Abstract
Binding of selectins to their glycan ligands is a prerequisite for successful leukocyte trafficking. During synthesis and transport through the secretory pathway, selectin ligands are constructed with the participation of one or more sialyltransferases of the ST3Gal subfamily. Previous studies established that ST3Gal-IV only partially contributes to selectin ligand formation, indicating that other ST3Gal-sialyltransferases are involved. By generating and analyzing St3gal6-null mice and St3gal4/St3gal6 double-deficient mice, in the present study, we found that binding of E- and P-selectin to neutrophils and L-selectin binding to lymph node high endothelial venules is reduced in the absence of ST3Gal-VI and to a greater extent in double-deficient mice. In an ex vivo flow chamber assay, P- and E-selectin–dependent leukocyte rolling was mildly reduced in St3gal6-null mice and more severely in double-deficient mice. In inflamed cremaster muscle venules of St3gal6-null mice, we found impaired P-selectin–dependent, but not E-selectin–dependent leukocyte rolling, whereas in double-deficient mice, E-selectin–dependent rolling was almost completely absent. Furthermore, neutrophil recruitment into the inflamed peritoneal cavity and lymphocyte homing to secondary lymphoid organs were impaired in St3gal6-null mice and more severely in double-deficient mice. The results of the present study demonstrate the coordinated participation of both ST3Gal-VI and ST3Gal-IV in the synthesis of functional selectin ligands.
Introduction
Research on selectins and their glycan ligands have underscored the importance of posttranslational protein glycosylation in leukocyte capture and rolling on activated endothelium, a prerequisite for neutrophil recruitment during inflammation and lymphocyte homing to the lymph nodes.1-7 Three different selectins are found in mammals. E- and P-selectin expression is induced on the vascular endothelium during inflammation, P-selectin is also present on activated platelets, and L-selectin is expressed on the surface of leukocytes.8 The absence of selectins can lead to reduced neutrophil recruitment to sites of inflammation, impaired lymphocyte trafficking, and decreased leukocyte turnover.9-13 The synthesis of selectin ligands requires multiple glycosyltransferases, including the fucosyltransferases FucT-VII and FucT-IV, the Core 2 N-acetylglucosaminyltransferase-I (C2GnT-I), and the sialyltransferase ST3Gal-IV. All of these enzymes contribute to the generation of sialyl Lewis X (sLex) or its sulfated form, 6-sulpho-sLex, 2 carbohydrate determinants displayed on glycoproteins or glycolipids with binding activity to selectins. The absence of one or more of these glycosyltransferases causes variable and sometimes pronounced defects in leukocyte rolling, which can diminish leukocyte recruitment during inflammation and lymphocyte homing into secondary lymphatic tissue.4,7,14-16
In mammals, 6 different sialyltransferases (ST3Gal-I–ST3Gal-VI) have been identified that generate α2-3 sialic acid linkages on glycans of glycoproteins and glycolipids, and theoretically all could contribute to the formation of sLex. Of those that are most likely to be involved, ST3Gal-IV and VI (and to a lesser degree, ST3Gal-III) can sialylate the type II glycan chains (Galβ1-4GlcNAc) that are part of the glycan linkages of sLex (Figure 1A).17,18 In fact, St3gal4-deficient mice have a partial and significant defect in selectin ligand function in vivo, including a mild reduction in E-selectin–dependent rolling, an increase in E-selectin–dependent rolling velocity, and a decrease in L-selectin–dependent rolling during inflammation (relying on P-selectin glycoprotein ligand-1 PSGL-1, the predominant selectin ligand expressed on leukocytes).16,19 In contrast, L-selectin–dependent rolling on high endothelial venules (HEVs), which depends on L-selectin ligands expressed on HEVs, is not affected by ST3Gal-IV deficiency.19
The contribution of sialylation to the formation of selectin ligands is well documented, but the enzymes involved have not been identified fully. Treatment of neutrophils with sialidase has indicated an essential role of sialic acids on selectin ligand function.16,20 However, because of the rather mild defect on selectin ligand activity observed in St3gal4-deficient mice and the dramatic effect of sialidase treatment, other sialyltransferases may contribute to the generation of selectin ligands. Earlier studies have shown that deficiency of ST3Gal-I, ST3Gal-II, or ST3Gal-III did not cause impairment of selectin ligand abundance or function.19 Because ST3Gal-V has specificity for glycolipid sialylation, ST3Gal-VI has remained a likely candidate in the synthesis of sLex on one or more glycoproteins. However, direct evidence for a role of ST3Gal-VI in selectin ligand synthesis and leukocyte rolling has been lacking. In the present study, we generated and characterized mice bearing a germline defect in the St3gal6 gene using Cre-loxP conditional mutagenesis. In addition, we have bred and analyzed in parallel mice deficient in both St3gal4 and St3gal6. We report that ST3Gal-VI has a critical role in E-, P-, and L-selectin ligand synthesis in vitro and in vivo, and that the coordinated expression of ST3Gal-IV and ST3Gal-VI together contribute to most, if not all, of the sialylation that is responsible for the synthesis and function of the selectin ligands.
Methods
St3gal6 expression and mutagenesis
Genomic clones of the St3gal6 were isolated from a 129/SvJ phage library for use in Cre/loxP gene-targeted mutagenesis procedures and mice bearing mutant genotypes were produced and bred as described previously.21 Genotyping was performed using PCR. St3gal6 wild-type allele was detected as a 190-bp fragment using primers MB1 (5′-AGACCCTGGAGTTCATCTGTGTAG) and MB3 (5′- CTGGGAGAAAGGTTCACTCCAT), whereas St3gal6 F (type 2) allele was detected as a 325-bp fragment. St3gal6 Δ allele was detected as a 360-bp fragment using primers MB1 and MB4 (5′-CCTTCCCAGTGCTCAGGTTA). In RNA expression analysis, total RNA from various mouse tissues was extracted using TRIzol reagent (Invitrogen). A mouse St3gal6 cDNA of the exon 7-9 region was used as a probe and total RNA was subjected to 1% formaldehyde-denaturing agarose gel electrophoresis for a Northern blot. Quantitative PCR (qPCR) was performed with Brilliant SYBR Green (Stratagene) as described previously.22 5′-AGTGCCATGTAAAAGGTGTG was used for the forward primer and 5′-CTGATCCTTGGGAAATACTC was used for the reverse primer in the qPCR reaction. In an RT-PCR experiment, cDNA was generated by the Superscript III first-strand synthesis system (Invitrogen) using total RNA (1 μg) from various tissues of wild-type and St3gal6 exon 3 deletion mice, and the exon 3-6 and exon 7-9 region were amplified by PCR. The St3gal6Δ alleles were bred into the C57BL/6 background for at least 5 generations before the experiments.
Hematology
Analysis of peripheral blood cells was performed as described previously.23 In brief, mice were anesthetized by a mixture of 3% isoflurane with oxygen in an induction chamber. Whole blood was collected from the tail veins of anesthetized mice into EDTA-Microtainer tubes (BD Biosciences). Blood cell counts and leukocyte differentials were performed in duplicate on Hemavet 850FS (Drew Scientific) programmed with mouse hematology settings. FACS analysis and lectin-binding studies were performed as described previously.16 For further details, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Intravital microscopy, cremaster muscle preparation, and flow chambers
Mice were prepared and anesthetized for intravital microscopy as described previously.24 All animal studies were approved by the institutional animal care and use committee of the University of California, Santa Barbara and by the Regierung of Oberbayern (Az.55.2-1-54-2531-80-07; Munich, Germany). Intravital microscopy was performed on an upright microscope (BX51; Olympus) with a saline immersion objective (40×/0.8 numerical aperture; Olympus). The surgical preparation of the cremaster muscle was performed as described previously.24 Recombinant murine TNF-α (R&D Systems) was injected into the scrotum at 500 ng per mouse 2-4 hours before intravital microscopy. Microscopic observation of murine cremaster muscle venules was recorded via CCD camera (CF8/HS; Kappa) on a Panasonic S-VHS recorder. Microvascular parameters (venular diameter, venular vessel segment length, and leukocyte rolling velocity) were assessed using an image processing system.24 Ex vivo and in vitro flow chamber experiments were conducted as described previously.24 For further details, please see supplemental Methods.
Lymphocyte trafficking
To determine the cellularity of secondary lymphoid organs, single-cell suspensions of lymphocytes from lymph nodes and Peyer patches were enumerated manually using a hematocytometer, as described previously.15 For lymphocyte homing assays, 2.5 × 107 cells were isolated from wild-type mesenteric lymph nodes and incubated with the CellTracker probe chloromethyl fluorescence diacetate (CFDA; Molecular Bioprobes/Invitrogen) before injection into the tail vein, as described previously.25 Lymphoid organs were harvested 1 hour after injection and T (CD3ϵ+) and B (CD45R/B220+) lymphocytes positive for CFDA were measured by flow cytometry. L-selectin expression on lymphatic tissue was analyzed following established protocols.15 For further details, please see supplemental Methods.
Statistical analysis
Data were analyzed as mean ± SEM. ANOVA on ranks or the Student t test was used to determine statistical significance, as appropriate.
Results
Tissue distribution and targeted mutagenesis of the St3gal6 gene
The murine ST3Gal-VI sialyltransferase is a type II transmembrane protein localized to the Golgi apparatus.18,26 Analysis of St3gal6 mRNA expression by Northern blot revealed a 2.0- and 2.5-kb mRNA sequence with wide expression and distribution among various normal tissues exhibiting high levels in BM, lung, small intestine, and colon (Figure 1B). In addition, analysis of St3gal6 mRNA relative expression by qPCR indicated high levels of St3gal6 mRNA n the lung, small intestine, colon and salivary glands, and this result was almost identical to the result from Northern blot (Figure 1C).
Activity, expression, and mutagenesis of ST3Gal-VI sialyltransferase. (A) ST3Gal sialyltransferases (ST3Gal-I-VI) add sialic acid to the terminus of type I, II, or III sugars. ST3Gal-IV, ST3Ga-VI, and, to a lesser degree, ST3Gal-III have substrate specificity for type II and are involved in formation of sialyl Lewis X, the prototypic ligand for selectins. (B-C) Expression of mouse St3gal6 RNA transcripts among total RNA samples in wild-type mouse tissues, as determined by Northern blot (B) and qPCR analysis (C). Data are represented as means ± SEM from 3 independent experiments. (D) Mouse genomic clone of St3gal6 bearing exon 3 (black box) used for constructing the targeting vector with the pflox plasmid as indicated. Homologous recombination generated the St3gal6 F[tkneo] allele. After Cre recombination and selection, ES cell clones were isolated containing the Δ (systemic deleted) and F (conditional) alleles. (E) Southern blot analysis of Avr II-digested (left panel) and Nhe I-digested (right panel) ES cell DNA probed with a genomic probe (left panel) and loxP probe (right panel). ES cell clones bearing the St3gal6 F[tkneo] allele were compared with R1 parental wild-type ES cells. (F) PCR analysis of ES cell DNA or tail DNA from the offspring of parental mice heterozygous for the St3gal6 Δ allele indicates the 190-bp wild-type (WT) fragment, the 360-bp Δ fragment, and the 325-bp F fragment. (G) RT-PCR analysis of total cDNA from several mice organs generating St3gal6 exon 3-6 product, St3gal6 exon 7-9 product, and Gapdh product.
Activity, expression, and mutagenesis of ST3Gal-VI sialyltransferase. (A) ST3Gal sialyltransferases (ST3Gal-I-VI) add sialic acid to the terminus of type I, II, or III sugars. ST3Gal-IV, ST3Ga-VI, and, to a lesser degree, ST3Gal-III have substrate specificity for type II and are involved in formation of sialyl Lewis X, the prototypic ligand for selectins. (B-C) Expression of mouse St3gal6 RNA transcripts among total RNA samples in wild-type mouse tissues, as determined by Northern blot (B) and qPCR analysis (C). Data are represented as means ± SEM from 3 independent experiments. (D) Mouse genomic clone of St3gal6 bearing exon 3 (black box) used for constructing the targeting vector with the pflox plasmid as indicated. Homologous recombination generated the St3gal6 F[tkneo] allele. After Cre recombination and selection, ES cell clones were isolated containing the Δ (systemic deleted) and F (conditional) alleles. (E) Southern blot analysis of Avr II-digested (left panel) and Nhe I-digested (right panel) ES cell DNA probed with a genomic probe (left panel) and loxP probe (right panel). ES cell clones bearing the St3gal6 F[tkneo] allele were compared with R1 parental wild-type ES cells. (F) PCR analysis of ES cell DNA or tail DNA from the offspring of parental mice heterozygous for the St3gal6 Δ allele indicates the 190-bp wild-type (WT) fragment, the 360-bp Δ fragment, and the 325-bp F fragment. (G) RT-PCR analysis of total cDNA from several mice organs generating St3gal6 exon 3-6 product, St3gal6 exon 7-9 product, and Gapdh product.
The production of St3gal6-deficient mice was initiated by conditional mutagenesis of the St3gal6 allele using Cre-loxP recombination in embryonic stem (ES) cells (Figure 1D). Exon 3, which encodes a large portion of the transmembrane domain of ST3Gal-VI, was flanked by loxP sites. The loxP-flanked alleles were confirmed by Southern blot analysis of ES-cell DNA (Figure 1E). Correctly targeted ES cells were then used to generate mutant mice bearing the deleted allelic structures, as described previously.21,27 The genotype of all alleles was assessed by PCR (Figure 1F). RT-PCR analysis was conducted to confirm the deletion of exon 3. As a result, exon 7-9 products were still observed, whereas exon 3-6 products were not detected (Figure 1G). Therefore, the deletion of exon 3 using mutagenesis induced a frameshift mutation resulting in the creation of a truncated form of ST3Gal-VI. Mice homozygous for the St3gal6Δ allele were produced at the expected Mendelian ratios without overt developmental abnormalities and were all fully fertile. Mice homozygous for the St3gal6Δ allele were further analyzed together with mice bred to homozygosity for null alleles of both St3gal4 and St3gal6.
ST3Gal-VI and ST3Gal-IV deficiency in hemostasis
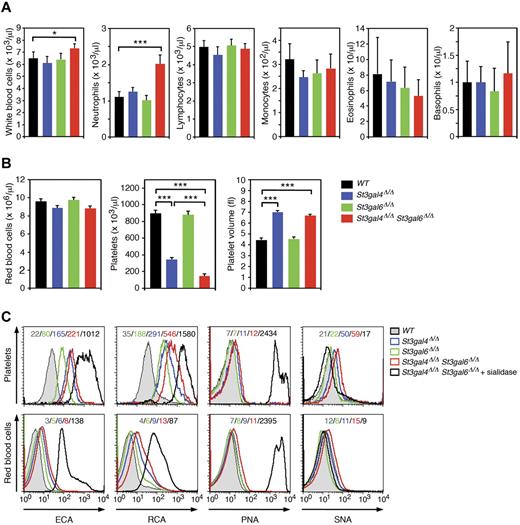
Leukocytosis is considered a hallmark of disorders with severe defects in leukocyte rolling and adhesion.4,28-30 Therefore, we compared WBC counts in St3gal4- and St3gal6-deficient mice both individually and combined. WBC counts in St3gal4 or St3gal6 single-deficient mice were unaltered from normal (Figure 2A). In contrast, double-deficient animals were markedly affected, with a 12% increase in WBC count due to an 80% increase in circulating neutrophils (Figure 2A). We did not observe any increase in the other circulating leukocyte populations (Figure 2A) and RBC counts were unaltered among St3gal4-deficient, St3gal6-deficient, and double-deficient mice (Figure 2B).
Analysis of peripheral blood cells in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A-B) Blood was collected from the tail vein of 8- to 12-week-old mice. Cell counts were carried out using the Hemavet 850FS multispecies hematology system. Values are presented as mean ± SEM (n = 12). ***P < .001; *P < .05. (C) ECA, RCA, PNA, and SNA lectin binding to circulating platelets (CD41+) and RBCs (Ter-119+) was assessed by flow cytometry. Mean fluorescence intensities are indicated in each histogram and data are representative of 3 independent experiments.
Analysis of peripheral blood cells in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A-B) Blood was collected from the tail vein of 8- to 12-week-old mice. Cell counts were carried out using the Hemavet 850FS multispecies hematology system. Values are presented as mean ± SEM (n = 12). ***P < .001; *P < .05. (C) ECA, RCA, PNA, and SNA lectin binding to circulating platelets (CD41+) and RBCs (Ter-119+) was assessed by flow cytometry. Mean fluorescence intensities are indicated in each histogram and data are representative of 3 independent experiments.
It has been reported that St3gal4 deficiency results in thrombocytopenia through clearance of circulating platelets by asialoglycoprotein receptor–dependent mechanisms,23,31 leading to a marked decrease in platelet counts to 39% of that of wild-type mice with a substantial increase in platelet volume (Figure 2B). Interestingly, blood of double-deficient mice indicated an additive decrease in platelet count to 16% of normal with a substantial increase in platelet volume, whereas St3gal6 deficiency alone did not alter platelet numbers and platelet volume (Figure 2B). This is most likely because of a larger galactose exposure via Galβ1-4GlcNAc-type II glycan structures on platelets among double-deficient mice. This was evident from measurements of Erythrina cristagalli aggutinin (ECA) and Ricinus communis agglutinin (RCA) lectin binding to platelets using flow cytometry (Figure 2C). St3gal6 deficiency produced a slight but significant increase in ECA and RCA binding to platelets (Figure 2C). In contrast, peanut agglutinin (PNA) binding (to Galβ1-3GalNAc) on platelets was similar between the groups (Figure 2C). Conversely, ECA, RCA, PNA, and Samhuca nigera (elderberry) agglutinin (SNA) binding to RBCs was mildly affected in St3gal4-deficient, St3gal6-deficient, and double-deficient mice compared with wild-type RBCs (Figure 2C). Sialidase treatment of double-deficient mice greatly increased ECA, RCA, and PNA lectin binding to RBCs (Figure 2C). These results further indicate that ST3Gal-VI and ST3Gal-IV participate in leukocyte sialylation, whereas other sialyltransferases participate in the sialylation of RBC glycans.
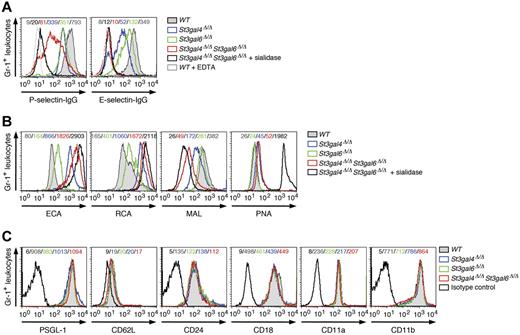
Absence of both ST3Gal-VI and ST3Gal-IV results in total loss of E-selectin ligands but incomplete loss of P-selectin ligands
The binding of selectin IgG Fc chimeric proteins was used in flow cytometric analyses to assess selectin ligand levels on circulating neutrophils.22 Loss of ST3Gal-IV resulted in a decrease in P- and E-selectin ligand expression, as described previously (Figure 3A).16 Among double-deficient neutrophils, E-selectin ligand expression was reduced similarly to wild-type neutrophils treated with sialidase or incubated with EDTA to prevent Ca2+-dependent selectin binding (Figure 3A and Table 1). These results indicate that ST3Gal-IV and ST3Gal-VI account for most, if not all, E-selectin ligand activity on neutrophils. P-selectin binding to selectin ligands on double-deficient neutrophils was strongly reduced compared with wild-type neutrophils (Figure 3A and Table 1). Interestingly, sialidase treatment of double-deficient neutrophils provoked a further reduction in P-selectin ligand expression, indicating that sialyltransferases other than ST3Gal-IV and ST3Gal-VI contribute to the residual generation of functional P-selectin ligands (Figure 3A). St3gal6 deficiency resulted in a moderate decrease in P- and E-selectin ligand expression (Figure 3A). As expected, binding of the lectins ECA and RCA to neutrophils of St3gal4-deficient, St3gal6-deficient, and double-deficient mice was greatly increased, whereas binding of the lectin Maackie amurensis lectin detecting Siaα2-3Galβ1-4GlcNAc was largely reduced in St3gal4-deficient mice and double-deficient mice and to a lesser degree in St3gal6-deficient mice (Figure 3B). No changes of expression occurred among the neutrophil-expressed adhesion molecules PSGL-1 (CD162), CD62L, CD24, CD18, CD11a, or CD11b (Figure 3C).
P- and E-selectin ligand expression on neutrophils in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) P- and E-selectin–Ig chimera binding to circulating Gr-1+ leukocytes was analyzed by flow cytometry. Loss of P- and E-selectin–Ig chimera binding in the presence of EDTA is also shown as a control. (B) ECA, RCA, Maackie amurensis lectin, and PNA lectin binding to circulating Gr-1+ cells was assessed by flow cytometry. (C) Expression of leukocyte adhesion molecules (PSGL-1/CD162, CD62L, CD24, CD18, CD11a, and CD11b) was analyzed by flow cytometry. Mean fluorescence intensity is indicated in each histogram and data are representative of 3 independent experiments.
P- and E-selectin ligand expression on neutrophils in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) P- and E-selectin–Ig chimera binding to circulating Gr-1+ leukocytes was analyzed by flow cytometry. Loss of P- and E-selectin–Ig chimera binding in the presence of EDTA is also shown as a control. (B) ECA, RCA, Maackie amurensis lectin, and PNA lectin binding to circulating Gr-1+ cells was assessed by flow cytometry. (C) Expression of leukocyte adhesion molecules (PSGL-1/CD162, CD62L, CD24, CD18, CD11a, and CD11b) was analyzed by flow cytometry. Mean fluorescence intensity is indicated in each histogram and data are representative of 3 independent experiments.
Selectin ligand function and leukocyte rolling in vitro and in vivo in St3gal4-, St3gal6-, and double-deficient mice compared with wild-type mice
| . | St3gal4Δ/Δ . | St3gal6Δ/Δ . | St3gal4Δ/ΔSt3gal6Δ/Δ . |
|---|---|---|---|
| In vitro | |||
| P-selectin binding to neutrophils | ↓ | ↓ | ↓↓ |
| E-selectin binding to neutrophils | ↓↓ | ↓ | ↓↓↓ |
| L-selectin binding to secondary lymphatic tissues | ↓ | = − ↓ | ↓↓ |
| P-selectin–dependent rolling (flow chamber) | ↓ 16 | ↓ | ↓↓↓ |
| E-selectin–dependent rolling (flow chamber) | ↓ 16 | = | ↓↓↓ |
| In vivo | |||
| P-selectin–dependent rolling | = 16 | ↓↓ | ↓↓ |
| P-selectin–dependent rolling velocities (TNF-α–stimulated cremaster muscle) | = 16 | = | = |
| E-selectin–dependent rolling | ↓ 16 | = | ↓↓↓ |
| E-selectin–dependent rolling velocities (TNF-α–stimulated cremaster muscle) | ↑↑ | = | ↑↑ |
| Lymphocyte homing (adoptive transfer of wild-type B and T cells) | ↓ | = | ↓↓ |
| . | St3gal4Δ/Δ . | St3gal6Δ/Δ . | St3gal4Δ/ΔSt3gal6Δ/Δ . |
|---|---|---|---|
| In vitro | |||
| P-selectin binding to neutrophils | ↓ | ↓ | ↓↓ |
| E-selectin binding to neutrophils | ↓↓ | ↓ | ↓↓↓ |
| L-selectin binding to secondary lymphatic tissues | ↓ | = − ↓ | ↓↓ |
| P-selectin–dependent rolling (flow chamber) | ↓ 16 | ↓ | ↓↓↓ |
| E-selectin–dependent rolling (flow chamber) | ↓ 16 | = | ↓↓↓ |
| In vivo | |||
| P-selectin–dependent rolling | = 16 | ↓↓ | ↓↓ |
| P-selectin–dependent rolling velocities (TNF-α–stimulated cremaster muscle) | = 16 | = | = |
| E-selectin–dependent rolling | ↓ 16 | = | ↓↓↓ |
| E-selectin–dependent rolling velocities (TNF-α–stimulated cremaster muscle) | ↑↑ | = | ↑↑ |
| Lymphocyte homing (adoptive transfer of wild-type B and T cells) | ↓ | = | ↓↓ |
The number of arrows indicates the magnitude of the change.
↑ indicates increases; ↓, decreases; =, no significant changes.
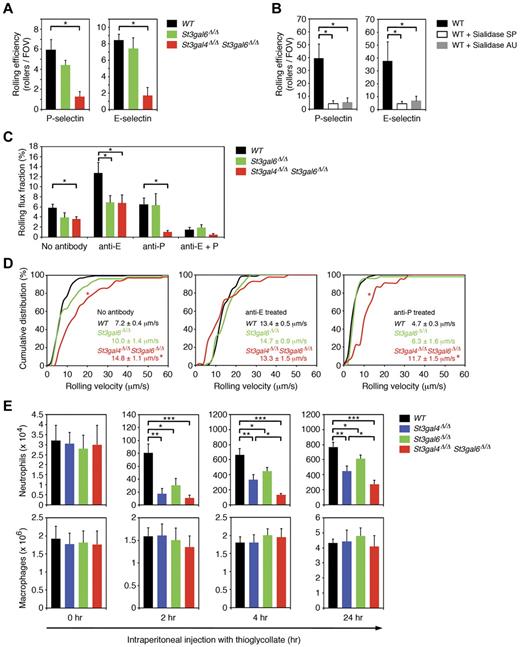
ST3Gal-VI participates in leukocyte rolling under dynamic flow conditions
We also investigated leukocyte rolling in an ex vivo flow chamber system in which flow chambers were coated with either recombinant murine P-selectin (rmP-selectin) or rmE-selectin and connected directly to the blood circulation of the mouse via a carotid artery catheter.32 In flow chambers coated with rmP-selectin and perfused with whole blood from wild-type mice, we observed 5.9 ± 1.1 (mean ± SEM) rolling leukocytes per field of view (Figure 4A). The number of cells rolling on rmP-selectin was reduced modestly in St3gal6-deficient mice and affected more severely in double-deficient mice (Figure 4A and Table 1), suggesting that ST3Gal-VI and ST3Gal-IV both contribute to the generation of P-selectin ligands in an overlapping fashion. To rule out nonspecific leukocyte rolling in our flow chamber assay, we performed additional experiments without rmP-selectin coating and did not observe any leukocyte rolling for the different groups (data not shown). We also investigated leukocyte rolling in flow chambers coated with rmE-selectin. The number of leukocytes rolling on rmE-selectin was similar in wild-type and St3gal6-deficient mice, but was strongly reduced in double-deficient mice (Figure 4A), suggesting that both ST3Gal-IV and ST3Gal-VI contribute to E-selectin–mediated rolling (Figure 4A and Table 1).
Leukocyte rolling and recruitment during inflammation in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) Leukocyte rolling in the autoperfused ex vivo microflow chamber. Microflow chambers were coated with recombinant murine (rm) P-selectin or rm E-selectin and perfused for 10 minutes with whole blood through a catheter connected to the mouse carotid artery (at least 5 chambers per group). Using an upright microscope, the number of rolling leukocytes calculated by rollers/(WBC count × 0.001) was assessed at 10 minutes. All values are shown as mean ± SEM (n = 6-12). *P < .05. (B) Effect of sialidase treatment on neutrophil rolling in the flow chamber. Isolated wild-type neutrophils were either left untreated for 1 hour (n = 15) or treated for 1 hour with Arthrobacter ureafaciens (AU) sialidase (n = 11) or with Streptococcus pneumonia (SP) sialidase (n = 14). Thereafter, neutrophils were perfused through flow chambers coated either with P- or E-selectin at a wall shear stress of 1 dyne/cm2. All values are shown as mean ± SEM (*P < .05). (C) Leukocyte rolling in TNF-α–treated cremaster muscle venules in vivo. Leukocyte rolling flux fraction (%) was assessed for each group. Blocking of E-selectin and P-selectin were performed by systemic injection of E-selectin–blocking mAb 9A9 and P-selectin blocking mAb RB40.34, respectively. All values are depicted as mean ± SEM (n = 3-6). *P < .05. (D) Leukocyte rolling velocities in TNF-α–treated cremaster muscle venules in vivo. Cumulative distribution of leukocyte rolling velocities are shown for no treatment (left), treatment with E-selectin–blocking mAb 9A9 (middle), and treatment with P-selectin–blocking mAb RB40.34 (right). Average rolling velocities are indicated as mean ± SEM with 3-6 mice per group. *P < .05 versus wild-type. (E) Total peritoneal leukocytes were collected and neutrophil and macrophage numbers analyzed by flow cytometry before (0 hours) or 2, 4, or 24 hours after IP injection of 3% thioglycollate in PBS to induce acute peritoneal inflammation. All values are presented as mean ± SEM (n = 6). ***P < .001; **P < .01; *P < .05.
Leukocyte rolling and recruitment during inflammation in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) Leukocyte rolling in the autoperfused ex vivo microflow chamber. Microflow chambers were coated with recombinant murine (rm) P-selectin or rm E-selectin and perfused for 10 minutes with whole blood through a catheter connected to the mouse carotid artery (at least 5 chambers per group). Using an upright microscope, the number of rolling leukocytes calculated by rollers/(WBC count × 0.001) was assessed at 10 minutes. All values are shown as mean ± SEM (n = 6-12). *P < .05. (B) Effect of sialidase treatment on neutrophil rolling in the flow chamber. Isolated wild-type neutrophils were either left untreated for 1 hour (n = 15) or treated for 1 hour with Arthrobacter ureafaciens (AU) sialidase (n = 11) or with Streptococcus pneumonia (SP) sialidase (n = 14). Thereafter, neutrophils were perfused through flow chambers coated either with P- or E-selectin at a wall shear stress of 1 dyne/cm2. All values are shown as mean ± SEM (*P < .05). (C) Leukocyte rolling in TNF-α–treated cremaster muscle venules in vivo. Leukocyte rolling flux fraction (%) was assessed for each group. Blocking of E-selectin and P-selectin were performed by systemic injection of E-selectin–blocking mAb 9A9 and P-selectin blocking mAb RB40.34, respectively. All values are depicted as mean ± SEM (n = 3-6). *P < .05. (D) Leukocyte rolling velocities in TNF-α–treated cremaster muscle venules in vivo. Cumulative distribution of leukocyte rolling velocities are shown for no treatment (left), treatment with E-selectin–blocking mAb 9A9 (middle), and treatment with P-selectin–blocking mAb RB40.34 (right). Average rolling velocities are indicated as mean ± SEM with 3-6 mice per group. *P < .05 versus wild-type. (E) Total peritoneal leukocytes were collected and neutrophil and macrophage numbers analyzed by flow cytometry before (0 hours) or 2, 4, or 24 hours after IP injection of 3% thioglycollate in PBS to induce acute peritoneal inflammation. All values are presented as mean ± SEM (n = 6). ***P < .001; **P < .01; *P < .05.
Comparison of ST3Gal deficiency and sialidase activity on in vitro leukocyte rolling under dynamic flow conditions
To investigate the role of sialylation on selectin ligand activity and rolling under dynamic flow conditions, we performed flow chamber experiments using isolated wild-type neutrophils that were either untreated or treated for 1 hour with Arthrobacter ureafaciens (AU) sialidase or with Streptococcus pneumonia (SP) sialidase. Whereas AU sialidase removes α2,3, α2,6 and α2,8-linked sialic acids, SP sialidase mostly acts on α2,3-linked sialic acids. We observed a severe reduction in rolling of sialidase-treated neutrophils in flow chambers coated with P- or E-selectin. At 10 minutes of perfusion and a wall shear stress of 1 dyne/cm2, rolling of AU sialidase- or SP sialidase-treated neutrophils on P-selectin was strongly reduced compared with wild-type neutrophils (Figure 4B). Similarly, rolling of AU sialidase and SP sialidase-treated neutrophils on E-selectin was severely impaired compared with wild-type neutrophils (Figure 4B). These results demonstrate that most of P- and E-selectin–mediated rolling is dependent on sialylation. Interestingly, the reduction in P- and E-selectin–mediated rolling of AU and SP sialidase-treated neutrophils was very similar to the reduction in rolling seen in St3gal6/St3gal4 double-deficient mice (Figure 4A-B), implying that ST3Gal-VI and ST3Gal-IV provide most, if not all, of the sialylation required for rolling on immobilized P- or E-selectin.
ST3Gal-IV and -VI contribute to leukocyte rolling during inflammation in vivo
To determine the effect of sialyltransferase deficiency in vivo, we investigated leukocyte rolling in TNF-α–treated cremaster muscle venules of wild-type, St3gal6-deficient, and double-deficient mice using intravital microscopy. As described previously, leukocyte rolling in this setting depends on endothelium-expressed P- and E-selectin.33 Leukocyte rolling was assessed as rolling flux fraction (RFF), which indicates the number of rolling leukocytes to all leukocytes passing through the vessel under observation in a given time period.34 Microvascular and hemodynamic parameters are depicted in Table 2 and show no differences between the groups. RFF in wild-type mice was reduced in St3gal6 deficiency and double deficiency to 67% and 62% of wild-type mice, respectively (Figure 4C). To determine the contribution of ST3Gal-VI on P-selectin–dependent rolling, we systemically injected the E-selectin–blocking mAb 9A9 into wild-type, St3gal6-deficient, and double-deficient mice. Interestingly, RFF in St3gal6-deficient mice treated with E-selectin blocking mAb 9A9 was reduced significantly to 54% of wild-type mice (Figure 4C), but did not further decrease in double-deficient mice (Figure 4C), suggesting that ST3Gal-VI contributes to the generation of P-selectin ligands in vivo, whereas ST3Gal-IV seems to not play any significant role in P-selectin–dependent rolling in vivo, which is in agreement with an earlier study (Table 1).16
Microvascular and hemodynamic parameters in TNF-α–treated postcapillary venules of the cremaster muscle
| Mouse genotype . | n . | Venules, n . | Vessel diameter, μm . | Centerline velocity, μm/s . | Wall shear rate, 1/s . | Systemic leukocyte counts, cells/μL . |
|---|---|---|---|---|---|---|
| Wild-type | 6 | 21 | 30 ± 1 | 1600 ± 100 | 1300 ± 80 | 2800 ± 400 |
| St3gal6Δ/Δ | 6 | 22 | 30 ± 1 | 1300 ± 70 | 1100 ± 60 | 3100 ± 400 |
| St3gal4Δ/ΔSt3gal6Δ/Δ | 8 | 25 | 29 ± 1 | 1400 ± 200 | 1200 ± 200 | 3100 ± 200 |
| Mouse genotype . | n . | Venules, n . | Vessel diameter, μm . | Centerline velocity, μm/s . | Wall shear rate, 1/s . | Systemic leukocyte counts, cells/μL . |
|---|---|---|---|---|---|---|
| Wild-type | 6 | 21 | 30 ± 1 | 1600 ± 100 | 1300 ± 80 | 2800 ± 400 |
| St3gal6Δ/Δ | 6 | 22 | 30 ± 1 | 1300 ± 70 | 1100 ± 60 | 3100 ± 400 |
| St3gal4Δ/ΔSt3gal6Δ/Δ | 8 | 25 | 29 ± 1 | 1400 ± 200 | 1200 ± 200 | 3100 ± 200 |
Vessel diameter, centerline velocity, wall shear rate, and systemic leukocyte counts are presented as mean ± SEM of all investigated venules.
Next, we studied E-selectin–dependent rolling in TNF-α–treated cremaster muscle venules by systemically injecting the P-selectin blocking mAb RB40.34. In St3gal6 deficiency, injection of the P-selectin blocking mAb RB40.34 led to RFF similar to wild-type mice (Figure 4C). In contrast, in double deficiency, E-selectin–dependent rolling was significantly reduced to 16% of wild-type mice (Figure 4C and Table 1). Because St3gal4 deficiency had been reported to exhibit only a mild E-selectin–dependent rolling defect,16 the strong reduction in E-selectin–dependent rolling observed in double-deficient mice indicates that both ST3Gal-IV and ST3Gal-VI contribute to the synthesis of E-selectin ligands.
To demonstrate that leukocyte rolling in this in vivo model is dependent on P- and E-selectins, the blocking mAbs 9A9 against E-selectin and RB40.34 against P-selectin were systemically injected into wild-type, St3gal6-deficient and double-deficient mice. This almost completely abolished leukocyte rolling in all 3 groups (Figure 4C).
To further characterize selectin ligand function in St3gal6-deficient and double-deficient mice, we investigated P- and E-selectin–dependent rolling velocities and their distribution in TNF-α–treated cremaster muscle venules in vivo. The mean leukocyte rolling velocity of wild-type was similar to St3gal6 deficiency (Figure 4D). In contrast, leukocyte rolling velocities were significantly increased in double deficiency (Figure 4D). Next, we analyzed P-selectin–dependent rolling velocities, which showed a similar distribution in wild-type mice, St3gal6 deficiency and double deficiency suggesting that both ST3Gal-IV and ST3Gal-VI do not regulate P-selectin–dependent rolling velocities (Figure 4D). For E-selectin–dependent rolling velocities, we found a similar velocity distribution in wild-type and St3gal6-deficient mice (Figure 4D). However, in double deficiency, E-selectin–dependent rolling velocities were strongly increased (Figure 4D and Table 1). Because an increase in E-selectin–dependent rolling velocities has also been reported in St3gal4-deficient mice,16 our findings in double-deficient mice indicate that ST3Gal-VI has little effect on E-selectin–dependent rolling velocities in vivo.
Leukocyte recruitment into the inflamed peritoneal cavity depends on ST3Gal-IV and ST3Gal-VI
Because mice deficient in both St3gal4 and St3gal6 demonstrated a severe decrease in selectin ligand activity and thus in leukocyte rolling, one could hypothesize that deficiency of these sialyltransferases would also lead to defective neutrophil recruitment to sites of inflammation. To test this hypothesis, we used the thioglycollate-induced peritonitis model in which IP injection of the chemical irritant thioglycollate leads to a strong influx of neutrophils into the peritoneal cavity (for further information on this model, please refer to supplemental Methods).15 Compared with wild-type mice, St3gal6 deficiency resulted in a moderate but significant decrease in neutrophil recruitment into the inflamed peritoneum at 2, 4, and 24 hours after injection, which was more pronounced in St3gal4 deficiency and severely defective in double deficiency (Figure 4E). We also investigated monocyte recruitment,35 which was not affected in St3gal4-deficient, St3gal6-deficient, or double-deficient mice 24 hours after injection (Figure 4E), suggesting that ST3Gal-IV and ST3Gal-VI have a critical role in neutrophil, but not in monocyte, recruitment during inflammation.
ST3Gal-VI and ST3Gal-IV have a critical role in L-selectin ligand synthesis in HEVs
Because L-selectin interacts with various glycoprotein ligands decorated with 6-sulfo-sLex carrying O-glycans present on the luminal surface of HEVs, these endothelial L-selectin ligands are generally termed peripheral node addressins (PNAds).36 To investigate the role of sialylation on selectin ligand function in view of the fact that ST3Gal-IV does not affect L-selectin ligand function on Peyer patch HEVs significantly,19 we first determined the expression of L-selectin ligands on HEVs by immunofluorescence studies using the binding of L-selectin–IgM chimeric proteins to HEVs of axillary lymph nodes (ALNs), inguinal lymph nodes (ILNs), mesenteric lymph nodes (MLNs), and Peyer patches (PPs; Figure 5A). The images of immunofluorescent cells were acquired and quantitated by TissueFAXS and TissueQuest Version 3.0.1120.0136 microscope software systems (Figure 5B). L-selectin ligands were reduced significantly in HEVs of St3gal4-deficient, St3gal6-deficient, or double-deficient mice compared with wild-type mice (Figure 5A). St3gal6 deficiency showed a mild reduction in L-selectin ligand expression in ALNs, ILNs, MLNs, and PPs. Conversely, in St3gal4 deficiency and in double deficiency, L-selectin ligand expression was more severely affected in those tissues (Figure 5B) with an additive decrease in double-deficient mice (Figure 5B). This indicates that ST3Gal-VI, in addition to ST3Gal-IV, contributes significantly to the synthesis of L-selectin ligands on HEVs in both a distinct and an overlapping manner.
L-selectin ligand expression in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) Frozen sections of ALNs, ILNs, MLNs, and PPs were stained with L-selectin–IgM (green) and αtubulin Ab (red). Serial sections were stained with H&E. Scale bars indicate 50 μm. (B) Scattergrams indicate the frequencies of L-selectin–IgM- and αtubulin-positive cells quantified using TissueQuest Version 3.0.1120.0136 microscope software systems. Data are representative of 10 independent experiments.
L-selectin ligand expression in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) Frozen sections of ALNs, ILNs, MLNs, and PPs were stained with L-selectin–IgM (green) and αtubulin Ab (red). Serial sections were stained with H&E. Scale bars indicate 50 μm. (B) Scattergrams indicate the frequencies of L-selectin–IgM- and αtubulin-positive cells quantified using TissueQuest Version 3.0.1120.0136 microscope software systems. Data are representative of 10 independent experiments.
Loss of ST3Gal-VI and ST3Gal-IV leads to reduced cellularity of secondary lymphatic tissue
Because St3gal4 and St3gal6 deficiency led to reduced binding of L-selectin to HEV-expressed L-selectin ligands in frozen sections of different lymphatic tissues, we next investigated whether their deficiencies may also affect lymphocyte homing. As a first approach, we quantified the cellularity of various lymphatic tissues. The number of lymphocytes in ALNs, ILNs, CLNs, MLNs, and PPs of St3gal4-deficient mice was reduced to 64%, 65%, 75%, 60%, and 52% of wild-type mice, respectively (Figure 6A). Interestingly, the number of lymphocytes in ALNs, ILNs, CLNs, MLNs, and PPs of double deficiency was additionally reduced to 39%, 44%, 58%, 39%, and 36% of wild-type mice, respectively, whereas no significant change in the number of lymphocytes was observed in ALNs, ILNs, CLNs, MLNs, and PPs of St3gal6-deficient mice (Figure 6A). These findings suggest that ST3Gal-IV might compensate for the loss of ST3Gal-VI, whereas ST3Gal-IV participates in the generation of L-selectin ligands in this setting, which is independent of ST3Gal-VI. Interestingly, no changes in the number of lymphocytes occurred within the spleen, thymus, or BM in the absence of ST3Gal-IV, ST3Gal-VI, or both enzymes (Figure 6A), suggesting lymphocyte homing mechanisms that may not require posttranslational sialylation. Similar results were found for posttranslational fucosylation.29
Lymphocyte trafficking in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) ALNs, ILNs, MLNs, PPs, spleen (SPL), thymus (THY), and BM aggregates were isolated from each genotype. Lymphocytes recovered from each organ were quantified manually using a hematocytometer. All values are presented as mean ± SEM (n = 6). ***P < .001; **P < .01; *P < .05. (B) CFDA-labeled lymphocytes (2.5 × 107) obtained from wild-type MLNs were injected into the tail vein of recipient mice of the indicated genotypes. Lymphoid aggregates were harvested 1 hour after injection, and CFDA+ B lymphocytes (B220+) and T lymphocytes (CD3ϵ+) were quantified by flow cytometry. All values are presented as means ± SEM (n = 6). ***P < .001; **P < .01; *P < .05.
Lymphocyte trafficking in St3gal4-deficient, St3gal6-deficient, and double-deficient mice. (A) ALNs, ILNs, MLNs, PPs, spleen (SPL), thymus (THY), and BM aggregates were isolated from each genotype. Lymphocytes recovered from each organ were quantified manually using a hematocytometer. All values are presented as mean ± SEM (n = 6). ***P < .001; **P < .01; *P < .05. (B) CFDA-labeled lymphocytes (2.5 × 107) obtained from wild-type MLNs were injected into the tail vein of recipient mice of the indicated genotypes. Lymphoid aggregates were harvested 1 hour after injection, and CFDA+ B lymphocytes (B220+) and T lymphocytes (CD3ϵ+) were quantified by flow cytometry. All values are presented as means ± SEM (n = 6). ***P < .001; **P < .01; *P < .05.
ST3Gal-VI and ST3Gal-IV regulate lymphocyte homing to LNs and PPs in vivo
We performed lymphocyte homing assays by systemically injecting CFDA-labeled wild-type B and T lymphocytes and assessing the number of injected cells in ALNs, ILNs, CLNs, MLNs, and PPs of wild-type, St3gal4-deficient, St3gal6-deficient, and double-deficient mice. Injection of CFDA-labeled wild-type B or T cells into St3gal4-deficient recipient mice resulted in a moderate reduction in the number of labeled B or T cells in the ALNs, ILNs, CLNs, MLNs, and PPs compared with labeled B or T cells in wild-type recipient mice (Figure 6B and Table 1). Interestingly, double deficiency had an additive effect on the reduction in the number of CFDA-labeled wild-type B or T lymphocytes in ALNs, ILNs, CLNs, MLNs, and PPs. Similar to the static L-selectin–binding assay, St3gal6 deficiency did not influence homing of injected wild-type B or T cells (Figure 6B and Table 1). These observations reveal that concomitant loss of the sialyltransferases ST3Gal-IV and ST3Gal-VI induces a marked reduction in L-selectin ligand activity in HEVs of ALNs, ILNs, CLNs, MLNs, and PPs, leading to a pronounced defect in L-selectin–dependent lymphocyte homing to secondary lymphoid organs.
Discussion
Sialyltransferases are required for the synthesis of selectin ligands. Earlier studies revealed that systemic injection of sialidase-treated neutrophils or HL-60 cells resulted in a dramatic reduction in P-selectin–dependent rolling in rat mesenteric venules in vivo.37 Similar results were found for binding of L-selectin–expressing lymphocytes to sialidase-treated LN frozen sections or sialidase-treated L-selectin ligands (PNAds) isolated from LN HEVs.20 Sialidase treatment of leukocytes under static conditions in vitro completely abolished binding of P- and E-selectin to selectin ligands.16 One or more of the 6 ST3Gal sialyltransferases have been implicated in contributing to the synthesis of the prototypical selectin ligand, sLex. Thus far, ST3Gal-IV has been found to contribute to the formation of some but not all selectin ligands in vivo, whereas ST3Gal-I, ST3Gal-II, and ST3Gal-III did not seem to play a role.16 With the exclusion of ST3Gal-V in selectin ligand formation, only ST3Gal-VI remains to be investigated. A role for ST3Gal-VI in the generation of functional E-selectin ligands has also been suggested in studies of the colon carcinoma cell line HCT15. These cells were shown to have increased levels of sLex and the St3gal6 gene when treated with a DNA-methyltransferase inhibitor.38 This was accompanied by a significant increase in interactions between HCT15 and immobilized E-selectin in a dynamic flow assay. In the present study, we have established ST3Gal-VI as a second sialyltransferase responsible for the formation of functional selectin ligands in vivo. Using St3gal6-deficient and St3gal4/St3gal6 double-deficient mice, we found that ST3Gal-VI contributes to the generation of P-, E-, and L-selectin ligands.
The most pronounced effect of ST3Gal-VI function was apparent in P-selectin ligand formation. P-selectin–dependent leukocyte rolling in the ex vivo flow chamber was reduced in St3gal6-deficient mice, and the additional loss of ST3Gal-IV led to a further decrease in P-selectin–dependent rolling to levels observed in sialidase-treated leukocytes. This suggests that ST3Gal-VI and ST3Gal-IV together provide most of the sialylation required for functional P-selectin ligands. However, in TNF-α-stimulated cremaster muscle venules, leukocyte rolling was only impaired but not eliminated in the absence of ST3Gal-VI, and the additional loss of ST3Gal-IV did not lead to a further reduction of P-selectin–dependent rolling. This implies that ST3Gal-IV does not seem to contribute to P-selectin–dependent rolling in this in vivo model, a finding that has been observed in other studies of St3gal4-deficient mice.16 The reason for the discrepancy between the flow chamber and in vivo findings is not known. In view of the fact that P-selectin–dependent rolling in the flow chamber is severely reduced in double-deficient mice, it can be speculated that different P-selectin ligands might contribute to P-selectin–dependent rolling under in vitro versus in vivo conditions. On the other hand, the flow chamber experiments differ from the in vivo studies by the absence of endothelial cells. Therefore, another potential explanation is that α2-3 sialylation among endothelial cells contributes to P-selectin–dependent rolling in vivo.
Because of the reduction in P-selectin–dependent rolling in TNF-α–stimulated cremaster muscle venules in the absence of ST3Gal-VI, it is likely that ST3Gal-VI is involved in the generation of functional PSGL-1 (the main P-selectin ligand in vivo). For mouse PSGL-1, a crucial O-glycosylation site required for binding to P-selectin has been identified at threonine residue 17 on the N-terminus of PSGL-1.39 Similar results were reported for human PSGL-1.40 It is likely that under in vivo conditions, this crucial O-glycan chain of PSGL-1 is modified directly by ST3Gal-VI, but not by ST3Gal-IV. This is supported by the observations that: (1) loss of ST3Gal-IV did not lead to significant changes in P-selectin–dependent rolling in vivo, and (2) the reduction in P-selectin–dependent rolling was similar between St3gal6-deficient and St3gal4/St3gal6 double-deficient mice.
E-selectin ligand synthesis is dependent on ST3Gal-IV, as described previously.16 We found herein that the loss of ST3Gal-VI alone did not have a marked effect on E-selectin ligand abundance. However, loss of both ST3Gal-IV and ST3Gal-VI was additive and resulted in an almost completely abolished neutrophil binding to E-selectin. A similar contribution of 2 isoenzymes in selectin ligand formation has been observed among the fucosyltransferases FucT-IV and FucT-VII.41 In the flow chamber, E-selectin–dependent rolling was not affected in the absence of ST3Gal-VI. Loss of both ST3Gal-IV and ST3Gal-VI reduced E-selectin–dependent rolling to a higher degree than absence of ST3Gal-IV alone, implying that ST3Gal-IV can partially compensate when ST3Gal-VI is absent. This was further supported by our in vivo experiments. No effect on E-selectin–mediated rolling was seen among St3gal6-deficient mice, and loss of both ST3Gal-IV and ST3Gal-VI abolished E-selectin–dependent rolling to the same extent as pretreatment with sialidases. These findings suggest that ST3Gal-VI, together with ST3Gal-IV, provides most, if not all, of the sialylation required to synthesize functional E-selectin ligands. In addition, the results of the present study indicate that both ST3Gal-VI and ST3Gal-IV are able to provide sufficient sialylation on E-selectin ligands for E-selectin–dependent capturing. Because PSGL-1 has been reported to mediate E-selectin–dependent capturing (but not E-selectin–dependent slow rolling velocities),42 it is likely that ST3Gal-VI and ST3Gal-IV provide most, if not all, of the sialylation necessary to generate the E-selectin–binding site on PSGL-1, a site distinct from the P- and L-selectin–binding sites.
Compared with E-selectin–dependent capturing, ST3Gal-VI does not appear to influence E-selectin–mediated slow rolling velocities. In contrast, E-selectin–dependent rolling velocities were increased significantly in the absence of ST3Gal-IV,16 but did not further increase in double-deficient mice. This indicates that ST3Gal-IV, but not ST3Gal-VI, is critical for the generation of E-selectin ligands such as CD44 and ESL-1, which are known to reduce E-selectin–mediated rolling velocities.43 CD44 and ESL-1 rely on N-glycans for binding to E-selectin,44 suggesting that ST3Gal-IV, but not ST3Gal-VI, provides sialylation of crucial N-glycan structures on ESL-1 and CD44.
In contrast to P- and E-selectin ligands, which are almost exclusively found on circulating blood cells, L-selectin ligands reside in large part on the luminal surface of HEVs of secondary lymphatic tissue.7,45 They comprise a group of sialomucins termed PNAds, are recognized by mAb MECA 79, and include GlyCAM-1, CD34, endomucin, and others.46,47 The PNAd group is considered a redundant carrier system with a protein backbone as scaffold for critical carbohydrate determinants such as 6-sulfo-sialyl Lewis X. Whereas several posttranslational modifications such as carbohydrate sulfation and α1,3-fucosylation have been investigated thoroughly,14,47-49 the contribution of sialylation for L-selectin ligand activity on HEV is less clear. ST3Gal-IV has been reported to be critical for L-selectin–dependent rolling during inflammation, whereas rolling on PP HEVs turned out to be unaffected in the absence of ST3Gal-IV.19 Our findings reveal that binding of L-selectin IgM chimera to L-selectin ligands on HEVs is slightly reduced in the absence of ST3Gal-IV or ST3Gal-VI. In addition, injection of wild-type B or T cells into St3gal4-deficient mice led to a moderate decrease in lymphocyte homing into the various secondary lymphatic tissues tested, whereas in St3gal6-deficient recipients, homing of injected wild-type B and T cells was normal, suggesting that ST3Gal-VI does not have a major role in L-selectin ligand–dependent lymphocyte homing. Interestingly, we observed a more pronounced defect in L-selectin binding and lymphocyte homing in double-deficient mice than in single-deficient mice, suggesting that ST3Gal-IV can compensate for the loss of ST3Gal-VI. Because L-selectin binding and lymphocyte homing could still be detected in the double-deficient mice, other sialyltransferases may be involved in the generation of functional L-selectin ligands on HEVs in vivo.
In the present study, we have identified the sialyltransferase ST3Gal-VI to be critically involved in the generation of functional selectin ligands both in vitro and in vivo. We could detect considerable participation of ST3Gal-VI on the formation and function of P-selectin ligands in vitro and in vivo. Conversely, the contribution to E- and L-selectin ligand activity was considerably less. We found that ST3Gal-VI participates in E-selectin–mediated capturing, but that this could only be unmasked by the concomitant absence of ST3Gal-VI and ST3Gal-IV. In contrast to ST3Gal-IV, ST3Gal-VI had no influence on the characteristically slow E-selectin–dependent rolling velocity. L-selectin ligand activity on HEVs was only slightly reduced in the absence of ST3Gal-VI and was affected more severely by the loss of both ST3Gal-VI and ST3Gal-IV.
In conclusion, the results of the present study demonstrate that ST3Gal-IV and ST3Gal-VI are the major sialyltransferases involved in the generation of functional selectin ligands in vivo, while at the same time implicating additional sialyltransferases that may further contribute in compensatory mechanisms to retain selectin ligand synthesis and function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Susanne Bierschenk for excellent help in performing the flow chamber experiments.
Funding for this research was provided by the National Institutes of Health (grants CA71932 and HL57345 to J.D.M.), a Korea Research Foundation grant (KRF-2008-357-C00101, W.H.Y.), and the Deutsche Forschungsgemeinschaft (SFB914) and LMU-Innovativ BioImaging (to M.S.).
National Institutes of Health
Authorship
Contribution: W.H.Y. and C.N. designed and performed the research, analyzed the data, and wrote the manuscript; P.K.G. designed the research and analyzed the data; and J.D.M. and M.S. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Sperandio, MD, Walter Brendel Center of Experimental Medicine, Ludwig-Maximilians-University, Marchioninistr 15, 81377 Munich, Germany; e-mail: markus.sperandio@lmu.de; or Jamey D. Marth, PhD, Center for Nanomedicine, 2324 Life Sciences Bldg, MCDB University of California, Santa Barbara, Santa Barbara, CA 93106-9625; e-mail: jmarth@sanfordburnham.org.

![Figure 1. Activity, expression, and mutagenesis of ST3Gal-VI sialyltransferase. (A) ST3Gal sialyltransferases (ST3Gal-I-VI) add sialic acid to the terminus of type I, II, or III sugars. ST3Gal-IV, ST3Ga-VI, and, to a lesser degree, ST3Gal-III have substrate specificity for type II and are involved in formation of sialyl Lewis X, the prototypic ligand for selectins. (B-C) Expression of mouse St3gal6 RNA transcripts among total RNA samples in wild-type mouse tissues, as determined by Northern blot (B) and qPCR analysis (C). Data are represented as means ± SEM from 3 independent experiments. (D) Mouse genomic clone of St3gal6 bearing exon 3 (black box) used for constructing the targeting vector with the pflox plasmid as indicated. Homologous recombination generated the St3gal6 F[tkneo] allele. After Cre recombination and selection, ES cell clones were isolated containing the Δ (systemic deleted) and F (conditional) alleles. (E) Southern blot analysis of Avr II-digested (left panel) and Nhe I-digested (right panel) ES cell DNA probed with a genomic probe (left panel) and loxP probe (right panel). ES cell clones bearing the St3gal6 F[tkneo] allele were compared with R1 parental wild-type ES cells. (F) PCR analysis of ES cell DNA or tail DNA from the offspring of parental mice heterozygous for the St3gal6 Δ allele indicates the 190-bp wild-type (WT) fragment, the 360-bp Δ fragment, and the 325-bp F fragment. (G) RT-PCR analysis of total cDNA from several mice organs generating St3gal6 exon 3-6 product, St3gal6 exon 7-9 product, and Gapdh product.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/5/10.1182_blood-2012-04-424366/4/m_zh89991294480001.jpeg?Expires=1769398048&Signature=jiPks8HsYz-YwSaMbBTGMxjVcvYQrnoFFmWO6rW3Oq1S8L~G06rRz6BWRqvNc7F8hFDRbmv3JDRFKenXsT5HiCgqKho-Uq-vfNWxfA~MEMV6TwazMmDE4YNDLJm2rWsN0-rAtAFpCwLBHwuXW8hV2jUt3pw5I57Vk8lrxEOCP4QUtSXjb1XFMYJxO80lwkwQQ2TpzhsDHVat1B9-s8d5XbJ94~yYdDgNKfO~34T12PWw9Mt9zVfQkMyRG-fX~NzH7HPlAecUpE8u95Zt3lONgxMoJ3u26H4KjG6TWOFZ2dZlEsF1ikLVqK4r2g9-krM9bkFJs0SY8Of-DEkzsHHaww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal