Abstract
Quantification of minimal residual disease (MRD) by real-time PCR directed to TCR and Ig gene rearrangements allows a refined evaluation of response in acute lymphoblastic leukemia (ALL). The German Multicenter Study Group for Adult ALL prospectively evaluated molecular response after induction/consolidation chemotherapy according to standardized methods and terminology in patients with Philadelphia chromosome-negative ALL. The cytologic complete response (CR) rate was 89% after induction phases 1 and 2. At this time point the molecular CR rate was 70% in 580 patients with cytologic CR and evaluable MRD. Patients with molecular CR after consolidation had a significantly higher probability of continuous complete remission (CCR; 74% vs 35%; P < .0001) and of overall survival (80% vs 42%; P = .0001) compared with patients with molecular failure. Patients with molecular failure without stem cell transplantation (SCT) in first CR relapsed after a median time of 7.6 months; CCR and survival at 5 years only reached 12% and 33%, respectively. Quantitative MRD assessment identified patients with molecular failure as a new high-risk group. These patients display resistance to conventional drugs and are candidates for treatment with targeted, experimental drugs and allogeneic SCT. Molecular response was shown to be highly predictive for outcome and therefore constitutes a relevant study end point. The studies are registered at www.clinicaltrials.gov as NCT00199056 and NCT00198991.
Introduction
Treatment results in adult acute lymphoblastic leukemia (ALL) have improved over the past 3 decades with complete remission rates increasing to 85%-90% and overall survival rates to 40%-50%.1,2 Nevertheless, 40%-50% of patients, including patients with standard risk without known poor-risk features, still develop relapse, which is associated with an extremely poor survival rate of < 10%.3 Reduction of relapse rate is therefore the main aim of treatment optimization in ALL. One approach is to identify patients in clinical complete remission (CR) with high risk of relapse as candidates for early treatment intensification, including allogeneic stem cell transplantation (SCT).
Response to standard induction treatment has been proven to be the most important factor in predicting outcome and risk of relapse. Cytologic confirmation of CR and time to achievement of CR are accepted as highly relevant prognostic factors and have been used as end points for clinical trials, including pivotal trials with new antileukemic agents.4 However, sensitivity of detecting leukemia cell reduction based on cytomorphology by definition is limited to 5%, and microscopic evaluation is prone to high interindividual variability.
In the past decade, more sensitive and precise methods have been developed, capable of detecting leukemic cells on a molecular level and to identify minimal residual disease (MRD) with a detection limit of 10−4 to 10−5 (0.01%-0.001%).5,6 Quantitative measurement of residual leukemic blasts is based on analysis of leukemia-specific characteristics such as individual gene rearrangements, fusion genes, or leukemia-specific immunophenotypes.5,6 Most experience and a high level of standardization have been accumulated for evaluation of leukemia-specific TCR and Ig gene rearrangements by quantitative PCR.7
The prognostic relevance of MRD for predicting relapse risk and survival has been reported in several clinical trials in pediatric8-12 and adult ALL.13-17 Overall, measurement of MRD (1) is a sensitive tool for assessing the response to treatment; (2) is well standardized and reproducible, if measured in qualified reference laboratories; (3) predicts overall outcome; and (4) has therefore been incorporated into most European ALL treatment protocols for risk stratification and serves as a basis to identify patients to be referred to SCT in first CR. Moreover, standardized definitions for MRD-based response evaluation and monitoring, such as “complete MRD response,” “MRD persistence,” and “MRD reappearance” have been established by the Consensus Development Workshop on MRD,18 allowing for comparison of MRD results between different trials.
Since 1999, the German Multicenter Study Group for Adult ALL (GMALL) has conducted 2 consecutive trials (GMALL 06/99 and 07/03) with prospective MRD evaluation. This report contains an analysis of the up-to-now largest cohort of MRD data in adult ALL. It aims to analyze the molecular response rates in correlation to cytologic response rates and to evaluate the prognostic effect of molecular response within risk groups defined according to conventional prognostic factors and the outcome of patients with molecular failure and thereby to establish reference data for future MRD-based clinical trials.
Methods
Patients
The analysis includes patients with Philadelphia chromosome (BCR-ABL)–negative ALL, aged 15-55 years, included in the GMALL trials 06/99 and the ongoing trial 07/03 between April 1999 and July 2009 to ensure a follow-up of 1 year in most of patients. Patients were diagnosed and treated in 130 participating centers of the GMALL study group. Patients with molecular response evaluation (n = 90) had already been reported in a previous publication.17 The studies were approved by the institutional review board of the University of Frankfurt, Germany, and are registered at clinicaltrials.gov (NCT00199056, NCT00198991). All patients have given signed informed consent in accordance with the Declaration of Helsinki.
Risk stratification and treatment
Both studies are based on a risk-adapted treatment strategy with prospective MRD monitoring at several time points during treatment and follow-up as described elsewhere.17 The treatment overview and chemotherapy schedule are detailed in supplemental Figure 1 and supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article. Patients were allocated to risk groups on the basis of conventional prognostic factors at diagnosis. High-risk features were white blood cell (WBC) count > 30 000/μL in B-lineage ALL, pro-B-ALL, early or mature T-cell ALL, MLL-AF4/t(4;11) translocation, or no achievement of cytologic CR after induction 1. In the beginning of study GMALL 06/99 also few patients with thymic T-ALL and WBC count > 100 000/μL were considered as high risk. Patients with any of these factors were allocated to the high-risk (HR) group. The remaining patients were allocated to the standard-risk (SR) group.
All patients received intensive 7-drug induction therapy with phase 1 and 2, followed by one uniform consolidation that was mainly based on high-dose methotrexate and high-dose cytarabine, starting at day 71 as described earlier.17 Patients in the SR group were scheduled to receive further intensive consolidation chemotherapy. Patients in the HR group were candidates for allogeneic SCT. In study GMALL 07/2003 patients in the SR group with persistent MRD > 10−4 until week 16 were candidates for transplantation in first CR as well.
Evaluation of MRD and definition of response
The main cytologic response evaluation took place after induction phase 2 (day 46/day 71). Standard criteria were used to evaluate response. CR was defined as no evidence of leukemic blasts in the BM (< 5%), complete resolution of extramedullary manifestations, and recovery of peripheral cell counts. Relapse was defined as reappearance of disease either as unequivocal blasts in the BM (> 5%), in the CNS, or at extramedullary sites after prior achievement of CR.
MRD evaluation took place during induction 1 (day 11), after induction 1 (day 26), after induction 2 (day 46), before consolidation 1 (day 71), after consolidation 1 (week 16), and at further time points during consolidation treatment and follow-up.17 Analysis for the essential time points at day 71 and week 16 was based on the patients with evaluable MRD results at the respective time point irrespective of prior or later MRD results. BM samples were sent to central reference laboratories at the University of Heidelberg and the University of Kiel. MRD was determined by real-time quantitative PCR of leukemia-specific Ig and TCR gene rearrangements with the use of clone-specific primers and a set of different germline TaqMan probes and germline primers. Quality control and standardized interpretation of quantification data were achieved in the frame of the European Study Group on MRD detection in ALL (EuroMRD, formerly ESG MRD ALL).7
Molecular response is only reported for patients in complete cytologic remission, with ≥ 1 marker for MRD analysis and samples available at the respective time points. Feasibility of MRD evaluation in the multicenter setting will be reported separately. Results were classified as “molecular CR” (molCR) in case of MRD negativity at the respective time point with an assay sensitivity of ≥ 10−4. Persistent quantifiable MRD positivity within the quantitative range (≥ 10−4) was defined as “molecular failure” (molFail), and reappearance of MRD within the quantitative range (> 10−4) after prior achievement of molecular CR was defined as molecular relapse. MRD positivity below quantitative range or < 10−4 was considered to be nonevaluable for molecular response assessment.
Statistical analysis
Data are presented as percentages for categorical variables and medians for continuous variables. Categorized variables were compared with the chi square test and medians with the Wilcoxon test to estimate significant differences. Survival analysis was performed with the Kaplan-Meier method. Overall survival was calculated from the date of diagnosis to the date of death or date of last follow-up in all included patients. Probability of continuous CR (CCR) was calculated from the date of CR to the date of relapse or date of last follow-up. Patients who died in CR or who were withdrawn from study treatment were censored at the respective dates. Disease-free survival was calculated from the date of CR to the date of last follow-up in case of CCR. Patients with withdrawal, relapse, death in CR, or secondary malignancy were counted as events. Comparisons of survival curves were performed with log-rank tests. The Cox model was used to test the prognostic role of molecular response in correlation to conventional prognostic factors. To evaluate the effect of SCT compared with chemotherapy, a landmark analysis was performed. For this analysis, all patients undergoing chemotherapy with remission duration shorter than the median time to SCT plus 1 month were excluded. A calculated P value < .05 was established to indicate statistical significance. All analyses were performed with the SAS program (SAS-PC Version 9; SAS Institute).
Results
Overall response after induction
A total of 1648 patients from 130 centers, with standard risk (n = 975) and high-risk (n = 673) features were evaluable. Overall, cytologic CR was achieved in 89% of patients, with significant differences between patients in the SR and HR groups (92% vs 85%; P < .0001). Significant differences in cytologic CR were also observed for immunophenotypic subtypes and age groups, whereas the WBC count at diagnosis had no effect on cytologic CR (Table 1).
Cytologic and molecular response rates after induction therapy (day 71) in correlation to prognostic factors
| . | Cytologic CR rate . | Molecular CR rate . | ||||
|---|---|---|---|---|---|---|
| N (%)* . | n (%) . | P† . | N (%)* . | n (%) . | P† . | |
| Total | 1648 | 1467 (89) | 580 | 407 (70) | ||
| Risk groups | .0001 | < .0001 | ||||
| Standard risk | 975 (59) | 895 (92) | 434 (75) | 335 (77) | ||
| High risk | 673 (41) | 572 (85) | 146 (25) | 72 (51) | ||
| Immunophenotype | ||||||
| B lineage | 1076 (65) | 961 (89) | NS | 383 (66) | 252 (66) | .001 |
| T lineage | 569 (35) | 504 (89) | NS | 197 (34) | 155 (79) | .001 |
| c-ALL | 912 (56) | 813 (89) | .0009 | 350 (61) | 236 (67) | < .0001 |
| Pro-B-ALL | 169 (10) | 148 (90) | .0009 | 33 (6) | 16 (48) | < .0001 |
| Early T-ALL | 151 (9) | 129 (85) | .0009 | 21 (4) | 10 (45) | < .0001 |
| Mature T-ALL | 97 (6) | 76 (78) | .0009 | 23 (4) | 9 (39) | < .0001 |
| Thymic T-ALL | 303 (19) | 282 (93) | .0009 | 151 (26) | 134 (89) | < .0001 |
| Age | .01 | NS | ||||
| 15-35 y | 982 (60) | 890 (91) | 375 (65) | 266 (71) | ||
| 35-55 y | 666 (40) | 577 (87) | 205 (35) | 141 (69) | ||
| Leukocyte count | ||||||
| B lineage | NS | .06 | ||||
| < 30 000/μL | 783 (73) | 706 (90) | 316 (83) | 215 (68) | ||
| > 30 000/μL | 288 (27) | 251 (87) | 66 (17) | 37 (56) | ||
| T-lineage | NS | NS | ||||
| < 100 000/μL | 416 (76) | 372 (89) | 145 (74) | 118 (81) | ||
| > 100 000/μL | 134 (24) | 116 (87) | 51 (26) | 36 (71) | ||
| . | Cytologic CR rate . | Molecular CR rate . | ||||
|---|---|---|---|---|---|---|
| N (%)* . | n (%) . | P† . | N (%)* . | n (%) . | P† . | |
| Total | 1648 | 1467 (89) | 580 | 407 (70) | ||
| Risk groups | .0001 | < .0001 | ||||
| Standard risk | 975 (59) | 895 (92) | 434 (75) | 335 (77) | ||
| High risk | 673 (41) | 572 (85) | 146 (25) | 72 (51) | ||
| Immunophenotype | ||||||
| B lineage | 1076 (65) | 961 (89) | NS | 383 (66) | 252 (66) | .001 |
| T lineage | 569 (35) | 504 (89) | NS | 197 (34) | 155 (79) | .001 |
| c-ALL | 912 (56) | 813 (89) | .0009 | 350 (61) | 236 (67) | < .0001 |
| Pro-B-ALL | 169 (10) | 148 (90) | .0009 | 33 (6) | 16 (48) | < .0001 |
| Early T-ALL | 151 (9) | 129 (85) | .0009 | 21 (4) | 10 (45) | < .0001 |
| Mature T-ALL | 97 (6) | 76 (78) | .0009 | 23 (4) | 9 (39) | < .0001 |
| Thymic T-ALL | 303 (19) | 282 (93) | .0009 | 151 (26) | 134 (89) | < .0001 |
| Age | .01 | NS | ||||
| 15-35 y | 982 (60) | 890 (91) | 375 (65) | 266 (71) | ||
| 35-55 y | 666 (40) | 577 (87) | 205 (35) | 141 (69) | ||
| Leukocyte count | ||||||
| B lineage | NS | .06 | ||||
| < 30 000/μL | 783 (73) | 706 (90) | 316 (83) | 215 (68) | ||
| > 30 000/μL | 288 (27) | 251 (87) | 66 (17) | 37 (56) | ||
| T-lineage | NS | NS | ||||
| < 100 000/μL | 416 (76) | 372 (89) | 145 (74) | 118 (81) | ||
| > 100 000/μL | 134 (24) | 116 (87) | 51 (26) | 36 (71) | ||
CR indicates complete response; cytCR, cytologic complete response; molCR, molecular complete response; and NS, not significant.
Percentages show distribution of characteristics within the patient group analyzed for cytologic CR rate.
χ2 test.
A total of 580 patients in CR were evaluable for analysis of molecular response defined by the level of MRD before consolidation 1 (day 71). The proportion of patients in the SR group was higher in the group of patients with evaluable MRD (75%) results than in the total patient cohort (59%; Table 1). MolCR was achieved in 70% of patients at this time point with significant differences between patients in the SR and HR groups (77% vs 51%; P < .0001). In addition, molCR rates for B-lineage and T-lineage ALL differed significantly (66% vs 79%; P = .001). A superior molCR rate was observed for thymic T-ALL compared with other subgroups of T-ALL (89% vs 65%; P < .0001). A higher molCR rate was also documented for patients with B-lineage ALL with WBC count < 30 000/μL compared with patients with higher WBC count (68% vs 56%; P = .06; Table 1).
Time to molecular CR
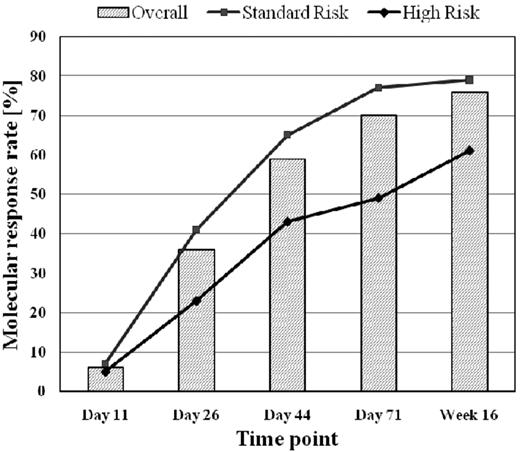
Molecular response to chemotherapy was assessed at different time points during and after induction and after first consolidation. MolCR rates increased from 6% on day 11 to 36% on day 26 and to 70% on day 71 during or after induction chemotherapy and reached 76% in week 16 after consolidation 1 (Figure 1).
Molecular response rate in relation to chemotherapeutic treatment phases.
The molCR rate was significantly lower for patients in the HR group than for patients in the SR group at all time points. Patients with T-ALL reached a significantly higher molCR rate on day 71 (79% vs 66%; P = .001) than patients with B-lineage ALL, whereas no difference was observed on day 26 (36% vs 36%) and in week 16 (75% vs 78%).
Nine percent of patients with molFail after induction on day 71 (N = 87) achieved molecular CR after first consolidation. This proportion was higher for patients in the SR group than for patients in the HR group (13% vs 0%; P = .06), whereas no difference was observed in B-lineage compared with T-lineage ALL.
Prognostic effect of molecular response
Because of initial treatment stratification, the prognostic effect of molCR and molFail was first analyzed within risk groups defined by conventional risk factors.
Patients in the SR group.
The probability of CCR after 5 years was significantly higher for patients with molCR than for patients who had molFail on day 71 (69% ± 3% vs 42% ± 6%; P < .0001) and in week 16 (74% ± 3% vs 37% ± 6%; P < .0001). When patients who underwent SCT in first CR were excluded from the analysis, the probability of CCR after 5 years further decreased for patients who had molFail on day 71 (69% ± 3% vs 32% ± 7%; P < .0001) and week 16 (74% ± 3% vs 13% ± 5%; P < .0001). Similar results were observed for disease-free survival.
At both time points, overall survival at 5 years was significantly higher for patients with molCR than for patients with molFail. On the basis of MRD evaluation in week 16, the probability of overall survival was 81% ± 3% vs 43% ± 6% (P < .0001). When patients who underwent SCT in first CR were excluded, survival of patients with molFail further decreased and was most unfavorable for patients with molecular failure in week 16 (31% ± 7%; Table 2).
Outcome after 5 years according to molecular response after induction (day 71) and consolidation (week 16)
| Parameter . | Day 71 after induction . | P . | Week 16 after consolidation . | P . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | MolCR . | MolFail . | N . | MolCR . | MolFail . | |||||||
| n . | % ± SD . | n . | % ± SD . | n . | % ± SD . | n . | % ± SD . | |||||
| Standard risk | ||||||||||||
| CCR | 434 | 335 | 69 ± 3 | 99 | 42 ± 6 | < .0001 | 424 | 335 | 74 ± 3 | 89 | 37 ± 6 | < .0001 |
| CCR SCT excluded | 384 | 317 | 69 ± 3 | 67 | 32 ± 7 | <.0001 | 376 | 322 | 74 ± 3 | 54 | 13 ± 5 | < .0001 |
| DFS | 434 | 335 | 62 ± 3 | 99 | 35 ± 6 | < .0001 | 424 | 335 | 68 ± 3 | 89 | 28 ± 5 | < .0001 |
| DFS SCT excluded | 384 | 317 | 62 ± 3 | 67 | 27 ± 6* | < .0001 | 376 | 322 | 68 ± 3 | 54 | 11 ± 5 | < .0001 |
| Overall survival | 434 | 335 | 80 ± 3 | 99 | 47 ± 6 | < .0001 | 424 | 335 | 81 ± 3 | 89 | 43 ± 6 | < .0001 |
| Overall survival SCT excluded | 384 | 317 | 80 ± 3 | 67 | 38 ± 7 | < .0001 | 376 | 322 | 81 ± 3 | 54 | 31 ± 7 | < .0001 |
| High risk | ||||||||||||
| CCR | 145 | 72 | 78 ± 8 | 73 | 41 ± 7 | < .0001 | 80 | 49 | 75 ± 8 | 31 | 29 ± 12 | < .0001 |
| CCR SCT excluded | 40 | 16 | 36 ± 26 | 24 | 21 ± 10 | .001 | 20 | 11 | 50 ± 35 | 9 | 19 ± 17 | < .0001 |
| DFS | 145 | 72 | 66 ± 8 | 73 | 32 ± 6 | < .0001 | 80 | 49 | 64 ± 8* | 31 | 18 ± 8* | < .0001 |
| DFS SCT excluded | 40 | 16 | 18 ± 15* | 24 | 6 ± 5* | .003 | 20 | 11 | 34 ± 26* | 9 | 11 ± 10* | .0009 |
| Overall survival | 146 | 72 | 72 ± 8 | 74 | 49 ± 7 | .0005 | 80 | 49 | 71 ± 9 | 31 | 41 ± 10 | .003 |
| Overall survival SCT excluded | 40 | 16 | 56 ± 17 | 24 | 21 ± 9 | .06 | 20 | 11 | 83 ± 15 | 9 | 44 ± 17 | NS |
| Overall | ||||||||||||
| CCR | 579* | 407 | 70 ± 3 | 172 | 39 ± 5 | < .0001 | 504 | 384 | 74 ± 3 | 120 | 35 ± 5 | < .0001 |
| CCR SCT excluded | 424 | 333 | 68 ± 3 | 91 | 26 ± 6 | < .0001 | 396 | 333 | 74 ± 3 | 63 | 12 ± 5 | < .000 |
| DFS | 579 | 407 | 63 ± 3 | 172 | 31 ± 4 | < .0001 | 504 | 384 | 67 ± 3 | 120 | 25 ± 5 | < .0001 |
| DFS SCT excluded | 424 | 333 | 60 ± 3 | 91 | 20 ± 5 | < .0001 | 396 | 333 | 68 ± 3 | 63 | 10 ± 4 | < .0001 |
| Overall survival | 580 | 407 | 79 ± 3 | 173 | 47 ± 5 | < .0001 | 504 | 384 | 80 ± 3 | 120 | 42 ± 5 | < .0001 |
| Overall survival SCT excluded | 424 | 333 | 80 ± 3 | 91 | 36 ± 6 | < .0001 | 396 | 333 | 81 ± 3 | 63 | 33 ± 7 | < .0001 |
| Parameter . | Day 71 after induction . | P . | Week 16 after consolidation . | P . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | MolCR . | MolFail . | N . | MolCR . | MolFail . | |||||||
| n . | % ± SD . | n . | % ± SD . | n . | % ± SD . | n . | % ± SD . | |||||
| Standard risk | ||||||||||||
| CCR | 434 | 335 | 69 ± 3 | 99 | 42 ± 6 | < .0001 | 424 | 335 | 74 ± 3 | 89 | 37 ± 6 | < .0001 |
| CCR SCT excluded | 384 | 317 | 69 ± 3 | 67 | 32 ± 7 | <.0001 | 376 | 322 | 74 ± 3 | 54 | 13 ± 5 | < .0001 |
| DFS | 434 | 335 | 62 ± 3 | 99 | 35 ± 6 | < .0001 | 424 | 335 | 68 ± 3 | 89 | 28 ± 5 | < .0001 |
| DFS SCT excluded | 384 | 317 | 62 ± 3 | 67 | 27 ± 6* | < .0001 | 376 | 322 | 68 ± 3 | 54 | 11 ± 5 | < .0001 |
| Overall survival | 434 | 335 | 80 ± 3 | 99 | 47 ± 6 | < .0001 | 424 | 335 | 81 ± 3 | 89 | 43 ± 6 | < .0001 |
| Overall survival SCT excluded | 384 | 317 | 80 ± 3 | 67 | 38 ± 7 | < .0001 | 376 | 322 | 81 ± 3 | 54 | 31 ± 7 | < .0001 |
| High risk | ||||||||||||
| CCR | 145 | 72 | 78 ± 8 | 73 | 41 ± 7 | < .0001 | 80 | 49 | 75 ± 8 | 31 | 29 ± 12 | < .0001 |
| CCR SCT excluded | 40 | 16 | 36 ± 26 | 24 | 21 ± 10 | .001 | 20 | 11 | 50 ± 35 | 9 | 19 ± 17 | < .0001 |
| DFS | 145 | 72 | 66 ± 8 | 73 | 32 ± 6 | < .0001 | 80 | 49 | 64 ± 8* | 31 | 18 ± 8* | < .0001 |
| DFS SCT excluded | 40 | 16 | 18 ± 15* | 24 | 6 ± 5* | .003 | 20 | 11 | 34 ± 26* | 9 | 11 ± 10* | .0009 |
| Overall survival | 146 | 72 | 72 ± 8 | 74 | 49 ± 7 | .0005 | 80 | 49 | 71 ± 9 | 31 | 41 ± 10 | .003 |
| Overall survival SCT excluded | 40 | 16 | 56 ± 17 | 24 | 21 ± 9 | .06 | 20 | 11 | 83 ± 15 | 9 | 44 ± 17 | NS |
| Overall | ||||||||||||
| CCR | 579* | 407 | 70 ± 3 | 172 | 39 ± 5 | < .0001 | 504 | 384 | 74 ± 3 | 120 | 35 ± 5 | < .0001 |
| CCR SCT excluded | 424 | 333 | 68 ± 3 | 91 | 26 ± 6 | < .0001 | 396 | 333 | 74 ± 3 | 63 | 12 ± 5 | < .000 |
| DFS | 579 | 407 | 63 ± 3 | 172 | 31 ± 4 | < .0001 | 504 | 384 | 67 ± 3 | 120 | 25 ± 5 | < .0001 |
| DFS SCT excluded | 424 | 333 | 60 ± 3 | 91 | 20 ± 5 | < .0001 | 396 | 333 | 68 ± 3 | 63 | 10 ± 4 | < .0001 |
| Overall survival | 580 | 407 | 79 ± 3 | 173 | 47 ± 5 | < .0001 | 504 | 384 | 80 ± 3 | 120 | 42 ± 5 | < .0001 |
| Overall survival SCT excluded | 424 | 333 | 80 ± 3 | 91 | 36 ± 6 | < .0001 | 396 | 333 | 81 ± 3 | 63 | 33 ± 7 | < .0001 |
MolCR indicates molecular complete response; molFail, molecular failure; CCR, continuous complete remission; SCT, stem cell transplantation; and DFS, disease-free survival.
Five years not reached.
Patients in the HR group.
Within the HR group, the probability of CCR after 5 years was significantly higher for patients with molCR at day 71 (78% ± 8% vs 41% ± 7%; P < .0001) and in week 16 (75% ± 8% vs 29% ± 12%; P < .0001) than for patients with molFail. When patients with SCT in first CR were excluded from the analysis, the probability of CCR after 5 years further decreased for patients with molFail on day 71 (36% ± 26% vs 21% ± 10%; P = .001) and in week 16 (50% ± 35% vs 19% ± 17%; P < .0001). Results were similar for disease-free survival. Overall survival at 5 years was significantly better for patients with molCR than for patients with molFail at both time points. When patients with SCT in first CR were omitted, no significant difference was observed between patient with molFail and patients with molCR, but patient numbers were small (Table 2).
Total patient cohort.
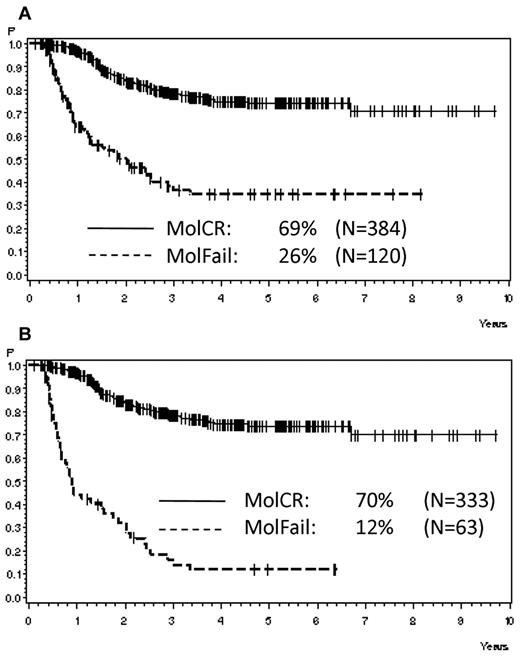
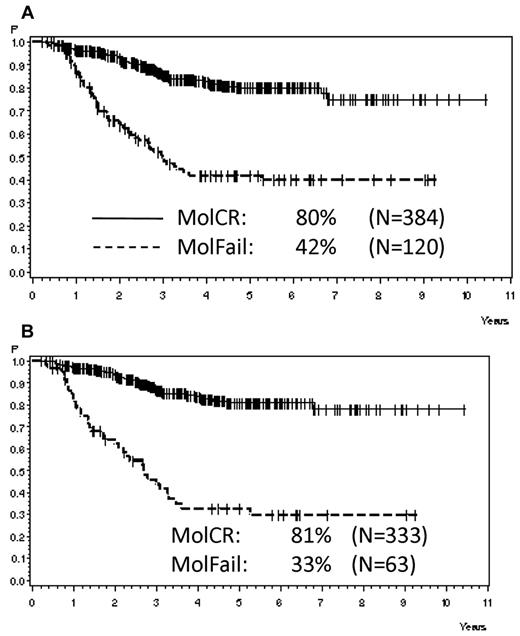
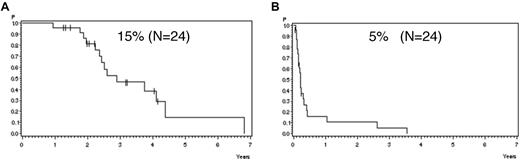
Analyzing all patients from both risk groups, molFail after consolidation chemotherapy (week 16) had the worst prognostic effect on CCR after 5 years (Table 2). The probability of CCR after 5 years was 74% ± 3% for patients with molCR versus 35% ± 5% for patients with molFail (P < .0001; Figure 2A). When patients who underwent SCT in first CR were omitted from analysis, the probability of CCR after 5 years decreased to 12% ± 5% (P < .0001; Figure 2B). Results for disease-free survival were similar. Overall survival was significantly inferior in molFail than in molCR (42% ± 5% vs 80% ± 3%; P < .0001; Figure 3A) and decreased further when patients with SCT in first CR were omitted from the analysis (33% ± 7% vs 81% ± 3%; P < .0001; Figure 3B).
Probability of CCR. Probability of CCR for patients in the SR and HR groups according to molecular response status in week 16, (A) overall (P < .0001) and (B) excluding SCT in first CR (P < .0001).
Probability of CCR. Probability of CCR for patients in the SR and HR groups according to molecular response status in week 16, (A) overall (P < .0001) and (B) excluding SCT in first CR (P < .0001).
Probability of survival. Probability of survival for patients in the SR and HR groups according to molecular response status in week 16, (A) overall (P < .0001) and (B) excluding SCT in first CR (P < .0001).
Probability of survival. Probability of survival for patients in the SR and HR groups according to molecular response status in week 16, (A) overall (P < .0001) and (B) excluding SCT in first CR (P < .0001).
A multivariate analysis of prognostic factors, including age, immunophenotype, risk group, and molecular response status in week 16, found that molecular response was the only parameter with significant prognostic effect on CCR after 5 years with a hazard ratio of 4.5 (P < .0001). In addition, the multivariate analysis confirmed age with a hazard ratio of 1.3 (P = .0007) and molecular remission status with a hazard ratio of 4.0 (P < .0001) to have significant effect on overall survival.
Effect of allogeneic SCT for patients with molecular failure
After first consolidation (week 16) 120 patients showed molFail (89 in the SR group and 31 in the HR group). In 47% of these patients with molFail, SCT was realized in first CR. The SCT performance rate was significantly higher for patients in the HR group than for patients in the SR group (71% vs 39%; P < .002). The median time from CR to SCT was 6.7 months (range, 2.4-44 months) and significantly shorter for patient in the HR group than for patients in the SR group (4.4 vs 9.1 month; P < .0001).
The probability of CCR after 5 years was significantly higher for patients with molFail and SCT in first CR than for those without SCT in first CR (66% ± 7% vs 12% ± 5%; P < .0001). For landmark analysis, patients with remission duration shorter than 232 days (median time to SCT plus 1 month) were excluded. The difference remained significant for the comparison of patients with (N = 29) versus without SCT (N = 40; 74% ± 9% vs 15% ± 6%; P < .0001), which also translated into a better survival for patients with SCT than for those without (54% ± 8% vs 33% ± 7%; P = .06). Results were similar for disease-free survival (Table 3).
Effect of SCT on 5-year outcome of patients in the SR and HR groups with molecular failure after consolidation (week 16)
| Parameter . | N . | No SCT . | SCT . | |||
|---|---|---|---|---|---|---|
| n . | % ± SD . | n . | % ± SD . | P . | ||
| Continuous complete remission | 120 | 63 | 12 ± 5 | 57 | 66 ± 8 | < .0001 |
| Continuous complete remission (landmark analysis) | 60 | 35 | 17 ± 7 | 25 | 73 ± 10 | .0001 |
| Disease-free survival | 120 | 63 | 11 ± 4 | 57 | 44 ± 8 | < .0001 |
| Disease-free survival (landmark analysis) | 60 | 35 | 16 ± 7 | 25 | 50 ± 10 | .004 |
| Overall survival | 120 | 63 | 33 ± 7 | 57 | 54 ± 8 | .06 |
| Parameter . | N . | No SCT . | SCT . | |||
|---|---|---|---|---|---|---|
| n . | % ± SD . | n . | % ± SD . | P . | ||
| Continuous complete remission | 120 | 63 | 12 ± 5 | 57 | 66 ± 8 | < .0001 |
| Continuous complete remission (landmark analysis) | 60 | 35 | 17 ± 7 | 25 | 73 ± 10 | .0001 |
| Disease-free survival | 120 | 63 | 11 ± 4 | 57 | 44 ± 8 | < .0001 |
| Disease-free survival (landmark analysis) | 60 | 35 | 16 ± 7 | 25 | 50 ± 10 | .004 |
| Overall survival | 120 | 63 | 33 ± 7 | 57 | 54 ± 8 | .06 |
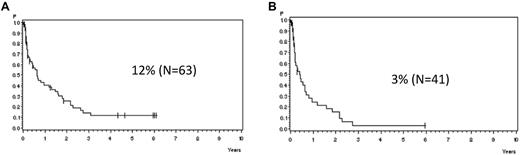
In patients with molFail without allogeneic SCT in first CR, the median time from detection of molFail to cytologic relapse was 7.6 months (Figure 4A). The median remission duration decreased to 4.9 months for patients (N = 41) with a higher MRD level (> 10−3; Figure 4B).
Probability of CCR. Probability of CCR for patients in the SR and HR groups with molecular failure from time point of detection with (A) MRD level > 10−4 (median, 7.6 months) and (B) MRD level > 10−3 in week 16, excluding SCT in first CR (median, 4.9 months).
Probability of CCR. Probability of CCR for patients in the SR and HR groups with molecular failure from time point of detection with (A) MRD level > 10−4 (median, 7.6 months) and (B) MRD level > 10−3 in week 16, excluding SCT in first CR (median, 4.9 months).
Outcome after molecular relapse
We observed a total of 34 molecular relapses for patients with continuous cytologic remission and without parallel extramedullary relapse. Of these, 38% (N = 13) occurred during the first year of treatment and 62% (N = 21) subsequently. All except 1 patient had been assigned to the SR group. The probability of continuous cytologic remission from the date of molecular relapse was 21% ± 9% at 5 years. If patients with SCT in first cytologic remission were excluded, the probability of CCR decreased to 5% ± 5% at 3 years (Figure 5A) compared with 80% ± 18% in the few patients (N = 10) who underwent SCT in ongoing first cytologic remission (P < .0001).
Probability of CCR and survival. Probability of (A) CCR and (B) survival after molecular relapse, excluding SCT in first CR.
Probability of CCR and survival. Probability of (A) CCR and (B) survival after molecular relapse, excluding SCT in first CR.
The median time from detection of molecular relapse for patients without SCT in first CR to subsequent clinical relapse (N = 24) was 2.6 months (range, 0.8-43 months). Overall survival of patients with molecular relapse was 36% ± 14% at 5 years. In patients without SCT in first CR survival was 15% ± 12% only (Figure 5B) compared with 80% ± 18% for patients (N = 10) with SCT in first CR (P = .02).
Discussion
This is the largest analysis to date of the prognostic effect of MRD on the outcome of adult ALL. MRD results are reported according to new internationally approved standards.18 We feel that it is essential to have a well-defined methodology and terminology to create reference data for future trials and to achieve comparability between different trials. The strict definition of molecular response criteria was one reason that only a proportion of the total patient cohort could be considered for the molecular response assessment. Patients without MRD test at the respective time point, with nonevaluable MRD (eg, low positive, nonquantifiable, or insufficient sensitivity) were excluded. This may have contributed to an overall positive selection of the analyzed patient cohort because, in addition, patients are excluded who relapsed or died before reaching the respective time points. Poorer results for patients without MRD testing have also been reported by others.16 The conclusion that MRD is a highly relevant prognostic factor for patients with MRD testing at the respective time points is not diluted by this effect. Furthermore, MRD testing was more frequently performed in patients with standard risk than in patients with high risk. The interpretation of results is however not hampered by this fact, because prognostic relevance was tested first within risk groups first and then, after similar prognostic effects had been shown, in the total cohort.
In this study, response to treatment significantly differed depending on the method of response assessment. A CR as assessed by cytologic evaluation was achieved in 89% of all patients, which is in line with other published ALL trials showing CR rates of 80%-90%.16,19,20 Measurement of MRD allowed a more sensitive assessment of response and found that 30% of the patients with cytologic CR did not achieve molecular CR. In addition considerable differences across specific ALL subtypes became apparent. Patients in the HR group achieved a lower molecular CR rate than patients in the SR group (51% vs 71%). Patients with thymic T-ALL showed a significantly better molecular response to treatment than other T-lineage subgroups (89% vs 65%), which correlates with the more favorable outcome of this subtype.21,22 Poorer MRD response of early T-ALL compared with other subtypes of T-ALL has also been reported in pediatric patients by MRD evaluation with the use of flow cytometry.23
MRD assessment at several early time points during treatment allowed evaluating the kinetics of molecular response. Additional patients with incomplete molecular response after induction 1 achieved a molecular CR after induction 2 (36% vs 70% molecular responses). However, with only 9% of patients who additionally entered molecular CR during first consolidation, the effect of further standard chemotherapy was limited. Future clinical application of molecular response evaluation may include the assessment of treatment modifications during induction and early consolidation therapy. Thus, it has been recently reported that the addition of rituximab to induction treatment in CD20+ ALL may contribute to an improved molecular CR rate.24
The most important time points for MRD evaluation were in our hands after induction (day 71) because it parallels the cytologic response evaluation and after consolidation 1 (week 16). Patients without a molecular CR at this time point have a high risk of relapse and little chance to obtain a molecular CR with conventional treatment. The most relevant time points have to be defined, however, depending on treatment protocol.
Detectable MRD after induction and first consolidation was associated with an unfavorable course of disease regardless of preexisting conventional risk factors, clearly validating the strong independent prognostic effect of molecular response as reported in several other smaller cohorts of adult patients with ALL.13-17 In this study only 12% of the patients with molecular failure after first consolidation who did not undergo SCT in first CR remained in CCR. Despite continued chemotherapy the median time to cytologic relapse was 7.6 months for patients with MRD > 10−4. In patients with MRD > 10−3 the median time to relapse was only 4.9 months.
A multivariate analysis proved that molecular response was the only significant prognostic factor for remission duration and survival throughout both conventional risk groups. Likewise, studies in pediatric ALL have found that molecular response is the most important independent prognostic factor.12 Even when using gene expression assays and testing for the presence of poor molecular aberrations such as Ikaros/IKZF1 deletions, MRD persistence after induction maintained its prognostic relevance as a functional biologic parameter.25
Results similar to those in molecular failure were observed for patients with molecular relapse.26 Without transplantation in first CR, the median time to cytologic relapse was 2.6 months and the probability of CCR was only 5%. Regular MRD follow-up tests are a prerequisite to detect molecular relapses. Because the overall relapse rate decreases after > 3 years from diagnosis, it may be sufficient to limit MRD follow-up to the first 2-3 years.
Molecular failure and relapse are not only associated with high relapse risk but also with significantly poorer survival. Because molecular failure indicates resistance to conventional chemotherapy, these patients also frequently respond poorly to salvage therapies after subsequent cytologic relapse. If no SCT is performed in first CR, the overall survival in molecular failure is only 33% at 5 years. This new high-risk group represents until now one of the most unfavorable subgroups within adult ALL.
Because of its prognostic relevance MRD has been implemented for treatment decisions in clinical trials. One option is to offer SCT to patients with molecular failure as to other patients with high risk. In this study, patients with molecular failure undergoing SCT in first CR had a significantly better probability of CCR than those without SCT (66% vs 11%), which again translated into a better survival (54% vs 32%). The Northern Italian Study group also reported an advantage for patients positive for MRD treated with SCT (N = 36) compared with non-SCT (N = 18) with approximately 50% compared with 10% long-term disease-free survival.16 These results indicate that, despite chemotherapy-resistant disease, the combination of conditioning regimen and donor-versus-leukemia effects could induce continuous remissions in patients positive for MRD. In our study, however, SCT in first CR could only be accomplished in 47% of the patients with molecular failure, often because of rapidly occurring relapses. Furthermore, it has been reported by several groups that a high level of MRD before SCT is associated with a higher relapse risk after allogeneic27,28 or autologous transplantation.29
There are 2 major options that may improve the outcome of patients with molecular failure. One is to optimize study logistics and to perform donor search in all patients to realize SCT as soon as possible after detection of molecular failure. The other option is to offer an interim therapy to reduce the tumor load and prohibit overt relapse. Treatment with drugs that use different mechanism of action is of particular interest in these chemotherapy-resistant patients. This may include subtype-specific chemotherapy such as the T cell–specific purine analog nelarabine,30 antibody treatment, or other immunologic therapies such as donor lymphocyte infusions in molecular relapse after SCT.31 Recently, it was reported in a small pilot study that for patients with persistent MRD a molecular remission rate of 80% could be achieved with a bispecific Ab that targets CD19.32
Treatment of patients with molecular failure is currently an unmet medical need. These patients are candidates for evaluation of new antileukemic agents that use MRD response as primary outcome. The results presented here for outcome of patients with molecular failure provide a reference database for future clinical trials enrolling patients with molecular failure for alternative treatment modalities to improve the patient's remission duration and overall survival.
In summary, the prospectively collected MRD data provide the proof of principle that evaluation of MRD with the use of standardized quantitative PCR as a measure of molecular response was feasible and clinically applicable in a multicenter treatment setting. The measurement of molecular response allowed the identification of a new subgroup of patients with an inadequate initial response and a high risk of relapse. Early assessment of MRD has not only been proven to be a strong and independent prognostic factor to predict the patients' outcome but also provided useful information for further treatment decisions and modifications. Because MRD was shown to directly correlate with clinical outcome, it is also an appropriate primary end point for future clinical trials that are evaluating new drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Regina Reutzel for study coordination and data management and the GMALL study group for entering patients into the trial and for follow-up documentation.
The work was supported by Deutsche Krebshilfe (702657Ho2) and BMBF (01GI9971/8).
Authorship
Contribution: N.G. and D.H. designed the research and coordinated the study; N.G. performed the statistical analysis and wrote the manuscript; M.B., M.K., H.T., T.R., and C.-R.B. surveyed and conducted the MRD analysis; and S.S. and E.T. surveyed and conducted the immunophenotyping. All coauthors supported the study as members of the protocol committee and approved the final report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola Gökbuget, Goethe University Hospital, Department of Medicine II, Theodor Stern Kai 7, D-60590 Frankfurt am Main, Germany; e-mail: goekbuget@em.uni-frankfurt.de.
References
Author notes
M.B. and D.H. contributed equally to this study.