In this issue of Blood, Rafei et al assess the requirement for γc cytokine signaling in postselection thymocytes and uncover distinct but nonredundant effects of several γc cytokines during CD8 lineage differentiation in vitro.1
T cells are generated in the thymus from bone marrow–derived progenitor cells by undergoing a series of selection and differentiation events. During these processes, the majority of developing thymocytes die and only a small fraction of cells survive to become mature T cells. The whole purpose of this exercise is to generate a random repertoire of T-cell receptor (TCR) specificities that are self-specific but not self-reactive. Identifying the few TCR specificities that are considered useful is referred to as positive selection, and positive selection permits survival and further differentiation of such thymocytes to become functionally mature T cells. Importantly, positive selection only permits and does not drive CD4/CD8 lineage choice of postselection thymocytes.2 Thus, a major quest in immunology has been to identify the cellular signals that control lineage fate of positive selected thymocytes, so that MHC-I–restricted cells always become CD8+ cytolytic T cells while MHC-II–restricted cells always become CD4+ helper T cells.3
Because thymic selection is focused on identifying useful TCR specificities, classically, thymocyte fate was presumed to be exclusively determined by the TCR. In agreement, TCR or TCR signaling–deficient thymocytes failed to mature, while transgenic expression of prearranged TCRs dramatically increased mature T-cell generation. More importantly, transgenic TCRs also imposed lineage fate on developing thymocytes so that enforced expression of MHC-I–restricted TCRs produced CD8+ T cells with cytolytic functions whereas MHC-II–restricted TCR expression generated CD4+ T cells with helper functions.4 However, how TCR specificities would coordinate such a 3-way match between MHC restriction, CD4/CD8 coreceptor expression, and acquisition of helper/cytolytic function remains unsolved. Recent data have now implicated co-receptor gene loci, instead of TCR, as key mechanisms in this process. Specifically, co-receptor gene imprinting posits that the Cd8 gene locus co-opts any co-receptor encoded within it to transiently terminate its expression upon positive selection and to impose cytotoxic lineage fate.5 Accordingly, cessation of the positive selecting TCR signal is a key event in CD8 lineage choice, which is necessary to permit cytokine signaling and to induce expression of the cytotoxic lineage specifying factor Runx3. In fact, Runx3 expression is up-regulated by IL-7 and other γc cytokine signaling, so that cytokines, not TCR signals, impose CD8 lineage fate.6 Collectively, CD4/CD8 lineage choice in postselection thymocytes has been proposed to be dependent on intrathymic γc cytokines.
On the other hand, γc cytokine–deficient mice do not show dramatic defects in CD4/CD8 lineage choice, and anti–IL-7 receptor antibody injection experiments failed to impact thymocyte lineage differentiation in vivo.7 Furthermore, γc deficiency results in severely impaired thymopoiesis during early thymocyte differentiation so that the role of γc signaling in postselection thymocytes remains uncertain.8 The current study by Rafei et al now addresses the role of γc cytokines using a simple but powerful in vitro model of thymocyte differentiation, and they document distinct roles for several γc cytokines on positive selection and during CD8 lineage differentiation (see figure).1
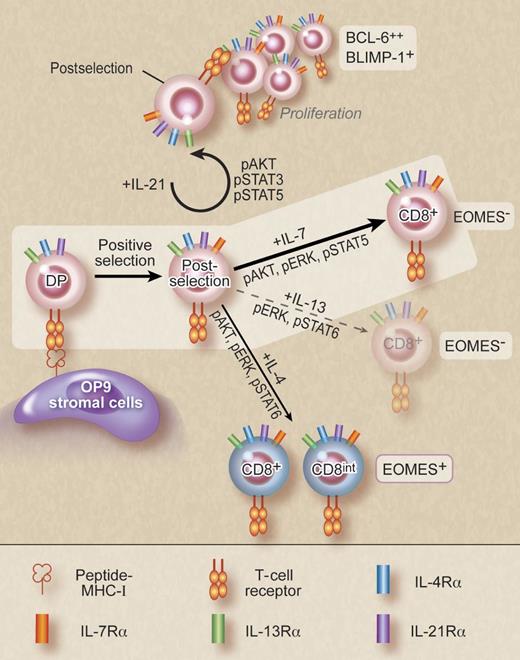
In vitro differentiation of postselection thymocytes by γc cytokine signaling. IL-7 is uniquely potent in driving CD8 cell differentiation. IL-21 signaling induces expansion of postselection thymocytes without CD8 lineage commitment, while IL-4 promotes differentiation of eomesodermin (EOMES) positive innate CD8 T cells. The non-γc cytokine IL-13, which uses the IL-4Rα, weakly induces CD8 cell differentiation. All other tested γc cytokines had no effect on postselection thymocytes. Professional illustration by Debra T. Dartez.
In vitro differentiation of postselection thymocytes by γc cytokine signaling. IL-7 is uniquely potent in driving CD8 cell differentiation. IL-21 signaling induces expansion of postselection thymocytes without CD8 lineage commitment, while IL-4 promotes differentiation of eomesodermin (EOMES) positive innate CD8 T cells. The non-γc cytokine IL-13, which uses the IL-4Rα, weakly induces CD8 cell differentiation. All other tested γc cytokines had no effect on postselection thymocytes. Professional illustration by Debra T. Dartez.
In a reductionist approach, the investigators used OP9 stromal cells loaded with synthetic peptides to present positive selecting signals, and then monitored lineage choice and differentiation of MHC-I–restricted OT-I TCR transgenic CD4, CD8 double positive thymocytes in coculture. Interestingly, in the absence of exogenous cytokines, CD8 lineage differentiation was blunted and minimal numbers of CD8 cells were produced. IL-7 treatment, however, dramatically improved the efficiency of CD8 cell generation, resulting in increased percentage and numbers of mature CD8 thymocytes. These results support a role for IL-7 on postselection CD8 lineage differentiation as previously proposed.6 Notably, in this in vitro system, no other γc cytokine than IL-7 was able to promote CD8 cell differentiation with the exception of IL-4. Even so, IL-4–induced CD8 cells had up-regulated expression of eomesodermin, PD-L1, and CD44, indicating that they were innate type CD8 cells of distinct function rather than regular CD8 thymocytes.9 All other γc cytokines, including IL-2, IL-9, IL-15, and IL-21 failed to show any improvement in CD8 lineage differentiation over medium treated cells. Of note, IL-21 signaling, while unable to promote CD8 lineage differentiation, induced proliferation and expansion of positively selected CD4, CD8 double positive thymocytes. Consequently, stimulation with both IL-21 and IL-7 resulted in significantly enhanced CD8 T-cell generation compared to IL-7 signaling alone. Thus, γc cytokines exert distinct effects on postselection thymocytes, and IL-7 is unique in promoting CD8 lineage differentiation (see figure).
These findings are significant because they demonstrate that positive selection alone is not sufficient to seal CD8 lineage fate and differentiation. They support a critical role for γc signaling in postselection thymocytes to drive functional and phenotypical maturation of CD8 cells. Whether such γc requirement also applies to CD4 lineage differentiation would be interesting to test, even if current models of CD4/CD8 lineage choice do not favor such an idea.3 Future studies with thymocytes deficient in γc cytokine signaling after positive selection should be able to clarify such questions. As such, this study documents that thymic education of immature thymocytes does not end with positive selection, but that their fate and functions are decided after positive selection. γc cytokines clearly can and do play a role in this process, and a role for non-γc cytokines also remains open. Collectively, positively selected thymocytes require cytokine signaling for further terminal differentiation, and the particular γc cytokine that they are exposed to will determine their cellular function and identity.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal