Abstract
Diagnosis of essential thrombocythemia (ET) has been updated in the last World Health Organization (WHO) classification. We developed a prognostic model to predict survival at diagnosis, named IPSET (International Prognostic Score for ET), studying patients with WHO-defined ET. Age 60 years or older, leukocyte count ≥ 11 × 109/L, and prior thrombosis significantly affected survival, by multivariable Cox regression. On the basis of the hazard ratio, we assigned 2 points to age and 1 each to leukocyte count and thrombosis. So, the IPSET model allocated 867 patients into 3 risk categories with significantly different survival: low (sum of points = 0; median survival not reached), intermediate (sum = 1-2; median survival 24.5 years), and high (sum = 3-4, median survival 13.8 years). The IPSET model was further validated in 2 independent cohorts including 132 WHO-defined ET and 234 Polycythemia Vera Study Group–defined ET patients. The IPSET model was able to predict the occurrence of thrombosis, and not to predict post-ET myelofibrosis. In conclusion, IPSET, based on age ≥ 60 years, leukocyte count ≥ 11 × 109/L, and history of thrombosis allows prognostic assessment of WHO-defined ET and the validation process makes IPSET applicable in all patients phenotypically appearing as ET.
Introduction
Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) characterized by elevated platelet count, vascular complications, and disease evolution into myelofibrosis (post-ET MF) and acute myeloid leukemia (AML).1 Life expectancy of ET patients is considered not far from that of healthy population,2 at least during the first decade from diagnosis.3
After the first Polycythemia Vera Study Group (PVSG) criteria,4 the World Health Organization (WHO) dictated the new criteria for diagnosis of ET, which are essentially based on platelet count, histopathologic features (normal age-matched bone marrow cellularity with dispersed large to giant megakaryocytes), and demonstration of clonality.1 The WHO histopathologic criteria help to distinguish ET from another entity presenting with thrombocytosis, named prefibrotic primary myelofibrosis (pPMF), characterized by increased age-matched bone marrow cellularity, dense to loose clustered atypical megakaryocytes, increased granulopoiesis, and reduced erythropoiesis. At first, the WHO histopathologic criteria appeared not easy to apply resulting in an insufficient distinction between ET and pPMF.5 More recently, an overall blinded consensus ranging from 88% (295 cases, 2 European centers) to 93% (1104 cases, 7 international centers) has been achieved among pathologists to recognize the 2 entities.6,7 The impact of a clear-cut identification of these 2 entities is emphasized by clinical results of the international-based data collection of 1104 patients with a clinical phenotype of ET: WHO-defined ET patients showed a lower risk of overt myelofibrosis, AML evolution, and, finally, a better survival compared with pPMF.7
The current risk stratification of ET is targeted at the definition of vascular events by advanced age (> 60 years of age) and history of thrombohemorrhagic events.8,9 Concerning prognostic models powered to predict survival, advanced age, leukocytosis, and anemia have been incorporated as risk factors.10-12
In this study, we developed a prognostic model to predict survival in WHO-defined ET, named International Prognostic Score for Essential Thrombocythemia (IPSET), starting from 891 patients diagnosed strictly according to WHO criteria.
Methods
This study includes 891 patients with WHO-defined ET. This cohort was derived from a clinicopathologic international database including 1104 patients with a clinical phenotype of ET after the central hemopathologist's revision. The main aspect of the project has already been published.7 The study was approved by the institutional review board of each institution. Diagnosis of post–ET-MF was made in accordance with the International Working Group on Myelofibrosis Research and Treatment (IWG-MRT) criteria.13 Diagnosis of AML was made according to WHO criteria, with a 20% blast threshold for diagnosis.
Statistical analysis
The Kaplan-Meier product-limit method was used to estimate univariate survival curves, and the Gehan-Wilcoxon test was adopted to compare survival curves. Cox proportional hazards regression was adopted to carry out uni- and multivariate survival analyses. The following parameters at diagnosis of ET were taken into consideration to find prognostic risk factors for survival in univariate and multivariate models: age, prior thrombosis, leukocyte count, platelet count, hemoglobin level, JAK mutational status, splenomegaly. The Harrell C concordance statistic was applied to set the best cutoff levels of continuous variables to predict survival. The cumulative incidence of leukemic evolution was estimated with a competing risk approach according to the Kalbfleisch-Prentice method.14 Death in absence of leukemia was considered as a competing event. All analyses were performed using Stata SE 11.2 (StataCorp LP) software, and Microsoft Excel.
Results
Initial characteristics and clinical course
Patients' characteristics are summarized in Table 1. Median follow-up from diagnosis was 6.2 years (range, 0-27). Cytotoxic drugs were assigned to 507 (65%) patients with available information along the course of the disease. During follow-up, 8 (1%) patients developed AML; all had received cytotoxic agents. The 10-year cumulative incidence of leukemic transformation, estimated with death as a competing risk, was 0.65% (95% confidence interval [CI]: 0.18%-1.81%). Most likely because of the low number of events, we did not identify prognostic factors for AML occurrence in ET: the log-rank test recognized only a borderline significance for white blood cell count higher than 15 × 109/L at diagnosis of ET (P = .05), and did not identify cytotoxic therapy as a risk factor for AML occurrence (P = .06).
Main clinical characteristics of 891 patients with WHO-defined essential thrombocythemia
| Characteristics . | . |
|---|---|
| Median age, y (range) | 56 (13-91) |
| Male/female | 370/521 |
| Median leukocyte count, ×109/L (range) | 8.6 (5.3-14.2) |
| Median hemoglobin, g/dL (range) | 14.1 (11.9-16.1) |
| Median platelet count, ×109/L (range) | 774 (500-1464) |
| Median LDH, mU/mL (range) | 298 (150-582) |
| Splenomegaly (proportion) | 146 (16%) |
| Median circulating CD34+, mcL (range) | 2 (0-15.2) |
| Grade 1 fibrosis (proportion) | 24 (3%) |
| Characteristics . | . |
|---|---|
| Median age, y (range) | 56 (13-91) |
| Male/female | 370/521 |
| Median leukocyte count, ×109/L (range) | 8.6 (5.3-14.2) |
| Median hemoglobin, g/dL (range) | 14.1 (11.9-16.1) |
| Median platelet count, ×109/L (range) | 774 (500-1464) |
| Median LDH, mU/mL (range) | 298 (150-582) |
| Splenomegaly (proportion) | 146 (16%) |
| Median circulating CD34+, mcL (range) | 2 (0-15.2) |
| Grade 1 fibrosis (proportion) | 24 (3%) |
LDH indicates lactate dehydrogenase.
Overall, 87 (10%) patients died, of whom 41 had available causes of death (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), which included thrombosis in 21 (51%) patients, hemorrhage in 4 (10%), leukemia in 7 (17%), and cancer in 9 (22%).
Identification of risk factors for survival
The Kaplan-Meier and Cox proportional hazards regression allowed us to identify the following parameters impacting survival by univariate analysis in 891 patients with WHO-ET: advanced age (P < .0001), higher platelet count (P = .003), higher white blood cell count (P < .0001), history of thrombosis (P < .0001), while splenomegaly, JAK2 mutational status, and hemoglobin level did not have any impact on survival. The Harrell C concordance statistics recognized age 60 years or older (P < .0001), white blood cell count ≥ 11 × 109/L (P < .0001), platelet count > 1000 × 109/L (P = .004), and anemia (hemoglobin value < 12 g/dL for women and < 13 g/dL for men; P = .04) as risk factors for worse survival.
Supplemental Table 2 reports results obtained from Cox multivariable regression: age 60 years or older, white blood cell count ≥ 11 × 109/L, and history of thrombosis retained their statistical significance on survival. To obtain a more accurate prediction, a further multivariable analysis including only these 3 parameters was carried out showing a hazard ratio (HR) of age 2-fold higher than that of leukocytosis or thrombosis (Table 2).
Result of the final multivariable analysis of predictors of survival in 891 patients with WHO-defined essential thrombocythemia
| Factors . | HR . | 95% CI . |
|---|---|---|
| Age ≥ 60 y | 6.5 | 3.9-10.7 |
| WBC ≥ 11 × 109/L | 3.1 | 2-4.7 |
| Thrombosis | 2.9 | 1.9-4.5 |
| Factors . | HR . | 95% CI . |
|---|---|---|
| Age ≥ 60 y | 6.5 | 3.9-10.7 |
| WBC ≥ 11 × 109/L | 3.1 | 2-4.7 |
| Thrombosis | 2.9 | 1.9-4.5 |
P < .0001.
HR indicates hazard ratio; CI, confidence interval; and WBC, white blood cell count.
The IPSET model
The IPSET model was built by assigning each factor (age 60 years or older, white blood cell count ≥ 11 × 109/L, and history of thrombosis) an integer weight close to the corresponding HR in the multivariable Cox regression (Table 3) in 867 patients (those with available all the risk factors). The higher HR associated with advanced age by multivariable Cox regression prompted us to assign a different weight to this parameter.
International Prognostic Scoring in essential thrombocythemia for survival in WHO-ET
| Risk factors . | Scores . | ||
|---|---|---|---|
| 0 . | 1 . | 2 . | |
| Age, y | < 60 | ≥ 60 | |
| WBC count, × 109/L | < 11 | ≥ 11 | |
| History of thrombosis | No | Yes | |
| Risk factors . | Scores . | ||
|---|---|---|---|
| 0 . | 1 . | 2 . | |
| Age, y | < 60 | ≥ 60 | |
| WBC count, × 109/L | < 11 | ≥ 11 | |
| History of thrombosis | No | Yes | |
Low risk implies a sum of scores equal to 0; intermediate risk, a sum of scores equal to 1-2; and high risk, a sum of scores equal to 3-4.
ET indicates essential thrombocythemia; and WBC, white blood cell count.
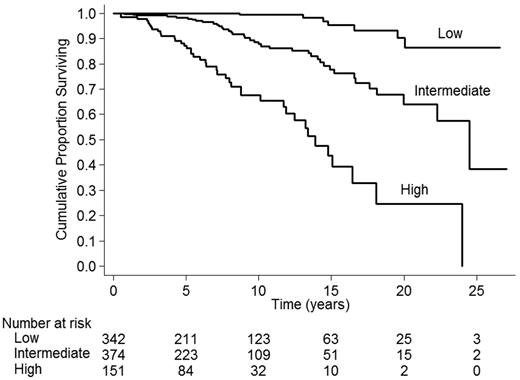
Kaplan-Meier survival curves (supplemental Figure 1) corresponding to the 5 scores were significantly different by log-rank test and test for trend (P < .001): score 0 (342 patients, median survival not reached [NR]), score 1 (146 patients, median survival 24.5 years, 95% CI: 22.3-NR), score 2 (228 patients, median survival 19.9 years 95% CI: 15.2-NR), score 3 (127 patients, median survival 16.5 years, 95% CI: 13.4-NR), score 4 (24 patients, median survival 8.8 years, 95% CI: 4.7-11.7). To facilitate the implementation of the score in clinical practice, we recoded it into 3 broader categories of adequate size by pooling consecutive score values, whose estimate of survival can be found in Figure 1. The resulting risk categories were low (sum of scores = 0), intermediate (sum of scores = 1 or 2), and high (sum of scores = 3 or 4) with significantly different survivals: not reached in low-risk patients (n = 342), 24.5 years (95% CI: 22.3-NR) in intermediate risk (n = 374), and 14.7 years (95% CI: 11.9-18) in high risk (n = 151). Fisher exact test did not reveal differences in the distribution of the causes of death among IPSET categories (supplemental Table 1). The model is to be applied for patients at diagnosis of ET.
Estimate of survival of 867 patients with essential thrombocythemia, by IPSET score. Three risk factors were taken into account: age ≥ 60 years (2 points), leukocyte count ≥ 11 × 109/L (1 point) and prior thrombosis (1 point). The resulting risk categories were low (sum of scores = 0), intermediate (sum of scores = 1 or 2), and high (sum of scores = 3 or 4) with significantly different survivals: not reached in low-risk patients, 24.5 years (95% CI: 22.3-NR) in intermediate risk, and 14.7 years (95% CI: 11.9-18) in high-risk patients.
Estimate of survival of 867 patients with essential thrombocythemia, by IPSET score. Three risk factors were taken into account: age ≥ 60 years (2 points), leukocyte count ≥ 11 × 109/L (1 point) and prior thrombosis (1 point). The resulting risk categories were low (sum of scores = 0), intermediate (sum of scores = 1 or 2), and high (sum of scores = 3 or 4) with significantly different survivals: not reached in low-risk patients, 24.5 years (95% CI: 22.3-NR) in intermediate risk, and 14.7 years (95% CI: 11.9-18) in high-risk patients.
Validation of the IPSET model
The IPSET model was then validated in 2 independent cohorts: one including 132 WHO-defined ET patients from Cologne (Germany) and a further including 234 PVSG-defined ET patients from Dijon (France), hence not diagnosed according to WHO criteria. These patients, whose clinical features at diagnosis are reported in supplemental Table 3, belong to a series of patients already published,6,11 but for this analysis we only considered patients who have all risk factors available.
Concerning the Cologne cohort, IPSET allowed a stratification of patients with significantly different survivals (P < .001) with median value not reached (95% CI: 15.6-NR) in the low risk, of 17.5 years (95% CI: 13.6-NR) in the intermediate risk, and of 9.4 years (95% CI: 5.8-13.4) in the high-risk categories (supplemental Figure 2). The IPSET model also consented to stratify the Dijon cohort into categories with significantly different survivals with median values ranging from 18.6 years (95% CI: NR) in the low-risk category, to 7.1 years (95% CI: 5.8-13.4) in the high-risk category (supplemental Figure 3).
Other applications of the IPSET model
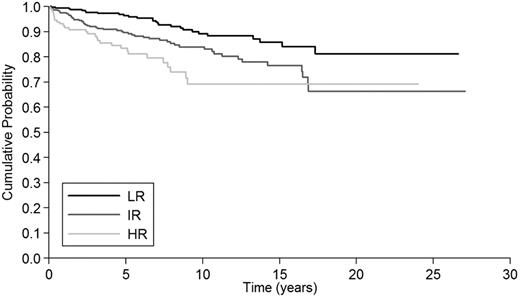
We also tested whether the IPSET model was able to predict thrombosis (n = 109) and myelofibrosis (n = 31) during follow-up. Among severe thrombosis, 27 (48%) occurred in the low-risk group, 53 (46%) in intermediate risk, and 29 (16%) in high risk. Figure 2 shows that the IPSET model predicted thrombosis during follow-up (P < .001). The 10-year thrombosis-free survival was 89% (95% CI: 84%-93%) for patients in the low-risk category, 84% (95% CI: 78%-88%) for those in the intermediate risk, and 69% (95% CI: 57%-78%) for those in the high risk. Concerning MF, IPSET categories did not predict this evolution.
Estimate of thrombosis-free survival in 867 patients with essential thrombocythemia, by IPSET score. Three risk factors were taken into account: age ≥ 60 years (2 points), leukocyte count ≥ 11 × 109/L (1 point) and prior thrombosis (1 point). The resulting risk categories were low (sum of scores = 0), intermediate (sum of scores = 1 or 2), and high (sum of scores = 3 or 4) with significantly different 10-year thrombosis-free survival: 89% (95% CI: 84%-93%) for patients in the low-risk category, 84% (95% CI: 78%-88%) for those in the intermediate risk and 69% (95% CI: 57%-78%) for those in the high risk.
Estimate of thrombosis-free survival in 867 patients with essential thrombocythemia, by IPSET score. Three risk factors were taken into account: age ≥ 60 years (2 points), leukocyte count ≥ 11 × 109/L (1 point) and prior thrombosis (1 point). The resulting risk categories were low (sum of scores = 0), intermediate (sum of scores = 1 or 2), and high (sum of scores = 3 or 4) with significantly different 10-year thrombosis-free survival: 89% (95% CI: 84%-93%) for patients in the low-risk category, 84% (95% CI: 78%-88%) for those in the intermediate risk and 69% (95% CI: 57%-78%) for those in the high risk.
Discussion
This is the first study on patients with WHO-ET aimed at defining a prognostic model for survival. The general outline of the ET project by the IWG-MRT was described elsewhere.7 The primary end point of the project was to assess the clinical relevance of applying WHO criteria to distinguish ET from pPMF. In the previous article,7 we showed that diagnosis of WHO-ET implies a lower risk of developing AML, overt myelofibrosis and actually a better survival compared with that of pPMF: the 10-year figures were 0.7%, 0.8%, and 89% for ET and 5.8%, 12.3%, and 76% for pPMF, respectively. This large cohort of WHO-ET patients, the international basis of the project, and the validation process allowed us to define a robust prognostic model for survival in WHO-ET.
The IPSET model is based on 3 parameters: age 60 years or older, history of thrombosis, and leukocyte count ≥ 11 × 109/L. Age older than 60 years and history of thrombosis are the 2 conventional risk factors for vascular events in ET.8 Age older than 60 years was also recognized as a risk factor for survival in large cohorts of ET patients.10,12,15,16 This study confirms previous observations on the association between leukocytosis and worse survival in ET.10,12,16 The role of leukocytosis on thrombosis prediction in ET has been largely discussed in the last years as many investigators found a correlation with thrombosis,3,17-20 others did not,21,22 and others found a relationship between increase of leukocyte count over time and thrombosis.23 Although all of these factors should ideally be assessed in a prospective study, the IPSET model undoubtedly states that leukocytosis > 11 × 109/L is a predictor of survival as well as history of thrombosis.
The IPSET database also allowed us to study the predictive role of JAK2 (V617F) mutation in 690 WHO-ET with assessed mutational status (61% V617F-positive). The presence of the mutation is associated with a higher risk of major and arterial thrombosis in WHO-ET,15 confirming the results of a recent meta-analysis in ET.24,25 Concerning survival, we did not find any association between the presence of the mutation and outcome: although JAK2 (V617F) is a risk factor for thrombosis, thrombosis accounts only for half of the causes of death; other causes have likely a lower association with the presence of the mutation.
We validated the IPSET model in 2 independent samples of patients from Europe. One set (Cologne cohort) includes patients with ET strictly diagnosed according to the WHO criteria and we found that IPSET stratifies patients into 3 risk categories with different survivals. This finding demonstrates that IPSET is useful in survival prediction of WHO-ET patients and may be applied in the case of diagnosis made according to WHO, mainly by MPN-expert pathologists. The difference in term of median survival between the IPSET and the Cologne cohort might be explained by the different sample of patients.
The second set (Dijon cohort) includes patients with diagnosis of ET performed outside of the WHO criteria, reflecting the common practice of diagnosis without the auxilium of MPN-expert pathologists. The IPSET model was also validated in this cohort of patients. Median survivals of these patients are lower than those of the IPSET database. This might be because of the inclusion of patients with early-phase PMF, known to have worse survival and to have higher leukocyte counts actually affecting risk-group distribution (more patients in higher risk categories), or because of the higher median age.7 Hence, the validation process showed that the IPSET model stratifies all patients with ET independently from the type of diagnostic classification applied. All this makes the IPSET model universally applicable in all patients phenotypically presenting as ET.
Another key observation from the current study involves the progression to AML in WHO-ET. We report reassuring data on this topic placing AML's prevalence at ∼ 1%. This figure looks like the data obtained in ET cohorts with a more accurate histopathologic assessment.12,21 We found that leukocytosis has a borderline impact on AML occurrence as the “myeloproliferative phenotype” exposes patients to higher risk of AML. Concerning the role of cytotoxic treatments, the IPSET database did not show any relationship, indicating a relative safety of current therapies at least in the short term (median time of observation: 6.2 years). The IPSET study may also guide future clinical trials: the very low frequency of AML suggests avoiding the inclusion of AML reduction as an end point.
It is known that in PMF some cytogenetic abnormalities may predict AML evolution,26,27 but the IPSET database was not finalized at the collection of cytogenetic data and we cannot address this point. However, investigators from the Mayo Clinic found cytogenetic abnormalities in 7% of 402 patients with ET without any impact on AML.
Defining survival in diseases known to be as long-lasting as ET is clinically relevant for patient-doctor communication, for treatment strategy, and for enrollment criteria of clinical trials. The international database used for the IPSET model reflects the current clinic practice in the European Union and in the United States, and defines what doctors may expect from current treatments in WHO-ET. Most patients who are at low and intermediate risk at diagnosis have a long survival, especially if cardiovascular risk factors are absent or controlled. Patients at high risk—that is, patients older than 60 years of age with leukocytosis or with a history of thrombosis—have a median survival of 14.7 years. Within this cohort of patients, new therapies might be taken into consideration in the subset of patients resistant or intolerant to hydroxyurea. This indication fits even more when diagnosis is done outside WHO classification, as shown by the validation process in the Dijon cohort (median survival of HR patients 7.1 years). As leukocytosis and thrombosis are the main risk factors in older patients with ET, new treatments should demonstrate activity against those targets.
As additional points, we found that the IPSET model predicts the occurrence of thrombosis, but not that of MF. Although this study was not designed to study prediction of thrombosis, we find the ability of the score to predict thrombosis in a simple manner useful.
In conclusion, the IPSET model, based on age of 60 years or older, leukocyte count ≥ 11 × 109/L, and history of thrombosis, allows prognostic assessment of WHO-defined ET at diagnosis and the validation process even made IPSET applicable in all patients phenotypically appearing as ET.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.T. and H.M.K. are greatly indebted to Prof Emeritus Dr H. C. V. Diehl, former director of the First Clinic of Medicine (University of Cologne, Cologne, Germany) and his associates for providing the clinical data of their patients (Cologne cohort).
F.P. was supported by a grant from Associazione Italiana contro Leucemie (AIL; Linfomi, Mieloma) Varese Onlus. Studies performed at the Department of Hematology Oncology, Fondazione Istituti di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo & University of Pavia (Pavia, Italy) were supported by Associazione Italiana per la Ricerca sul Cancro (Special Program Molecular Clinical Oncology 5x1000, project 1005), Fondazione Cariplo, Regione Lombardia, and the Italian Ministry of Health. A.M.V. was supported by a special grant from Associazione Italiana per la Ricerca sul Cancro (AIRC; Milano, Italy) Special Program Molecular Clinical Oncology 5x1000 (no. 1005) to AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM).
Authorship
Contribution: F.P., T.B., and A.T. designed the research; F.P. interpreted the results and wrote the manuscript; J.T. reviewed bone marrow slides; F.P., F.G., E.R., A.C., H.G., H.M.K., M.R., M.L.R., N.G., A.M.V., A.G., B.G., L.M., F.R., E.S.G.d'A., I.B., C.A.H., E.B., F.M., M.M., D.C., E.A., V.C., V.B.-A., M.C., T.B., and A.T. performed research and revised the manuscript; and C.P. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Passamonti, MD, Division of Hematology, Department of Internal Medicine, University Hospital Ospedale di Circolo e Fondazione Macchi, Viale L. Borri 57, 21100 Varese, Italy; e-mail francesco.passamonti@ospedale.varese.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal