of these patients recovered without sequelae.
Occurrence of a second malignancy
The diagnosis of second malignancies was unexpectedly high during follow-up of this patient cohort, with 11 patients experiencing 12 other cancers. Analysis of primary site of cancer showed a high rate of renal carcinoma of various histologies (5 patients). Others were colon adenocarcinoma (2 patients), lung carcinoma, prostate carcinoma, male breast carcinoma, diffuse large B-cell lymphoma (with IgH rearrangement different from initial MCL), and hamarthochondroma (1 patient for each site). No patient was diagnosed with myelodysplasia or secondary acute leukemia during follow-up.
Discussion
MCL is a distinct entity characterized by initial indolent features contrasting with refractoriness to classical therapy, such as anthracycline-based therapy, leading to death of patients in most cases. To date, there is still controversy about the initial regimen for younger fit patients, and our study provides valuable data to suggest that the best treatment should include induction strategy with cytarabine-based regimen and incorporation of rituximab in association with chemotherapy during induction phase followed in patients with chemosensitive disease by an intensification with high-dose therapy and ASCT (Table 3).
Summary of studies, including rituximab and intensive induction therapy
| . | Nordic12 . | MDACC11 . | HOVON13 . | GOELAMS14 . | GELA . |
|---|---|---|---|---|---|
| No. of patients | 160 | 97 | 62 | 17 | 60 |
| CR/CRu rate, % | 60 | 87 | 64 | 92 | 78 |
| ORR rate, % | 96 | 97 | 70 | 96 | 82 |
| PFS/TTF/EFS | 4-year PFS: 73% | Median TTF: 5.9 y | 4-year FFS: 46% | 3-year EFS: 75% | 5-year EFS: 64% |
| OS | 4-year: 81% | Projected 10-year: 64% | 4-year: 79% | 3-year: 76% | 5-year: 75% |
| . | Nordic12 . | MDACC11 . | HOVON13 . | GOELAMS14 . | GELA . |
|---|---|---|---|---|---|
| No. of patients | 160 | 97 | 62 | 17 | 60 |
| CR/CRu rate, % | 60 | 87 | 64 | 92 | 78 |
| ORR rate, % | 96 | 97 | 70 | 96 | 82 |
| PFS/TTF/EFS | 4-year PFS: 73% | Median TTF: 5.9 y | 4-year FFS: 46% | 3-year EFS: 75% | 5-year EFS: 64% |
| OS | 4-year: 81% | Projected 10-year: 64% | 4-year: 79% | 3-year: 76% | 5-year: 75% |
Our study provides more evidence that cytarabine-based therapy induce high response rate and, particularly, is able to convert a high proportion of PR obtain with (R)-CHOP to CR. These results were already shown in our previous study and have been subsequently confirmed by others. Although less demonstrative to assess specifically the role of high-dose cytarabine, hyper-CVAD alternating with HD-methotrexate–HD-cytarabine provides high response rate and prolonged PFS and OS either as initial therapy or for relapsing patients with MCL.11 In this protocol, it is difficult to assess whether the improvement of CR and PFS is related to dose intensity of alkylating agents and anthracyclines, the addition of high-dose cytarabine, or both. More recently, the Nordic Lymphoma Group reported in a large group of patients that introduction of both high-dose of cytarabine and rituximab (MCL2 study) has dramatically improved the result of their previous study with CHOP (MCL1 study).12 However, in this study, it was not possible to assess the respective role of high-dose cytarabine and rituximab because both were added. Nevertheless, some other recent data have also suggested impact of early consolidation with cytarabine (HOVON study) before ASCT,13 and even initial treatment with R-DHAP without anthracyclines.14 Taken together, our results provide further data suggesting strongly that high-dose cytarabine-based regimen have dramatically changed the prognosis of MCL.
Compared with our previous study, although based on retrospective analysis, our study provides additional evidence about the impact of rituximab in MCL. Whether or not rituximab addition to conventional chemotherapy improves response rate or survival of patients with MCL is still debated. When rituximab was tested as monotherapy, it did not show significant activity with 27% of responders at week 12 and only 2% of CR.6 In addition, in the European Mantle Cell Lymphoma randomized trial, the combination of CHOP and rituximab (R-CHOP) was significantly superior to CHOP in terms of overall response rate (94% vs 75%; P = .0054) and CR rate (34% vs 7%; P = .0002) but did not result in an improved PFS.15 However, a recent meta-analysis suggests that rituximab may improve PFS and OS.8 In relapsing disease, a fludarabine-containing regimen FCM (fludarabine, cyclophosphamide, and mitoxantrone) in combination with rituximab (R-FCM) not only improved the overall response rate (58% vs 46%) and CR rate (29% vs 0%), but also significantly prolonged OS (P = .0042).7 In the present study, the patient received only 4 infusions of rituximab, the first when the third course of CHOP was administered. This schedule was developed to prevent infusion reactions secondary to rituximab, such as cytokine-release syndrome, because half of these patients were diagnosed with high peripheral lymphocyte count. Finally, no patient has developed adverse event secondary to rituximab administration.
Our study emphasizes the role of ASCT for younger, fit patients with chemosensitive disease. The impact of ASCT was demonstrated 5 years ago by a previous study from the European MCL network, with an improvement in PFS but also OS over classical consolidation therapy with interferon. In our study, aggressive myeloablative high-dose radiochemotherapy and high-dose cytarabine led to notable improvement of CR rate, which rose from 69% to 96%. On the other hand, this treatment did not induce unacceptable toxicity with usual hematologic recovery and without toxic death during the procedure. Finally, with an extended follow-up, we did not observe myelodysplastic syndrome or secondary acute leukemia, confirming the feasibility of this regimen in a multicentric setting. In our study, few patients received either high-dose cytarabine or TBI because they were not fit. EFS was shorter in this group of patients, suggesting that either TBI or high-dose cytarabine or both may improve EFS. A retrospective study from the EBMT suggests that TBI may improve PFS, mainly in patients in PR.16 Further work will be warranted to assess the role of TBI because MCL cells are particularly radiosensitive.17
A high incidence of second malignancies was observed in our cohort. With a limited follow-up, 11 of 60 patients were diagnosed with other malignancy, mostly renal cancer. This high incidence of solid tumors, and particularly renal cancer, was already reported in a cohort of patients treated at the MD Anderson Cancer.18 On the other hand, there was no diagnosis of myelodysplastic syndrome or secondary acute leukemia in our cohort, despite the use of TBI. Taken together, these data suggest that a common link should explain the occurrence of MCL and other solid tumors, rather than a side effect of lymphoma therapy, including a predisposing genetic background both for MCL and solid tumors. For example, a high frequency of germline ATM mutations was reported in MCL,19 suggesting a role for tumor suppressor gene alterations in lymphomagenesis. Heterozygote mutation of ATM is known to be associated with breast cancers,20 but little is known about other solid tumors. Whether a genetic susceptibility could explain the increased risk of MCL and solid tumors should be studied.
In conclusion, our data provide strong evidence to use high-dose cytarabine and rituximab in induction regimen of MCL previous high-dose chemotherapy and autologous BM transplantation. However, it is difficult to compare our results obtained with our therapeutic strategy with previous MCL studies because, at the beginning of this clinical trial, prognosis factors, including Mantle Cell Lymphoma International Prognostic Index and Ki-67 expression, were not required and difficult to assess retrospectively. Thus, this regimen is currently tested in a phase 3 study against the best arm (R-CHOP21 followed by ABMT) of the previous European Mantle Cell Lymphoma protocol.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marion Fournier for statistical analysis.
Authorship
Contribution: R.D., C.H., V.R., A.V.H., F.L., and O.H. designed the study; R.D., C.H., V.R., P.B., A.D., H.T., G.S., A.V.H., O.C., and O.H. provided patients; N.B. was responsible for pathological analysis; F.L. provided complementary data; R.D. collected, assembled, and analyzed data; R.D. and O.H. prepared the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: R.D., C.H., A.D., H.T., G.S., and O.C. received honoraria from Roche. C.H., A.D., H.T., and O.C. received research funding from Roche. G.S. and O.C. are consultants/members of the advisory board of Roche. The remaining authors declare no competing financial interests.
For a list of members of the GELA, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Olivier Hermine, Service d'Hematologie, Hopital Necker, 149 rue de Sevres, 75743 Paris, cedex 15, France; e-mail: ohermine@gmail.com; and Richard Delarue, Service d'Hematologie, Hopital Necker, 149 rue de Sevres, 75743 Paris, cedex 15, France; e-mail: richard.delarue@nck.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal