Abstract
Improved supportive care, more precise risk stratification, and personalized chemotherapy based on the characteristics of leukemic cells and hosts (eg, pharmacokinetics and pharmacogenetics) have pushed the cure rate of childhood acute lymphoblastic leukemia to near 90%. Further increase in cure rate can be expected from the discovery of additional recurrent molecular lesions, coupled with the development of novel targeted treatment through high-throughput genomics and innovative drug-screening systems. We discuss specific areas of research that promise to further refine current treatment and to improve the cure rate and quality of life of the patients.
Introduction
Optimal use of existing antileukemic agents and improved supportive care in contemporary clinical trials have improved the 5-year survival rate of childhood acute lymphoblastic leukemia (ALL) above 85% in developed countries (Table 1).1-8 Further advances in survival and quality of life will require a better understanding of ALL pathobiology, the mechanisms of drug resistance, and drug disposition in the host, together with the development of innovative therapeutics. To this end, the advent of high-resolution genome-wide analyses of gene expression, DNA copy number alterations, and epigenetic changes, and more recently, next-generation whole-genome and transcriptome sequencing have provided new insights into leukemogenesis, drug resistance, and host pharmacogenomics, identified novel subtypes of leukemia, and suggested potential targets for therapy.9,10 Paralleling these advances has been the development of novel monoclonal antibodies, small molecule inhibitors, chemotherapeutics, and cell-based treatment strategies.9 Here we discuss some of the current challenges and future directions in pediatric ALL research.
Patient characteristics and treatment results from selected clinical trials enrolling children with ALL
| Study group . | Years of study . | No. of patients . | Age, y, range . | T-cell ALL, % . | 5-y outcome, % . | Data source . | ||
|---|---|---|---|---|---|---|---|---|
| Cumulative CNS relapse rate . | EFS . | Survival . | ||||||
| AIEOP-95 | 1995-2000 | 1743 | 0-18 | 11 | 1.2 ± 0.3 | 75.9 ± 1.0 | 85.5 ± 0.8 | Conter et al1 |
| BFM-95 | 1995-1999 | 2169 | 0-18 | 13 | 4.0 ± 0.4 | 79.6 ± 0.9 | 87.0 ± 0.7 | Möricke et al2 |
| COG | 2000-2005 | 7153 | 0-21 | 7 | NA | NA | 90.4 ± 0.5 | Hunger et al3 |
| DCOG-9 | 1997-2004 | 859 | 1-18 | 11 | 2.6 ± 0.6 | 80.6 ± 1.4 | 86.4 ± 1.2 | Veerman et al4 |
| DFCI 00-01 | 2000-2004 | 492 | 1-18 | 11 | NA | 80.0 ± 2 | 91 ± 1 | Vrooman et al5 |
| NOPHO-2000 | 2002-2007 | 1023 | 1-15 | 11 | 2.7 ± 0.6 | 79.4 ± 1.5 | 89.1 ± 11 | Schmiegelow et al6 |
| SJCRH 15 | 2000-2007 | 498 | 1-18 | 15 | 2.7 ± 0.8 | 85.6 ± 2.9 | 93.5 ± 1.9 | Pui et al7 |
| UKALL 97/99 | 1999-2002 | 938 | 1-18 | 11 | 3.0 ± 0.6 | 80.0 ± 1.3 | 88.0 ± 1.1 | Mitchell et al8 |
| Study group . | Years of study . | No. of patients . | Age, y, range . | T-cell ALL, % . | 5-y outcome, % . | Data source . | ||
|---|---|---|---|---|---|---|---|---|
| Cumulative CNS relapse rate . | EFS . | Survival . | ||||||
| AIEOP-95 | 1995-2000 | 1743 | 0-18 | 11 | 1.2 ± 0.3 | 75.9 ± 1.0 | 85.5 ± 0.8 | Conter et al1 |
| BFM-95 | 1995-1999 | 2169 | 0-18 | 13 | 4.0 ± 0.4 | 79.6 ± 0.9 | 87.0 ± 0.7 | Möricke et al2 |
| COG | 2000-2005 | 7153 | 0-21 | 7 | NA | NA | 90.4 ± 0.5 | Hunger et al3 |
| DCOG-9 | 1997-2004 | 859 | 1-18 | 11 | 2.6 ± 0.6 | 80.6 ± 1.4 | 86.4 ± 1.2 | Veerman et al4 |
| DFCI 00-01 | 2000-2004 | 492 | 1-18 | 11 | NA | 80.0 ± 2 | 91 ± 1 | Vrooman et al5 |
| NOPHO-2000 | 2002-2007 | 1023 | 1-15 | 11 | 2.7 ± 0.6 | 79.4 ± 1.5 | 89.1 ± 11 | Schmiegelow et al6 |
| SJCRH 15 | 2000-2007 | 498 | 1-18 | 15 | 2.7 ± 0.8 | 85.6 ± 2.9 | 93.5 ± 1.9 | Pui et al7 |
| UKALL 97/99 | 1999-2002 | 938 | 1-18 | 11 | 3.0 ± 0.6 | 80.0 ± 1.3 | 88.0 ± 1.1 | Mitchell et al8 |
EFS indicates event-free survival; AIEOP, Associazione Italiana di Ematologia ed Oncologia Pediatrica; BFM, Berlin-Frankfurt-Münster; NA, not available; DCOG, Dutch Childhood Oncology Group; DFCI, Dana-Farber Cancer Institute consortium; NOPHO, Nordic Society of Pediatric Hematology and Oncology; SJCRH, St Jude Children's Research Hospital; and UKALL, United Kingdom Medical Research Council Working Party on Childhood Leukaemia.
Emerging leukemia subtypes
Traditionally, ALL has been classified into precursor T (or T-cell), precursor B, and B-cell (Burkitt) phenotypes, which are then further subdivided according to recurrent karyotypic abnormalities, including aneuploidy and translocations.9,11 Detailed profiling of submicroscopic alterations and mutational analyses have allowed refinement of these classification schema, identification of genetic alterations that coexist and cooperate with chromosomal alterations in leukemogenesis, and discovery of new ALL subtypes that lack alterations on cytogenetic analysis. Like acute myeloid leukemia,12 many ALL subtypes are characterized by genetic alterations that perturb multiple key cellular pathways, including hematopoietic development, signaling or proliferation, and epigenetic regulation. Recent studies have identified a novel high-risk immature T-cell subtype, termed “early T-cell precursor” ALL, which is characterized by immunologic markers and a gene expression profile reminiscent of double-negative 1 thymocytes that retain the ability to differentiate into both T-cell and myeloid, but not B-cell, lineages.13 Whole genome sequencing showed that the mutational spectrum of this subtype shares characteristics of acute myeloid leukemia, and the transcriptional profile of the blasts is similar to that of normal hematopoietic stem cells and granulocyte-macrophage precursors, suggesting that this subtype of leukemia is a stem cell disease.14 The mutations frequently involve genes regulating hematopoietic development (GATA3, ETV6, RUNX1, IKZF1, and EP300), cytokine receptor and RAS signaling (NRAS, KRAS, FLT3, IL7R, JAK3, JAK1, SH2B3, and BRAF), and histone modification (EZH2, EED, SUZ12, SETD2, and EP300). These findings suggests that patients with early T-cell precursor ALL may benefit from new therapies that exploit the myeloid or stem cell features of this leukemia, such as high-dose cytarabine or epigenetic therapy,14 a hypothesis that remains to be proven.
Although several specific genetic abnormalities have been recognized to have prognostic or therapeutic relevance,9 there is no consensus on the specific genotypes used for treatment stratification. Thus, different favorable (hyperdiploidy > 50; trisomies 4, 10, and 17; ETV6-RUNX1) and unfavorable (hypodiploidy < 44; intrachromosomal amplification of chromosome 21; BCR-ABL1) genotypes have been used by various study groups to direct therapy.9,15-18 Pre-B ALL with the t(1;19) (q23;p13) and expression of the TCF3-PBX1 fusion, once considered a high-risk entity, is not even prospectively identified by some study groups because of the improved outcome with contemporary treatment regimens. We assert that this genotype should still be identified for intensification of intrathecal therapy because of its associated risk of CNS relapse in the context of clinical trials with improved systemic control.7,19 However, this is not a consistent finding in other clinical trials; we hypothesized that improved hematologic control might have increased the risk of CNS relapse in these patients because bone marrow relapse and CNS relapse can be competing events. Indeed, this hypothesis could also explain the seemingly paradoxical findings in a randomized study of the Children's Oncology Group (COG), showing improved CNS control but inferior survival of patients treated with triple intrathecal therapy compared with those treated with intrathecal methotrexate therapy20 (as discussed in “The case for complete omission of prophylactic cranial irradiation”).
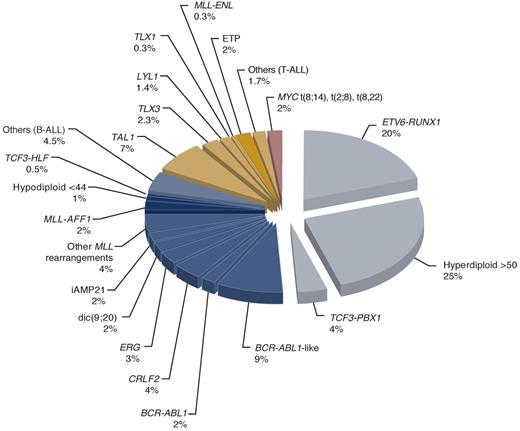
Primary genetic abnormalities can be identified in 75% to 80% of childhood ALL cases with standard chromosomal and molecular genetic analyses,9 but in virtually all cases with the addition of genome-wide analyses (Figure 1). Of several newly discovered subtypes, one is characterized by increased CRLF2 expression with or without a corresponding genomic lesion (IGH@-CRLF2, P2RY8-CRLF2, and CRLF2 F232C) and commonly with a concomitant JAK1/2 sequence mutation, and occurs in 5% to 7% of children with precursor B-cell ALL and, remarkably, in approximately 50% of the cases with Down syndrome.21-26 The non-Down syndrome patients with this genotype probably require more intensive therapy because they generally have a poor outcome in the reported series (particularly among the National Cancer Institute high-risk patients).26 The prognostic impact of this genotype among patients with Down syndrome remains to be determined.
Estimated frequency of specific genotypes in childhood ALL. Data were modified from Pui et al9 by including recently identified genotypes. The genetic lesions that are exclusively seen in cases of T-cell ALL are indicated in gold and those commonly associated with precursor B-cell ALL in blue. The darker gold or blue color indicates those subtypes generally associated with poor prognosis. BCR-ABL1–like cases can be separated into one group with CRLF2 dysregulation and the other with activating cytokine receptor and kinase signaling.
Estimated frequency of specific genotypes in childhood ALL. Data were modified from Pui et al9 by including recently identified genotypes. The genetic lesions that are exclusively seen in cases of T-cell ALL are indicated in gold and those commonly associated with precursor B-cell ALL in blue. The darker gold or blue color indicates those subtypes generally associated with poor prognosis. BCR-ABL1–like cases can be separated into one group with CRLF2 dysregulation and the other with activating cytokine receptor and kinase signaling.
Another novel high-risk subtype, termed “BCR-ABL1–like” ALL, also has a precursor B-cell phenotype, exhibits a gene expression profile similar to that of BCR-ABL1–positive ALL with an IZKF1 alteration, and occurs in approximately 10% of children with ALL.27,28 As many as half of the BCR-ABL1–like cases have a CRLF2 rearrangement,29 with concomitant JAK mutations in one-third of the CRLF2-rearranged cases.22-26 In a recent transcriptome and whole genome sequencing study, many of the BCR-ABL1–like cases lacking CRLF2 dysregulation were found to have alternative genetic alterations activating cytokine receptor and kinase signaling.30 This genotype is associated with a high risk of relapse, independent of age, leukocyte count at diagnosis, cytogenetics, and levels of minimal residual disease (MRD) after remission induction.27,28 Partly because considerable expertise is needed to identify these subtypes and partly because of their recent discovery, they have yet to be incorporated into risk stratification systems in the contemporary clinical trials. However, we would test patients with refractory or relapsed leukemia for these subtypes, as they may benefit from targeted therapy (discussed in the next section).
Potential therapeutic targets: the search for personalized medicine
The remarkable improvement of early treatment outcome in children with BCR-ABL1–positive ALL with the addition of an ABL1 tyrosine kinase inhibitor (imatinib) to an intensive treatment regimen of the COG,18 including a high cumulative dose of alkylating agent and cranial irradiation, has fueled enthusiasm for developing an even more effective targeted therapy for this ALL subtype. An international study has been initiated to test whether the intensity of chemotherapy can be reduced and cranial irradiation limited to only patients with overt CNS leukemia at diagnosis by substitution of imatinib with a more potent second-generation inhibitor (dasatinib) that penetrates readily to the CNS.
Recent insights gained from genome-wide analyses have identified novel genetic alterations, some of which have clinical relevance and others could also serve as therapeutic targets in childhood ALL (Table 2).21-24,27,28,30-47 The identification of activating mutations of the Janus kinases (primarily JAK2, but also JAK1 and JAK3) in high-risk ALL22-25,39-42 has led to a COG phase 1 clinical trial of JAK inhibitor (ruxolitinib) for relapsed and refractory malignancy. Among genetic abnormalities identified in BCR-ABL1–like cases, EBF1-PDGFRB or NUP214-ABL1 fusion responded to ABL1 tyrosine kinase inhibitors (which also inhibit PDGFRB), and BCR-JAK2 or mutated IL7R responded to JAK2 inhibitor in preclinical studies.30
Novel genomic alterations with potential prognostic or therapeutic relevance
| Gene . | Alteration . | Frequency . | Pathway and consequences of alteration . | Clinical relevance . | References . |
|---|---|---|---|---|---|
| PAX5 | Focal deletions, translocations, sequence mutations | 31.7% of precursor B-cell ALL | Transcription factor required for B-lymphoid development Mutations impair DNA binding and transcriptional activation | Role in pathogenesis of precursor B-ALL; not related to outcome | 31,–33 |
| IKZF1 | Focal deletions or sequence mutations | 15% of all pediatric precursor B-cell ALL cases | Transcription factor required for lymphoid development Deletions and mutations result in loss of function or dominant negative isoforms | 31 | |
| > 80% BCR-ABL1 ALL and 66% chronic myeloid leukemia in lymphoid blast crisis | Associated with poor outcome | 32,34,35 | |||
| One-third of high-risk BCR-ABL1 negative ALL | 3-fold increased risk of relapse | 27,28,36 | |||
| Inherited variants | Increased risk of ALL | 37,38 | |||
| JAK1/2 | Pseudokinase and kinase domain mutations | 18%-35% Down syndrome ALL | Constitutive JAK-STAT activation | May be responsive to JAK inhibitors | 39,40,–42 |
| 10.7% high-risk BCR-ABL1 negative ALL | 39 | ||||
| CRLF2 | Rearrangement as IGH@-CRLF2 or P2RY8-CRLF2 resulting in overexpression | 5%-16% pediatric and adult precursor B-cell ALL, and > 50% Down syndrome-ALL | Associated with mutant JAK in up to 50% of cases | 21,22,44,45 | |
| 14% pediatric high-risk ALL | Associated with IKZF1 alteration and JAK mutations | Associated with poor outcome | 23,24 | ||
| CREBBP | Focal deletion and sequence mutations | 19% of relapsed ALL; commonly acquired at relapse | Mutations result in impaired histone acetylation and transcriptional regulation | Associated with glucocorticoid resistance | 45,46 |
| TP53 | Deletions and sequence mutations | Up to 3% precursor B-cell ALL, commonly acquired at relapse | Loss of function or dominant negative | Associated with poor outcome | 47 |
| Gene . | Alteration . | Frequency . | Pathway and consequences of alteration . | Clinical relevance . | References . |
|---|---|---|---|---|---|
| PAX5 | Focal deletions, translocations, sequence mutations | 31.7% of precursor B-cell ALL | Transcription factor required for B-lymphoid development Mutations impair DNA binding and transcriptional activation | Role in pathogenesis of precursor B-ALL; not related to outcome | 31,–33 |
| IKZF1 | Focal deletions or sequence mutations | 15% of all pediatric precursor B-cell ALL cases | Transcription factor required for lymphoid development Deletions and mutations result in loss of function or dominant negative isoforms | 31 | |
| > 80% BCR-ABL1 ALL and 66% chronic myeloid leukemia in lymphoid blast crisis | Associated with poor outcome | 32,34,35 | |||
| One-third of high-risk BCR-ABL1 negative ALL | 3-fold increased risk of relapse | 27,28,36 | |||
| Inherited variants | Increased risk of ALL | 37,38 | |||
| JAK1/2 | Pseudokinase and kinase domain mutations | 18%-35% Down syndrome ALL | Constitutive JAK-STAT activation | May be responsive to JAK inhibitors | 39,40,–42 |
| 10.7% high-risk BCR-ABL1 negative ALL | 39 | ||||
| CRLF2 | Rearrangement as IGH@-CRLF2 or P2RY8-CRLF2 resulting in overexpression | 5%-16% pediatric and adult precursor B-cell ALL, and > 50% Down syndrome-ALL | Associated with mutant JAK in up to 50% of cases | 21,22,44,45 | |
| 14% pediatric high-risk ALL | Associated with IKZF1 alteration and JAK mutations | Associated with poor outcome | 23,24 | ||
| CREBBP | Focal deletion and sequence mutations | 19% of relapsed ALL; commonly acquired at relapse | Mutations result in impaired histone acetylation and transcriptional regulation | Associated with glucocorticoid resistance | 45,46 |
| TP53 | Deletions and sequence mutations | Up to 3% precursor B-cell ALL, commonly acquired at relapse | Loss of function or dominant negative | Associated with poor outcome | 47 |
A candidate gene sequencing study disclosed frequent involvement of the TP53/RB1 signaling, B-cell development pathway, RAS signaling, and JAK/STAS signaling pathways in high-risk ALL, suggesting RAS/MAPK signaling as a potential target for therapy.48 The association of high expression of FLT3 with a poor outcome in infant ALL cases without MLL rearrangement suggests that these infants could also be included in clinical trials testing an FLT3 inhibitor.49 It may also be of interest to test the multikinase inhibitors sorafenib and crenolanib, which have significant activity in acute myeloid leukemia with mutated FLT3,50 in infant cases. The findings of aberrant DNA methylation in the majority of MLL-rearranged infant ALL cases51,52 and mutations of CREBBP encoding histone acetyltransferase CREB-binding protein in relapsed ALL cases45 raise the possibility of using epigenetic treatment (eg, DNA methyltransferase inhibitor and histone deacetylase inhibitor) in these patients. Although none of the newly discovered genetic abnormalities are part of the routine workup in current front-line clinical trials, it would seem reasonable to search for them in refractory or relapsed cases so that the affected patients could benefit from targeted therapy.
Toward more precise risk stratification
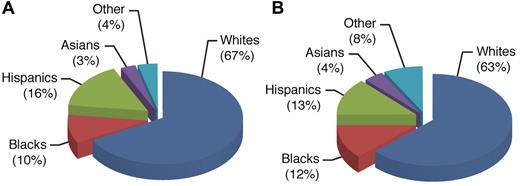
Precise assessment of the risk of relapse in individual patients is essential to ensuring that intensive treatment is limited primarily to high-risk cases, thus sparing low-risk cases from undue toxicities. It is well recognized that effective treatment can abolish the adverse impact of many clinical and biologic features once associated with a poor prognosis. For example, treatment with intensive dexamethasone, vincristine, and asparaginase, as well as high-dose methotrexate, has resulted in high cure rates for older adolescents and black patients treated in our Total Therapy XV study, comparable with the best results reported for other children.53,54 There is substantial ancestral diversity among children with ALL (Figure 2). A recent study showed that the Native American ancestry is significantly associated with a poor outcome among patients of Hispanic ethnicity, and even within patients self-reporting as white, and that its adverse prognosis could be abrogated by an additional course of delayed intensification therapy.55
Use of germline variation to define ancestry(> 90% European ancestry for whites, > 10% Native American ancestry for Hispanics, > 70% African ancestry for blacks, and > 90% Asian ancestry for Asians). The population of children (n = 2534) with ALL in the United States (A)55 displays the ancestral diversity that is comparable to that observed in the entire United States population (B; based on self-declared status; United States Census Bureau, 2000), with a slightly lower proportion of blacks and a slightly higher proportion of Hispanics among patients with ALL (reflecting lower incidence of ALL in black children and higher incidence of ALL in Hispanic children).
Use of germline variation to define ancestry(> 90% European ancestry for whites, > 10% Native American ancestry for Hispanics, > 70% African ancestry for blacks, and > 90% Asian ancestry for Asians). The population of children (n = 2534) with ALL in the United States (A)55 displays the ancestral diversity that is comparable to that observed in the entire United States population (B; based on self-declared status; United States Census Bureau, 2000), with a slightly lower proportion of blacks and a slightly higher proportion of Hispanics among patients with ALL (reflecting lower incidence of ALL in black children and higher incidence of ALL in Hispanic children).
Despite their loss of adverse prognostic impact in the context of contemporary effective treatment, many features still have therapeutic implications, with their presence indicating the need for modified therapy. Table 3 lists some characteristics of the leukemic cells and host that can be used for therapeutic intervention. To this end, even low-risk patients may require some degree of intensified therapy to achieve a high cure rate. This point is well illustrated by recent experience in our Total Therapy XV study, where treatment with intensive asparaginase and perhaps also high-dose methotrexate might have contributed to the outstanding outcome of patients with ETV6-RUNX1 fusion, especially those with poor early treatment response.56
Selected characteristics with therapeutic implications
| Characteristics . | Associated features . | Potential therapeutic intervention . |
|---|---|---|
| Infants with rearranged MLL | Hyperleukocytosis, CD10− B-cell precursor phenotype, increased CNS leukemia, poor prednisone response | FLT3 inhibitor (eg, lestaurtinib), tyrosine kinase inhibitor (eg, sorafenib), demethylating agents (eg, 5-azacytidine, decitabine), novel nucleoside analogs (eg, clofarabine) |
| Older adolescents | T-cell phenotype, male, increased MLL-AF4 | Intensive glucocorticoids, vincristine and asparaginase treatment, high-dose methotrexate; close monitoring of treatment adherence |
| T-cell | Hyperleukocytosis, increased CNS leukemia, male | Intensive glucocorticoids, vincristine and asparaginase treatment, high-dose methotrexate, intensive intrathecal therapy |
| Early T-cell precursor | CDla−, CD8−, CD5weak, stem cell or myeloid markers, older age, dismal prognosis | Myeloid-directed therapy (eg, high-dose cytarabine); epigenetic therapy |
| t(9;22)/BCR-ABL1 | Hyperleukocytosis, older age, precursor B-cell phenotype, poor prednisone response, IKZF1 alterations | Tyrosine kinase inhibitor (imatinib, dasatinib, nilotinib) |
| t(1;19)/TCF3-PBX1 | Pre-B phenotype, black race, increased CNS relapse | Intensive intrathecal therapy |
| t(17;19) /TCF3-HLF | Precursor B-cell phenotype, hypercalcemia, coagulopathy, dismal prognosis | Allogeneic transplant |
| Hypodiploidy < 44 chromosomes | Precursor B-cell phenotype, increased risk of relapse | Intensive treatment with very high-risk protocol |
| iAMP21 | Older age, low white blood cell count | Intensive glucocorticoids; vincristine and asparaginase treatment |
| Host TPMT activity | TPMT activity is inversely related to accumulation of active thioguanine nucleotides | Adjust thiopurine dose based on TPMT genotype or phenotype |
| High methotrexate clearance | Younger age, male | Adjust methotrexate dose based on estimated clearance |
| Presence of serum IgG antiasparaginase antibodies during therapy | Allergy to asparaginase; silent inactivation | Consider use of alternative form of asparaginase |
| Characteristics . | Associated features . | Potential therapeutic intervention . |
|---|---|---|
| Infants with rearranged MLL | Hyperleukocytosis, CD10− B-cell precursor phenotype, increased CNS leukemia, poor prednisone response | FLT3 inhibitor (eg, lestaurtinib), tyrosine kinase inhibitor (eg, sorafenib), demethylating agents (eg, 5-azacytidine, decitabine), novel nucleoside analogs (eg, clofarabine) |
| Older adolescents | T-cell phenotype, male, increased MLL-AF4 | Intensive glucocorticoids, vincristine and asparaginase treatment, high-dose methotrexate; close monitoring of treatment adherence |
| T-cell | Hyperleukocytosis, increased CNS leukemia, male | Intensive glucocorticoids, vincristine and asparaginase treatment, high-dose methotrexate, intensive intrathecal therapy |
| Early T-cell precursor | CDla−, CD8−, CD5weak, stem cell or myeloid markers, older age, dismal prognosis | Myeloid-directed therapy (eg, high-dose cytarabine); epigenetic therapy |
| t(9;22)/BCR-ABL1 | Hyperleukocytosis, older age, precursor B-cell phenotype, poor prednisone response, IKZF1 alterations | Tyrosine kinase inhibitor (imatinib, dasatinib, nilotinib) |
| t(1;19)/TCF3-PBX1 | Pre-B phenotype, black race, increased CNS relapse | Intensive intrathecal therapy |
| t(17;19) /TCF3-HLF | Precursor B-cell phenotype, hypercalcemia, coagulopathy, dismal prognosis | Allogeneic transplant |
| Hypodiploidy < 44 chromosomes | Precursor B-cell phenotype, increased risk of relapse | Intensive treatment with very high-risk protocol |
| iAMP21 | Older age, low white blood cell count | Intensive glucocorticoids; vincristine and asparaginase treatment |
| Host TPMT activity | TPMT activity is inversely related to accumulation of active thioguanine nucleotides | Adjust thiopurine dose based on TPMT genotype or phenotype |
| High methotrexate clearance | Younger age, male | Adjust methotrexate dose based on estimated clearance |
| Presence of serum IgG antiasparaginase antibodies during therapy | Allergy to asparaginase; silent inactivation | Consider use of alternative form of asparaginase |
iAMP21 indicates intrachromosomal amplification of chromosome 21; and TPMT, thiopurine methyltransferase.
It is well recognized that there is heterogeneity within each genetic subtype of ALL, partly because of differences in cooperating mutations or the target cell that undergoes malignant transformation, and partly because of variable host factors.11 Not surprisingly, the response to remission induction therapy as determined by MRD level is the most important prognostic indicator in patients with ALL because it accounts for the entire constellation of leukemic-cell biologic features (intrinsic drug sensitivity), host pharmacodynamics and pharmacogenomics, treatment adherence, and efficacy of the treatment regimen.11 If so, why did the level of MRD at the end of remission induction lack prognostic significance in a recent COG study for Philadelphia chromosome-positive ALL,18 and remission induction failure did not predict a dire outcome of precursor B-cell ALL without other adverse features in an international cooperative group study?57 A possible explanation is that the ABL1 tyrosine kinase inhibitor was not used during remission induction in the COG study, and antimetabolites (including high-dose methotrexate), which are effective treatment components for precursor B-cell ALL (especially those with hyperdiploidy), tend to be used only after remission induction. These 2 scenarios should prompt reassessment of the current indications for hematopoietic stem cell transplantation in first complete remission, which should be reserved for patients with leukemia that is refractory to contemporary most effective combination chemotherapy, as documented by the MRD assay. In this regard, the St Jude Total Therapy XV study used MRD level at day 46 of remission induction (after treatment with prednisone, vincristine, daunorubicin, asparaginase, cyclophosphamide, cytarabine, and mercaptopurine) for final risk assessment.7 Likewise, in the AIEOP-BFM-ALL 2000 study, MRD at the second time point on day 78 (after treatment with the same 7 drugs) was found to be the most important predictor of treatment outcome, superseding the MRD result at the first time point on day 33 (after treatment with the first 4 drugs).58
Optimizing remission induction therapy
Although protocols for pediatric ALL rely on the same classes of drugs for remission induction, there is no consensus on what constitutes an optimal regimen. Virtually all study groups in developed countries can achieve an overall complete remission rate of 98% to 99%. Whether there are differences in the rate of “molecular” or “immunologic” remission (ie, < 0.01% leukemic cells in bone marrow) among different clinical trials is unknown. However, it should be emphasized that neither molecular nor immunologic remission after induction therapy is required for cure. Indeed, early studies showed that intensive induction therapy may not be necessary for standard-risk patients, provided that they receive adequate postremission intensification therapy.59,60 In our Total Therapy XV study, patients with an MRD level of 0.01% to 0.99% at the end of induction received intensified postremission therapy and achieved a 5-year survival of 90.9% plus or minus 5.4%, a rate similar to that (94.5% ± 1.9%) for patients who had negative MRD at the end of induction therapy.7 In view of the many examples of increased early morbidity and mortality in protocols featuring intensive induction therapy,59,61 remission induction should be moderate in intensity for standard-risk patients, especially in low-income countries where resources are less abundant and among patients with Down syndrome who are more susceptible to fatal infectious complications.62 Indeed, myelosuppressive therapy should be temporarily delayed in the presence of infection during induction, even in high-risk patients. To this end, we measure MRD level after 2 weeks of remission induction, and use the result to guide the intensity of the subsequent induction therapy. This approach is especially desirable in low-income countries where identification of good early responders to avoid overtreatment can pay large dividends in terms of reduced morbidity and mortality. Based on the finding of exquisite sensitivity of normal bone marrow lymphoid progenitors (CD19+, CD10+, and/or CD34+) to corticosteroids and other antileukemic drugs, a simple and inexpensive assay for MRD detection has been developed to identify such patients during remission induction therapy.63
Remission induction therapy invariably includes a glucocorticoid, vincristine, and asparaginase, not only because they are nonmyelosuppressive but also because they have distinct mechanisms for their antileukemic effects and may act synergistically.56,64 Prednisone has traditionally been the glucocorticoid most commonly used in remission induction, but dexamethasone has increasingly replaced prednisone in recent clinical trials.65 Two recent studies showed that dexamethasone, given at 10 mg/m2 per day during remission induction, improved outcome in patients with T-cell ALL and a good response to 7 days of upfront prednisone treatment, and in children under 10 years of age with precursor B-cell ALL, compared with prednisone administered at 60 mg/m2 per day.66,67 However, it should be noted that the efficacy of prednisone and dexamethasone is dose dependent; and when the dose ratio of prednisone to dexamethasone is greater than 7, event-free survival (EFS) estimates are comparable with the 2 drugs, although dexamethasone still appears to yield improved CNS control.65 These findings notwithstanding, an excellent outcome can also be achieved with a relatively low dose of prednisone (40 mg/m2 per day) during remission induction, provided that postremission treatment is adequate and includes dexamethasone, as shown in our Total Therapy XV study.7 Dexamethasone at a dose of 10 mg/m2 is not recommended for remission induction in children 10 years of age or older with precursor B-cell ALL because of the high rates of toxicity and toxic death associated with the treatment,66 a finding partly related to the slower clearance of dexamethasone in this age group.68
Selecting effective consolidation/intensification/reinduction therapy
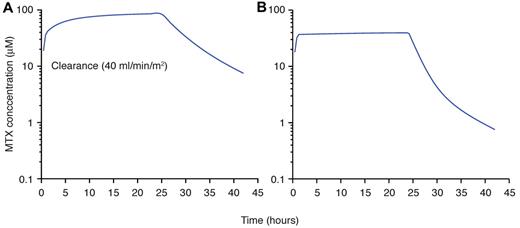
This phase of therapy is essential for all patients with ALL, but there is no consensus on the best regimens and their duration. Intensification of methotrexate treatment clearly improves outcome in patients with intermediate-risk or high-risk ALL, but its utility in low-risk (or so-called standard-risk) patients is still disputed. Among different strategies of intensification of methotrexate, extended intravenous infusion (ie, over 24 hours) at high doses (eg, 5 g/m2) has been widely used for patients with T-cell ALL.69,70 Although the addition of escalating intravenous methotrexate (initial dose 100 mg/m2, increasing by 50 mg/m2 every 10 days for 4 doses) without leucovorin rescue has been shown to improve the outcome of standard-risk ALL,71 it was not as effective or less toxic than high-dose methotrexate with leucovorin rescue in a recent COG study for high-risk ALL.72 Although the optimal dosage and number of courses of high-dose methotrexate remain to be determined for individual subtypes of ALL, based on current evidence and pharmacologic studies in ALL subtypes, we recommend the infusion of high dose (∼ 5 g/m2) over 24 hours for T-cell cases and those with TCF3-PBX1 fusion.69,73 For patients who receive such high dose, we would also individualize the dosage to achieve the desired steady-state plasma concentration (65μM) not only to optimize antileukemic effects but also to reduce toxicity (Figure 3).
At St Jude Children's Research Hospital, dosages of high-dose methotrexate are individualized based on the estimates of clearance to achieve a desired systemic exposure to the drug. As shown, in a patient estimated to have low clearance (40 mL/min per m2; A), the dosage should be lowered to achieve the desired steady-state plasma concentration to reduce potential toxicities (B). Those patients with high clearance have dosages increased.
At St Jude Children's Research Hospital, dosages of high-dose methotrexate are individualized based on the estimates of clearance to achieve a desired systemic exposure to the drug. As shown, in a patient estimated to have low clearance (40 mL/min per m2; A), the dosage should be lowered to achieve the desired steady-state plasma concentration to reduce potential toxicities (B). Those patients with high clearance have dosages increased.
Delayed intensification with asparaginase, vincristine, and dexamethasone, with or without anthracycline, mercaptopurine, and methotrexate, is the most widely used strategy in ALL protocols and is beneficial to all patients.7,73-75 In COG studies, intensification treatment for 6 months is as effective as 10 months of such therapy for standard-risk patients and high-risk patients with a rapid early response.74 Whether high-risk slow early responders would benefit from prolonged intensification therapy remains uncertain. Notably, altered dosing of dexamethasone during delayed intensification, by giving it on days 1 to 7 and days 15 to 21 for 2 courses rather than on days 1 to 21 for 1 course, significantly reduced the incidence of osteonecrosis in a COG study.74
One of the key components of this phase of treatment is asparaginase, which is available in several formulations with different pharmacokinetic profiles.76 In terms of leukemia control, the dose intensity and duration of asparaginase therapy are more important than the type of asparaginase used. Because of lower immunogenicity, less frequent administration, and the feasibility of intravenous administration, the pegylated form of Escherichia coli asparaginase (PEG-asparaginase) has replaced the native E coli product as the first-line treatment for children in the United States and is being used increasingly in other clinical trials around the world. In general, the preparation derived from Erwinia chrysanthemia, which lacks cross-reactivity with E coli preparation, is used as second- or third-line therapy for patients with hypersensitivity reactions to native E coli or PEG-asparaginase.76
Depending on the preparation used, the treatment schedule, and concomitant immunosuppressive therapy, 10% to 60% of the patients would develop hypersensitivity reactions because of the production of antiasparaginase antibody.76 One-third of the patients without clinical hypersensitivity reactions may also develop IgG antibodies that can inactivate asparaginase, leading to suboptimal asparagine depletion, a phenomenon commonly referred to as “silent inactivation.”77 The presence of IgG antibodies, with or without clinical hypersensitivity, can lead not only to decreased exposure to asparaginase but also to high clearance of dexamethasone when given concomitantly, presumably because of lower asparaginase effects on the production of hepatic enzymes involved in dexamethasone metabolism.64 However, the presence of asparaginase antibody is an inconsistent prognostic indicator of leukemia-free survival, perhaps because its adverse effect can be mitigated by the use of an alternative asparaginase preparation or by the overall efficacy of the treatment regimen.76
Routine antibody monitoring is not being implemented in current practice, partly because the prognostic impact of the presence of antibody is variable and partly because there are no commercially available kits to measure it. These issues notwithstanding, antibody testing could be useful in some clinical conditions. First, measurement of antibodies is an excellent tool for diagnosing clinical hypersensitivity in patients with ambiguous symptoms or signs of allergy.77,78 Second, PEG-asparaginase treatment can still yield a therapeutic level of asparaginase in patients with low to intermediate antibody levels against E coli asparaginase.78 Third, some patients may have antibodies to the nonprotein PEG moiety, resulting in rapid clearance of PEG-asparaginase,79 but they can still respond to native E coli asparaginase. As noted earlier, antibody testing is not readily available, and there are technical difficulties in measuring the serum asparagine level; thus, measuring the asparaginase level is considered a relatively reliable and feasible way to monitor treatment. In the absence of a monitoring tool, the serum albumin level may serve as a biomarker of asparaginase activity.64
Pancreatitis and thrombosis are 2 of the most serious and most frequent dose-limiting asparaginase-related toxicities, occurring more often in patients 10 years of age or older than in younger patients.80,81 Concomitant administration of other drugs may potentiate or contribute to the risk and severity of pancreatitis (eg, glucocorticoid, mercaptopurine, trimethoprim sulfamethoxazole) and thrombosis (glucocorticoid). Although these complications rarely resulted in mortality, they pose significant morbidity and often recur with rechallenge.80,81 With low-molecular-weight heparin prophylaxis and close monitoring, most patients with thrombotic complications can complete the scheduled asparaginase treatment.81 In one study, patients with mild pancreatitis were rechallenged after resolution of symptoms and normalization of pancreatic enzymes, with two-thirds having a second episode of pancreatitis after receiving an average of 7 or 8 additional doses of asparaginase.80 Whether the use of octreotide or short-acting Erwinia asparaginase can prevent or reduce the risk of recurrence of pancreatitis82 requires additional studies.
Pharmacogenomics-guided continuation (maintenance) treatment: gaining a therapeutic edge
The combination of weekly low-dose methotrexate and daily mercaptopurine, with or without pulses of dexamethasone and vincristine, constitutes the standard “backbone” of ALL continuation regimens. Tailoring the dosages of methotrexate and mercaptopurine to the limits of tolerance has been associated with a better outcome.73 In most clinical trials, both drugs are increased or decreased in parallel without ascertaining the genetic polymorphism status of thiopurine methyltransferase, even though the relationship between its genotype or phenotype and the clinical effects of mercaptopurine is well established.83 The enzyme catalyzes S-methylation of thiopurines to inactive methylated metabolites. Thus, patients with an inherited deficiency of this enzyme are at increased risk of mercaptopurine-induced toxicities because more parent drug is available for anabolism to active metabolites. We argue that the time has come to customize the dosage of mercaptopurine based on preemptive testing for thiopurine methyltransferase status. First, among patients with poor tolerance to continuation treatment, mercaptopurine dosage can be selectively reduced, whereas methotrexate can still be given at full dosage in those with a deficiency of the enzyme. This approach has reduced the likelihood of acute myelosuppression without compromising disease control.6,83,84 Second, patients with thiopurine methyltransferase deficiency are at greater risk for the development of mercaptopurine-induced myeloid malignancy, particularly if they receive high-dose mercaptopurine (eg, 75 mg/m2 per day).85
Thioguanine is more potent than mercaptopurine, but its prolonged use at a dose more than 40 mg/m2 has been associated with profound thrombocytopenia, an increased risk of death, and an unacceptable rate of hepatic veno-occlusive disease (∼ 20%).86,87 Hence, thioguanine is no longer used for continuation treatment; however, whether its short-term use can improve outcome, especially in terms of CNS control, without causing undue toxicity remains to be determined. Notably, thiopurine methyltransferase also has a significant impact on the pharmacokinetics of thioguanine, and patients with the enzyme deficiency are at an increased risk of developing hepatic veno-occlusive disease.88 We recommend implementation of the guidelines for thiopurine therapy (updates at http://www.pharmgkb.org), based on the association between clinical effects and genotype/phenotype of the enzyme, as recently developed by the Clinical Pharmacogenetics Implementation Consortium.83
In many clinical trials, weekly methotrexate is given orally for convenience and cost savings. We prefer to give it intravenously because this route offers a way to partly circumvent the problems of variable bioavailability and poor treatment adherence. Indeed, an adherence rate of less than 95% was associated with an increased risk of relapse in a recent COG study.89 Although there are various methods to monitor treatment adherence to antimetabolite therapy, such as measurement of erythrocyte mercaptopurine metabolite (thioguanine nucleotide) level or mean corpuscular volume, pill counts, or electronic monitoring device, parenteral administration of methotrexate is a certain way to assure that the patient received at least one drug. We think that this approach was partly responsible for the improved prognosis of older adolescents treated in our Total Therapy XV study.53 The dosages of methotrexate used in continuation treatment of various ALL protocols range from 20 mg/m2 orally to 40 mg/m2 intravenously per week. Whether the use of higher dosage given intravenously would improve outcome remains to be determined.
Ongoing pharmacogenomics studies hold great promise to yield additional genetic polymorphisms that can be used to further individualize the dosages of other antileukemic agents, building on the early success of thiopurine methyltransferase and mercaptopurine.90
The case for complete omission of prophylactic cranial irradiation
Studies of survivors of childhood ALL have found that prophylactic cranial irradiation can cause many late-occurring sequelae, including second cancers, neurocognitive impairment, and multiple endocrinopathy.91,92 Recognizing the devastating complications of cranial irradiation, pediatric oncologists have steadily reduced the use of this treatment modality since the late 1970s. Two studies have shown that prophylactic cranial irradiation can be successfully omitted from virtually all patients, regardless of their presenting features, in the context of effective systemic therapy (including high-dose intravenous methotrexate) and intrathecal therapy.4,7 The isolated CNS relapse rates in the trials were 2.6% and 2.7% and the 5-year EFS rates 81% and 85.6%, respectively.4,7 Notably, both studies used triple intrathecal therapy: 13 doses for the non–high-risk patients and 15 to 17 doses for the high-risk patients in the Dutch Childhood Oncology Group protocol ALL-9,4 and 13 to 18 doses in low-risk cases and 16 to 25 doses in standard- or high-risk cases in the St Jude Total Therapy XV study.7 Despite these results, many leukemia therapists still prefer intrathecal methotrexate and are reluctant to omit cranial irradiation from protocols for high-risk patients.
The mixed results of a randomized COG study (CCG 1952) for standard-risk ALL20 has led to an ongoing debate over the characteristics of optimal intrathecal therapy. In that study, triple intrathecal therapy resulted in a significantly lower incidence of isolated CNS relapse than did treatment with intrathecal methotrexate (6-year cumulative risk: 3.4% ± 1.0% vs 5.9% ± 1.2%). The impact was even more pronounced in patients with a CNS2 status (6-year cumulative risk: 7.7% ± 5.3% vs 23.0% ± 9.5%), a subset requiring intensified treatment to avert a high hazard of relapse.93 However, significantly more hematologic and testicular relapses occurred among patients treated with triple intrathecal therapy than among those receiving intrathecal methotrexate, resulting in a significantly inferior survival for the former group (90.3% ± 1.5% vs 94.4% ± 1.1%).20 The COG investigators raised the possibility that intrathecal cytarabine or hydrocortisone somehow interferes with the egress of methotrexate from cerebrospinal fluid into blood, leading to less systemic exposure to methotrexate than does treatment with intrathecal methotrexate alone.20 We favor another explanation: that so-called isolated CNS relapse could be an early manifestation of systemic relapse, and the improved CNS control secured with triple intrathecal treatment sets the stage for overt leukemic relapse in other sites subsequently.93 Whatever the explanation, we would argue that triple intrathecal therapy used together with effective systemic chemotherapy, at least in patients at high risk of relapse, would allow cranial irradiation to be omitted in this subgroup. In this regard, a recent meta-analysis of randomized trials of CNS-directed therapy showed that adding intravenous methotrexate for patients treated with triple intrathecal therapy improves outcome by reducing both CNS and non-CNS relapses, whereas adding it for those treated with intrathecal methotrexate yields little benefit.94
The case for eliminating prophylactic cranial irradiation can be summarized as follows. First, even among patients treated before 2000 with less effective chemotherapy regimens than those in contemporary use, the substitution of intravenous and intrathecal methotrexate therapy could yield EFS comparable with that of cranial irradiation with additional intrathecal treatment.94 Second, the remission retrieval rate is high for patients with isolated CNS relapse who did not receive prior prophylactic cranial irradiation. In this regard, all 11 patients with an isolated CNS relapse in our Total Therapy XV study7 remain in subsequent remission after retrieval therapy for 4 to 11 years and, in all likelihood, are cured of their leukemia. Third, patients who receive irradiation are at life-long risk of second cancers and other complications91,92 ; indeed, any short-term gain in EFS with use of radiation may be abolished with prolonged follow-up. Fourth, there is no safe dose of cranial irradiation. In the ALL-BFM 90 study, which used 12 Gy cranial irradiation, the cumulative risk of second neoplasms had already reached 1.7% (95% CI, 0.1%-3.4%) at 15 years after induction,95 and is expected to increase with longer follow-up. In our current study, we further intensify triple intrathecal therapy during the early phase of remission induction in patients identified to have a higher risk of CNS relapse from Total Therapy XV study (ie, T-cell immunophenotype, t(1;19)/TCF3-PBX1, and any CNS involvement by leukemic cells),7 in an effort to prevent CNS relapse entirely.
Even though encouraging neuropsychologic results, with the exception of adverse effects on complex fine-motor functioning, were reported for patients treated on the Dutch Childhood Oncology Group protocol ALL-9 with chemotherapy only,96 we realize that intensive systemic chemotherapy as well as a high cumulative dose of intrathecal therapy can impair neurocognitive and neuromuscular function.97-100 Thus, future studies should focus on determining the optimal doses of chemotherapy needed to avoid overtreatment or undertreatment.
Future directions
Treatments developed and refined over the past several decades have resulted in high cure rates and a large population of leukemia survivors. Studying the long-term health complications of these adult survivors will help to develop less toxic treatments while preserving earlier gains in efficacy. The remarkable advances in biomedical technology, next-generation genome sequencing of leukemic cells and normal host cells, and high-throughput screening systems for new drugs should bring the promise of personalized treatment with targeted agents to fruition, resulting in more effective and less toxic treatments for patients with ALL. Recent genome-wide association studies have begun to identify a number of inherited polymorphisms, such as ARID5B, IKZF1, CEBPE, and CDKN2A,37,38,101,102 which are associated with the risk of childhood ALL in different ethnic and racial groups, paving the way for development of potential preventive measures, at least for certain subtypes of leukemia.
Acknowledgments
This work was supported in part by the National Institutes of Health (CA36401, CA21765, and GM92666) and American Lebanese Syrian Associated Charities.
National Institutes of Health
Authorship
Contribution: C.-H.P. conceived, wrote, and revised the manuscript; and C.G.M., W.E.E., and M.V.R. contributed comments and edited the manuscript.
Conflict-of-interest disclosure: St Jude Children's Research Hospital receives royalty income from licensing patent rights related to thiopurine methyltransferase polymorphisms, for which W.E.E. is an inventor and previously received royalty income. M.V.R receives funding for investigator-initiated research on the pharmacology of asparaginase from Sigma-Tau Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ching-Hon Pui, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: ching-hon.pui@stjude.org.