Recombinant factor VIIa (rFVIIa) is used for treatment of hemophilia patients with inhibitors, as well for off-label treatment of severe bleeding in trauma and surgery. Effective bleeding control requires supraphysiological doses of rFVIIa, posing both high expense and uncertain thrombotic risk. Two major competing theories offer different explanations for the supraphysiological rFVIIa dosing requirement: (1) the need to overcome competition between FVIIa and FVII zymogen for tissue factor (TF) binding, and (2) a high-dose–requiring phospholipid-related pathway of FVIIa action. In the present study, we found experimental conditions in which both mechanisms contribute simultaneously and independently to rFVIIa-driven thrombin generation in FVII-deficient human plasma. From mathematical simulations of our model of FX activation, which were confirmed by thrombin-generation experiments, we conclude that the action of rFVIIa at pharmacologic doses is dominated by the TF-dependent pathway with a minor contribution from a phospholipid-dependent mechanism. We established a dose-response curve for rFVIIa that is useful to explain dosing strategies. In the present study, we present a pathway to reconcile the 2 major mechanisms of rFVIIa action, a necessary step to understanding future dose optimization and evaluation of new rFVIIa analogs currently under development.
Introduction
The use of a recombinant factor VIIa (rFVIIa) product, NovoSeven, which is licensed for the treatment of hemophilia patients with inhibitory Abs against factor VIII (FVIII) or factor IX (FIX), has proven to be safe and efficacious, but its dosing remains problematic.1,,–4 The recommended dosing schedule is a supraphysiological dose of 90 μg/kg every 2-3 hours until hemostasis is achieved, producing an approximately 250-fold increase above basal plasma concentrations of FVIIa (0.1 to 25nM).5 Not only does such a treatment regimen incur a high cost, but ineffective drug responses and thrombotic complications have also been reported.6,,–9 However, our current understanding of the mechanism of rFVIIa action is unclear, limiting our ability to optimize the safety and cost of treatment.10,11
Off-label use of rFVIIa for nonhemophilia patients at similar high doses indicates that the high dose required for hemophilia treatment cannot be explained by deficiencies of FVIII or FIX alone.5,12 FVIIa is a weak enzyme and its activity requires either of 2 cofactors, tissue factor (TF) or negatively charged phospholipids.13 Disagreement over which of the 2 cofactors explains the high pharmacologic dose of the drug has led to TF- and phospholipid-dependent theories of rFVIIa action. Although these mechanisms may appear to be nonexclusive, the 2 theories support opposing approaches to dose adjustment.
The TF-dependent mechanism suggests that the hemostatic effect of rFVIIa is mediated by its binding to TF expressed on cell surfaces at the site of injury,14 forming the extrinsic tenase complex, which activates factor X (FX), leading to thrombin generation (TG).11 Physiologic amounts of FVIIa were found previously to compete with FVII for TF,15 which is further supported by the finding that both bind to TF with comparable affinities.16 Therefore, van't Veer and Mann suggested that relatively high doses of rFVIIa are needed to shift the competition between rFVIIa and FVII for TF.17 However, the theory argues that dose increases would be wasteful and clinical doses could even be reduced because the high binding affinity of the TF/rFVIIa interaction means that subpharmacologic doses of rFVIIa should saturate all available TF molecules,18 which are believed to be exposed at picomolar concentrations.14
The alternative phospholipid/platelet-dependent mechanism proposed by Monroe et al postulates that hemostasis is achieved by direct activation of FX by rFVIIa bound to phospholipids exposed on the surface of activated platelets, and is therefore entirely TF independent.19 The binding affinity of FVIIa to phospholipids and platelets is low and likely does not contribute to hemostasis at physiologic FVIIa levels,20 but the low binding may explain the need for high doses.21 In contrast to the TF-dependent mechanism, platelet activation at the site of vessel injury ensures that the TF-independent effect should never become saturated, suggesting a benefit of dose increases.
Despite the similarity of their experimental models of ex vivo thrombin generation, Hoffman and Mann were unsuccessful in reproducing the opposing mechanism, which only reinforced the divide between the 2 schools of thought. Butenas et al showed that platelets did not help rFVIIa to restore normal TG in hemophilia in both a reconstituted coagulation model containing coagulation proteins, platelets, and TF-bearing phospholipid vesicles and in a model of minimally altered “acquired” hemophilia blood.20 Conversely, the Hoffman cell-based model of hemostasis (purified coagulation proteins, platelets, and TF-bearing monocytes) did not reveal zymogen inhibition of TG.22 The 2 groups could only agree that the variable presence of zymogen inhibition might be attributed in part to the source of TF.10,21
To explore the modes of rFVIIa action, in the present study, we designed a novel experimental model using fluorogenic substrate–based thrombin generation and clotting in human plasma. We found conditions for the presence and absence of zymogen inhibition, the key controversy among previous studies, by testing over a wide range of FVII and rFVIIa concentrations. Using zymogen inhibition as a probing tool, we found that the disagreements between previous studies were because of their experimental limitations. Under conditions in which zymogen inhibition is present, we are the first to show the simultaneous existence of TF-dependent and phospholipid-dependent mechanisms and, in doing so, found that each mechanism independently contributes to rFVIIa-induced coagulation. Moreover, our experimental results were confirmed with mathematical simulations based on simple kinetic interactions of rFVIIa-induced FXa generation. We provide a theoretical framework to explain how rFVIIa may act under various dosing strategies depending on the level of TF exposed and rFVIIa infused.
Methods
Human congenital FVII- or FVIII-deficient plasma was from HRF. Immunodepleted FVII and FVIII double-deficient plasma was from Affinity Biologicals. rFVIIa was from Novo Nordisk (NovoSeven). Human plasma-derived factor VII and factor VIIa with a blocked active site (inactivated FVIIa or FVIIai) were from Enzyme Research. Recombinant lipidated TF (rTF; Innovin) was from Dade Behring. Some experiments also used lipidated rTF from American Diagnostica, with identical results. Lipidated recombinant soluble TF (sTF) was from Diagnostica Stago (StaClot kit). TF density experiments were done using full-length rTF from Haematologic Technologies. CaCl2 (2M) was from Quality Biological. Fluorogenic substrate for thrombin Z-Gly-Gly-Arg-AMC was from Bachem. Phospholipid vesicles were prepared by extrusion from synthetic DOPC:DOPS (7:3) phospholipids (Avanti Polar Lipids). Corn trypsin inhibitor (CTI) was from Haematologic Technologies.
Characterization of protein preparations
Activities of commercial lipidated TF preparations, rTF Innovin (6nM) and sTF StaClot (800nM), were determined using a chromogenic activity assay (Actichrome TF; American Diagnostica) against 2 TF standards of known molar concentration: full-length TF from American Diagnostica, and sTF provided by Dr Alireza R. Rezaie (St Louis University, St Louis, MO).
Specific activity of aliquoted rFVIIa (42 000 U/mg) was determined using the StaClot FVIIa activity assay (Diagnostica Stago). The same assay was used to determine FVIIa contamination in preparations of FVII and inactivated FVIIai. FVIIa activity in FVII was 86 U/mg (approximately a 1% contamination of FVII with FVIIa), and FVIIa activity in FVIIai was 78 U/mg (approximately a 0.6% contamination).
The purities of FVII and FVIIa preparations were analyzed further using SDS-PAGE (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). FVIIa contained approximately 1% of zymogen (wt/wt); FVII contained less than 0.5% of FVIIa; FVIIai contained approximately 9% of FVII and approximately 10% of other impurities (wt/wt).
Preparation of density-variable rTF
Relipidation of rTF at different densities was prepared using the octyl-β-D-glucopyranoside dialysis method.23 Briefly, different rTF amounts (2000-17 nmol) were incubated with octyl-β-D-glucopyranoside at a constant phospholipid concentration (approximately 2.6mM) and then dialyzed. Functional TF activity was quantified using thrombin peak heights (TG/fibrin generation [FG] in FVII-deficient plasma supplemented with 6nM of rFVIIa, see “TG/FG assay”) and rTF (Innovin) as a standard. A chromogenic TF assay (Actichrome) was used to confirm the activity.
TG/FG assay
Initial studies were performed in FVII-deficient, FVIII-deficient, and FVII/FVIII double-deficient plasma. No specific effect of FVIII deficiency on FVII/rFVIIa interactions was found, so the experiments presented here in used only FVII-deficient plasma. Different sources of plasma were tested, but because all sources gave similar results, experiments reported herein were performed using a single source of FVII-deficient plasma (HRF).
Clotting was initiated with rTF, and TG/FG was monitored over time using a fluorogenic substrate and clot absorbance as described previously.24 Briefly, FVII-deficient plasma (90% vol/vol) was first mixed with fluorogenic substrate Z-Gly-Gly-Arg-AMC (1.25%, 800μM final concentration), then with FVII, rFVIIa, and buffer (6.25%). Recalcification/activation was initiated by the addition of rTF diluted in 0.8M CaCl2 solution (2.5%) and transferred to a microplate reader regulated at 37°C. Fluorescence (380 nm excitation, 430 nm emission) and absorbance (492 nm) were recorded in a 96-well plate using the Infinite F500 reader (Tecan) for at least 2 hours. Phospholipid vesicles were added to some experiments.
In previously published studies, whole blood or synthetic plasma, rFVIIa, and FVII were prepared together before the initiation of TG. However, the activation of FVII by FVIIa is known to occur.13,23 To minimize the interaction between FVII and FVIIa and to reduce the effects of sampling errors, we used the following experimental design: (1) FVII-deficient plasma was supplemented with phospholipid vesicles, fluorogenic substrate, and buffer; (2) equal portions of plasma were divided using paired vials; (3) FVII and FVIIa were added to separate paired vials of plasma (ie, FVII and FVIIa were never added to the same plasma vial); (4) these paired vials of FVII- and FVIIa-containing plasma were mixed together just before the start of the experiment; (5) the reaction was started by the addition of a rTF/CaCl2 mixture to paired wells (with or without FVII); and (6) multichannel pipettes were used to ensure that paired FVII wells received the same amount of rTF simultaneously. The TG/FG data were processed as described previously24 with the help of scripts written by M.V.O. for Origin Pro software (OriginLab).
Thrombin and fibrin generation on TF-bearing cells
The human lung fibroblast line WI-38 was purchased from the ATCC and was used at passages 3-5. Different amounts of cells (from sparse cells up to a confluent monolayer) were set in 96-well microtiter plates and used in experiments the following day. Fibroblasts were propagated in MEM (ATCC) supplemented with 10% FBS (Cambrex) at 37°C. Cells were washed 3 times with Dulbecco PBS just before the experiment. TG/FG on cells was performed as described under “TG/FG assay” except that phospholipids were not used and rTF was omitted from the recalcification mixture.
The functional TF activity of cell monolayers was estimated from peak thrombin height concentrations using a calibration curve obtained with rTF preparation (Innovin). Therefore, this method does not determine the concentration of TF per cell, but presents the number of lipidated TF molecules needed to achieve an equivalent TG response in plasma.
Direct thrombin-generation assay using Western blot
TG was initiated in 1.5-mL Eppendorf tubes with 1pM rTF, 6nM rFVIIa, 4μM phospholipid PC:PS vesicles in plasma with or without 100nM FVII at 37°C. Individual reactions were quenched by the addition of 100 μL of 1.1× SDS-PAGE sample buffer with 1.1× reducing agent at the indicated time points. Quenched samples were diluted 1:4 in reducing SDS-PAGE loading buffer, incubated at 70°C for 10 minutes, and subjected to SDS-PAGE using Bis-Tris gels (4%-12%, 1 or 1.5 mm) from Invitrogen in MOPS or MES running buffer (Invitrogen). Western blot was performed with Thermo Scientific Pierce Fast Semi Dry Blotter. Proteins were marked with sheep anti–human thrombin Abs PAHT-S from Haematologic Technologies and peroxidase-conjugated donkey anti-sheep IgG (H+L) secondary Abs from Jackson ImmunoResearch. The polyclonal Ab detected prothrombin, thrombin, and the thrombin-antithrombin complex.25 Blot images were visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and acquired with a Kodak ImageStation 4000 mm Pro (Carestream Healthcare).
Mathematical models of extrinsic coagulation pathway
A detailed description of the model equations can be found in the supplemental materials. Briefly, the majority of simulations used a model of FXa generation that describes the initial stages of the coagulation cascade; that is, the interactions among TF, FVII, FVIIa, TF pathway inhibitor, antithrombin III, FX, FXa, and phospholipids (Figure 2B). In some initial simulations, a model of thrombin generation was used that consisted of the FXa model supplemented with equations describing prothrombinase complex formation, thrombin generation, and inactivation (supplemental Figure 6A). However, these additional equations were found not to be necessary because the simpler FXa model was sufficient to describe the experiments of this paper.
Simulation conditions corresponded to platelet-rich plasma (phospholipid concentration was 4μM) and platelet-free plasma (phospholipid concentration was approximately 1% that of platelet-rich plasma). To model the experimental conditions, we incorporated the contamination of FVII with FVIIa and vice versa. This correction had a minor effect on the results of calculations. As an output parameter for each simulation experiment, we used the total amount of FXa produced during the first 40 minutes of simulation, ∫040Xa · dt.
The following parameters were used in the mathematical experiments:
To disable FVII and FVIIa competition, the constant of FVII and TF association kaFVII,TF was set to zero.
To disable FVII activation by FXa and FVIIa, the constants KactFVII_TF,FXa, KactFVII,FXa, KactFVII,FVIIa_TF, and KactFVII_TF,FVIIa_TF were zeroed.
To model the experiments with inactivated FVIIa, a new variable, FVIIai, was introduced, which was different from FVIIa in the following respects:
It had a 5× higher binding affinity to TF26 and
it had no proteolytic activity.
To model experiments with soluble TF, we changed the following parameters:
The constant of inactive extrinsic Xase (FVII/TF) activation by FXa was reduced by almost 20× (KactFVII_TF,FXa = 0.0034nM−1min−1)27 and there was no activation of FVII/TF complex by FVIIa/TF.
The constant of FVII activation by FVIIa/TF complex was zeroed.
The constants of FVII/TF and FVIIa/TF complexes dissociation were increased by 20× (from kdFVIIaTF = 0.0138 min−1 for TF to kdFVII_TF = 0.354 min−1 for sTF; from kdFVIIa_TF = 0.0138 min−1 for TF to kdFVIIa_TF = 0.354 min−1 for sTF).28
The constants of FVII/TF and FVIIa/TF association were reduced by 5× (from kaFVII,TF = 0.052nM−1min−1 for TF to kaFVII,TF = 0.0285nM−1min−1 for sTF; from kaFVIIa,TF = 0.156nM−1min−1 for TF to kaFVIIa,TF = 0.0285nM−1min−1 for sTF).28
To model experiments with high and low densities of TF per phospholipid vesicle, we changed the TFdensity from 0.02nM/m2 (for low TF) to 0.5nM/m2 (for high TF).
Results
Finding the optimal conditions of zymogen inhibition in our plasma model
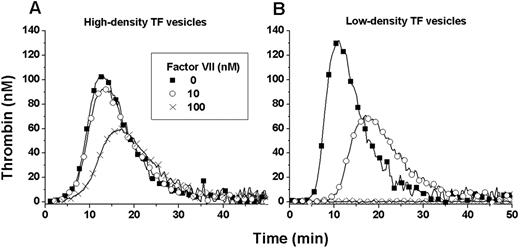
To determine the conditions that would reveal zymogen inhibition, we used the fluorogenic substrate-based TG assay to test wide ranges of FVII, rFVIIa, and relipidated rTF concentrations with and without additional procoagulant phospholipids. TF preparations relipidated at different surface densities of rTF per phospholipid vesicle were also tested. The conditions for the presence of zymogen inhibition were found to depend on the concentration of FVII and rFVIIa and the density of TF (Figure 1). The optimal conditions shown were obtained with plasma supplemented with 4μM phospholipids, 6nM rFVIIa, 0-100nM FVII and coagulation was initiated with either 1pM high-density (1:4000; TF:PSPC concentration ratio) or low-density (1:26 000) TF. High-density TF with 100nM FVII and low-density TF with 10nM FVII reduced the peak height of the TG curve by approximately 50%. For technical reasons, all subsequent experiments were performed with a commercially available rTF reagent (Innovin), which has an apparent high TF surface density (supplemental Figure 3B).
Optimal conditions of zymogen inhibition. FVII-deficient plasma was supplemented with phospholipids (4μM), rFVIIa (6nM), and FVII (0, 10, or 100nM). Coagulation was activated with a 1pM concentration of either high-surface-density TF (1:4000 TF: phospholipid molar concentration ratio) or low surface-density TF (1:26 000). The presence of zymogen inhibition depends on the concentrations of FVII and rFVIIa and the strength of inhibition is related to the surface density of TF.
Optimal conditions of zymogen inhibition. FVII-deficient plasma was supplemented with phospholipids (4μM), rFVIIa (6nM), and FVII (0, 10, or 100nM). Coagulation was activated with a 1pM concentration of either high-surface-density TF (1:4000 TF: phospholipid molar concentration ratio) or low surface-density TF (1:26 000). The presence of zymogen inhibition depends on the concentrations of FVII and rFVIIa and the strength of inhibition is related to the surface density of TF.
FVII inhibition was confirmed in a similar reaction without fluorogenic substrate that was monitored simultaneously using SDS-PAGE analysis of the reaction products and fibrin generation, ensuring that the fluorogenic substrate did not interfere with the kinetics of rFVIIa-dependent coagulation to produce false artifacts of zymogen inhibition29 (supplemental Figure 4).
Unlike previous work,22 our model plasma system revealed the inhibitory effect of zymogen on rFVIIa under both high and low TF concentrations, although it was greater at lower TF concentrations with (not shown) or without phospholipids (supplemental Figure 2). The optimal TF concentration was set at 1pM because lower concentrations gave inconsistent TG. Both FVII and rFVIIa were found to be TF-dependent procoagulants when tested independently, and zymogen inhibition was not observed in the absence of TF (supplemental Figure 5); confirming that the interaction among TF, FVII, and rFVIIa was responsible for the zymogen inhibition.
Zymogen inhibition is regulated by the ratio of rFVIIa and FVII
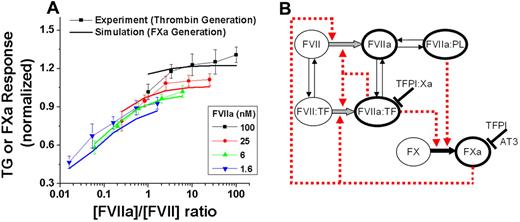
The dose-dependent effect of zymogen inhibition was found over the FVII (0-100nM) and rFVIIa (0.4-100nM) ranges (Figure 2A symbols). The molar ratio of rFVIIa to FVII appeared to determine the peak of TG (ie, the coagulation response to rFVIIa).
The concentration ratio of rFVIIa to FVII regulates thrombin and FXa-generation responses to rFVIIa. (A) Comparison of experimental TG peak height data (dotted lines, n = 8) and FXa-generation model simulations (lines). Simulation output is the integral of FXa generation (total FXa generation after 40 minutes) and is proportional to TG peak heights (supplemental Figure 6B). Activation was with 1pM TF (Innovin). Experimental data are normalized to an internal control (signal at 6nM rFVIIa without FVII) to reduce effects of variability on dose-dependent curves. (B) The reactions modeled in our FXa-generation theoretical simulations. These reactions are the minimum set needed to predict our TG experimental data.
The concentration ratio of rFVIIa to FVII regulates thrombin and FXa-generation responses to rFVIIa. (A) Comparison of experimental TG peak height data (dotted lines, n = 8) and FXa-generation model simulations (lines). Simulation output is the integral of FXa generation (total FXa generation after 40 minutes) and is proportional to TG peak heights (supplemental Figure 6B). Activation was with 1pM TF (Innovin). Experimental data are normalized to an internal control (signal at 6nM rFVIIa without FVII) to reduce effects of variability on dose-dependent curves. (B) The reactions modeled in our FXa-generation theoretical simulations. These reactions are the minimum set needed to predict our TG experimental data.
Simulation model of FXa generation can predict in vitro TG experiments
To determine whether known FVII- and FVIIa-dependent reactions are sufficient to explain the TG experiments, we simulated TG using published constants of kinetic interactions from the initial reactions of the blood coagulation cascade (supplemental Figure 6A). The mathematical model of TG was further reduced to only reactions important for FXa generation, limiting the potential number of reactions important for zymogen inhibition (Figure 2B). FXa generation can be used to model in vitro TG experiments because the integral of FXa generation is proportional to peak thrombin height (supplemental Figure 6B). Simulation under the same conditions as the in vitro experiment using FXa generation as the output showed strong agreement with the predictive effect of rFVIIa/FVII concentration ratio on TG (Figure 2A solid lines).
Inhibition is dependent on the balance between competition for TF and FVII activation
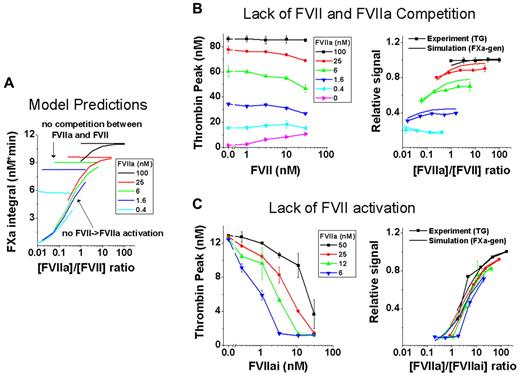
The simplified FXa-generation model was used to predict which reactions are mechanistically essential for zymogen inhibition by simulating, one-by-one, conditions in which one of the model reactions was switched off or enhanced (Table 1). Simulations suggested that the effect of FVII is regulated by only 2 reaction sets (Figure 3A): (1) the competition of FVII and FVIIa for TF, which inhibits the activity of the extrinsic Xase; and (2) the activation of FVII/TF to FVIIa/TF, which increases the Xase activity. To test these simulation predictions, we designed experiments that modeled the effects of neutralized inhibition and increased inhibitory capability of FVII.
Reactions underlying the FVII-dependent inhibition of FXa generation
| Reaction . | Effect on FVII-dependent inhibition . | |
|---|---|---|
| Reaction was disabled . | Reaction was accelerated . | |
| Competition of FVII and FVIIa for TF | No inhibition | Increased inhibition |
| Activation of FVII or FVII/TF | Higher inhibition | No inhibition |
| Activation of FX by FVIIa/TF | No inhibition | No effect |
| Activation of FX by FVIIa/phospholipid | No effect but FXa integral was lower at the highest rFVIIa concentrations | No effect on inhibition but FXa integrals were higher at higher rFVIIa |
| Inactivation of FXa by AT3 and TFPI | No effect but FXa integral was higher | No effect but FXa integral was lower |
| Inactivation of FVIIa/TF by TFPI/Xa | No effect but FXa integral was higher | No effect but FXa integral was lower |
| Reversal of inactivation of FVIIa/TF by TFPI/Xa | No effect but FXa integral was lower | No effect but FXa integral was higher |
| Reaction . | Effect on FVII-dependent inhibition . | |
|---|---|---|
| Reaction was disabled . | Reaction was accelerated . | |
| Competition of FVII and FVIIa for TF | No inhibition | Increased inhibition |
| Activation of FVII or FVII/TF | Higher inhibition | No inhibition |
| Activation of FX by FVIIa/TF | No inhibition | No effect |
| Activation of FX by FVIIa/phospholipid | No effect but FXa integral was lower at the highest rFVIIa concentrations | No effect on inhibition but FXa integrals were higher at higher rFVIIa |
| Inactivation of FXa by AT3 and TFPI | No effect but FXa integral was higher | No effect but FXa integral was lower |
| Inactivation of FVIIa/TF by TFPI/Xa | No effect but FXa integral was higher | No effect but FXa integral was lower |
| Reversal of inactivation of FVIIa/TF by TFPI/Xa | No effect but FXa integral was lower | No effect but FXa integral was higher |
Experiments were simulated under conditions in which each of the reactions was disabled or enhanced.
TFPI indicates TF pathway inhibitor.
FVII/rFVIIa competition for TF and activation of FVII dictate zymogen inhibition. (A) Theoretical simulations of FXa generation in an ideal system in which FVII and rFVIIa do not compete for TF (horizontal lines) and FVII cannot be activated to FVIIa (sloped curves). (B) Left panel shows activation of coagulation with sTF (133pM), which possesses low affinity for FVII and rFVIIa and mimics a system that lacks competition for TF (representative TG experiment from n = 2; data from 2 wells). Phospholipids (4μM) were added to compensate for the lower cofactor activity of sTF. Right panel shows comparison of the experimental data (dotted lines indicate measured TG peak height) from left panel to simulations under the same conditions (lines indicate simulated integral of FXa generation). (C) Left panel shows the strong inhibitory effect on rFVIIa by FVIIai, (FVIIai replaces FVII) mimicking a system without FVII activation (representative experiment from n = 2; data from 2 wells). Right panel shows comparison of experimental data (dotted lines indicate measured TG) from left panel to simulations under the same conditions (lines indicate simulated integral of FXa generation).
FVII/rFVIIa competition for TF and activation of FVII dictate zymogen inhibition. (A) Theoretical simulations of FXa generation in an ideal system in which FVII and rFVIIa do not compete for TF (horizontal lines) and FVII cannot be activated to FVIIa (sloped curves). (B) Left panel shows activation of coagulation with sTF (133pM), which possesses low affinity for FVII and rFVIIa and mimics a system that lacks competition for TF (representative TG experiment from n = 2; data from 2 wells). Phospholipids (4μM) were added to compensate for the lower cofactor activity of sTF. Right panel shows comparison of the experimental data (dotted lines indicate measured TG peak height) from left panel to simulations under the same conditions (lines indicate simulated integral of FXa generation). (C) Left panel shows the strong inhibitory effect on rFVIIa by FVIIai, (FVIIai replaces FVII) mimicking a system without FVII activation (representative experiment from n = 2; data from 2 wells). Right panel shows comparison of experimental data (dotted lines indicate measured TG) from left panel to simulations under the same conditions (lines indicate simulated integral of FXa generation).
To neutralize FVII inhibition, competition between FVII and rFVIIa for TF was reduced by replacing full-length rTF with a soluble truncated mutant of TF30 (Figure 3B). Soluble TF mimics a lack of competition because it has a low binding affinity to both FVII and rFVIIa and must be used at much higher molar concentrations to exhibit activity. The thrombin peak no longer decreased as the concentration of FVII was increased at the various concentrations of rFVIIa, as demonstrated by the nearly horizontal lines. Mathematical simulations of this experiment used affinity constants appropriate for soluble TF and were in good agreement with the in vitro experiment (Figure 3B).
To enhance the inhibition of rFVIIa by FVII, we disabled the ability of FVII to be activated to FVIIa by using a FVIIa derivative with an inhibited active site (FVIIai).26 FVIIai revealed strong inhibition at all rFVIIa concentrations tested (Figure 3C). Mathematical simulations were in good agreement with the in vitro experiment, confirming the validity of our model assumptions (Figure 3C).
FVII inhibition exists in a TF-bearing cell-based model of hemostasis
Others10,31 have suggested that the reported contradictions in the experimental observations of rFVIIa action can be attributed to experimental design: specifically, the use of relipidated rTF versus TF-bearing living cells. We changed our source of TF from rTF-bearing vesicles to high-surface-density TF-bearing32 lung fibroblasts and monitored TG/FG activated by cell monolayers grown at high to low cell counts per well. Lower cell numbers revealed zymogen inhibition, as evidenced by a decrease in TG and delayed clotting time in the presence of FVII, whereas higher cell numbers not only overcame FVII inhibition, but also increased TG (Figure 4 averaged experiments and supplemental Figure 7 individual experiments). Mathematical simulations of the inhibitory effect over a wide range of TF concentrations (0-100pM) showed the same effect: as the TF concentration increased, zymogen inhibition decreased until zymogen became procoagulant at TF concentrations above 50pM (supplemental Figure 9). Functionally similar ranges of TF activity were observed on fibroblast monolayers compared with TG induced by TF-bearing vesicles (supplemental Figure 8). Simulations suggest that high concentrations or density of TF on cells accelerates FVII activation, preventing rFVIIa/FVII competition for TF.
High cell count prevents presence of zymogen inhibition in a TF-bearing cell model. Experiments were carried out in 96-well cell culture-treated plates in the presence of rFVIIa (6nM) with (100nM) or without FVII, and clotting was activated by fibroblast cells grown on the bottom of each well. Means and SD are shown for n = 5 experiments. Data from individual experiments are presented in supplemental Figure 7. (A) Dependence of thrombin peak height on the presence of FVII activated at different cell numbers. Individual cell counts per well ranged from minimal (approximately 1-5 cells/well), low (approximately 100-500 cells/well), medium (approximately 1000-5000 cells/well), to high (approximately 10 000-50 000 cells/well). *Statistically significant difference between presence of FVII (0 or 100nM; P < .05 by paired t test). (B) Dependence of clot time on the presence of FVII in the same experiments shown in panel A.
High cell count prevents presence of zymogen inhibition in a TF-bearing cell model. Experiments were carried out in 96-well cell culture-treated plates in the presence of rFVIIa (6nM) with (100nM) or without FVII, and clotting was activated by fibroblast cells grown on the bottom of each well. Means and SD are shown for n = 5 experiments. Data from individual experiments are presented in supplemental Figure 7. (A) Dependence of thrombin peak height on the presence of FVII activated at different cell numbers. Individual cell counts per well ranged from minimal (approximately 1-5 cells/well), low (approximately 100-500 cells/well), medium (approximately 1000-5000 cells/well), to high (approximately 10 000-50 000 cells/well). *Statistically significant difference between presence of FVII (0 or 100nM; P < .05 by paired t test). (B) Dependence of clot time on the presence of FVII in the same experiments shown in panel A.
Phospholipid-dependent action of rFVIIa is independent in our zymogen-inhibition model
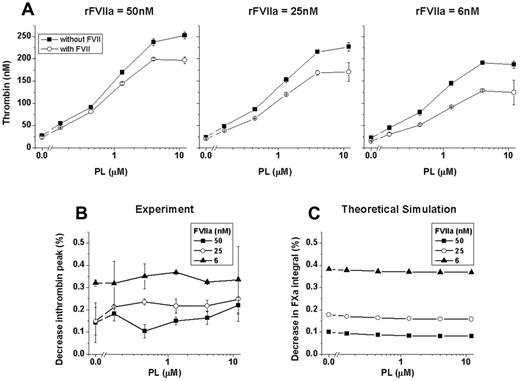
To investigate the role of TF-independent rFVIIa action in our model, we studied the effect of phospholipid concentration under the optimal conditions we found for the presence of zymogen inhibition. Synthetic procoagulant phospholipid vesicles (0-12μM) in the presence of rFVIIa (6, 24, and 50nM) and activated by low rTF concentration (1pM) led to a dose-dependent increase in both the presence (100nM) and absence of FVII (Figure 5A). However, the relative inhibitory effect of FVII, the ratio of TG peak heights in the presence of FVII to the absence of FVII, revealed no change as a function of phospholipid concentration (Figure 5B). Moreover, inhibition was stronger at lower rFVIIa concentrations, confirming our previous conclusion that the rFVIIa/FVII ratio determines the procoagulant action of rFVIIa. Simulations of these in vitro experiments were in good agreement (Figure 5C). Optimal conditions for zymogen inhibition in our model demonstrated that the phospholipid-dependent action of rFVIIa increased TG and, although it acted simultaneously, phospholipid-mediated rFVIIa action functioned independently from any TF-dependent contribution.
Phospholipid-mediated rFVIIa action functions independently from the TF-dependent mechanism. (A) Dependence of thrombin peak heights on phospholipid concentration in the presence of 50, 25, or 6nM rFVIIa with or without FVII (closed symbols indicate 0nM FVII; open symbols, 100nM FVII). Data shown are from averaged duplicate wells. Experiments were carried out in the presence of TF (1pM; Innovin). (B) Data from panel A are represented as the percentage decrease in thrombin peak value of FVII-supplemented plasma compared with FVII-free plasma. Decrease in thrombin peak caused by the presence of FVII depends on rFVIIa concentration regardless of the phospholipid concentration. (C) Theoretical simulation of the inhibitory effect of FVII on FXa production in the presence of phospholipids.
Phospholipid-mediated rFVIIa action functions independently from the TF-dependent mechanism. (A) Dependence of thrombin peak heights on phospholipid concentration in the presence of 50, 25, or 6nM rFVIIa with or without FVII (closed symbols indicate 0nM FVII; open symbols, 100nM FVII). Data shown are from averaged duplicate wells. Experiments were carried out in the presence of TF (1pM; Innovin). (B) Data from panel A are represented as the percentage decrease in thrombin peak value of FVII-supplemented plasma compared with FVII-free plasma. Decrease in thrombin peak caused by the presence of FVII depends on rFVIIa concentration regardless of the phospholipid concentration. (C) Theoretical simulation of the inhibitory effect of FVII on FXa production in the presence of phospholipids.
Synthesis of phospholipid- and TF-dependent theories
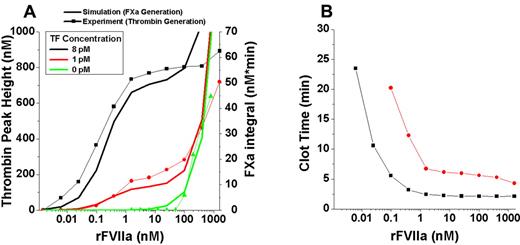
Because our model is capable of revealing both mechanisms of rFVIIa action simultaneously, we extended our TG experiments along the entire range of physiologic to suprapharmacologic rFVIIa doses to map when contributions of each mechanism become important for hemostasis. A range of rFVIIa doses (0-1600nM) was added to plasma supplemented with phospholipids, CTI, and activated with 0, 1, and 8pM TF (Figure 6A).
Range of TG responses to rFVIIa doses reveals independent contributions of TF-dependent and phospholipid-dependent mechanisms. FVII-deficient plasma was supplemented with phospholipids (4μM), CTI (50 μg/mL), and rFVIIa (0-1600nM) and activated with 0, 1, and 8pM TF (dotted lines). (A) Comparison of experimental TG (dotted lines) to theoretical simulations (solid lines). (B) Clot time (TF-initiated experiments from panel A) is insensitive to the upper supraphysiological dose range of rFVIIa.
Range of TG responses to rFVIIa doses reveals independent contributions of TF-dependent and phospholipid-dependent mechanisms. FVII-deficient plasma was supplemented with phospholipids (4μM), CTI (50 μg/mL), and rFVIIa (0-1600nM) and activated with 0, 1, and 8pM TF (dotted lines). (A) Comparison of experimental TG (dotted lines) to theoretical simulations (solid lines). (B) Clot time (TF-initiated experiments from panel A) is insensitive to the upper supraphysiological dose range of rFVIIa.
When TF was present, TG increased from 0-1nM rFVIIa and then TG plateaued until 50nM rFVIIa caused a sharp increase in TG that appeared to never saturate. Higher TF concentration increased the magnitude of TG compared with low TF, but did not affect the overall shape of the dose-response (rFVIIa vs TG peak height) curve. When this experiment was reproduced without phospholipids (supplemental Figure 10A lines with symbols), the TG signal was reduced by > 5×, as expected, but did not change the overall relative dose responses observed.
In the absence of TF, phospholipid-driven contributions of rFVIIa only increased TG sharply after 50nM rFVIIa was reached (Figure 6A). Interestingly, TF-initiated clot times were insensitive to the sharp increase in TG associated with the phospholipid-dependent effects of rFVIIa at concentrations above 50nM (Figure 6B). Clot times were slower without phospholipids and confirmed the insensitivity to phospholipid-dependent effects (supplemental Figure 10B). All mathematical simulations of FXa generation under the same conditions were well correlated with the observed in vitro data (Figure 6A and supplemental Figure 10A lines without symbols).
Discussion
Resolving the ongoing debate over which mechanism of rFVIIa action can explain the need for high doses is a necessary and critical step to both the justification and reevaluation of current NovoSeven dosing strategies. Even though similar experimental models were used, other investigations have differed on the existence of FVII zymogen inhibition of rFVIIa activity. To address this controversy, our experimental model was designed with conditions that would permit observation of zymogen inhibition. Consistent with the synthetic plasma models used previously, we used a FVII-deficient plasma model because it provides all of the coagulation enzymes and inhibitors but without the confounding presence of platelets and also permits the addition of FVII and rFVIIa over a wide range of concentrations, which is necessary to capture the upper and lower limits of the dose-response curve. Moreover, this plasma-based fluorogenic TG assay has been used to monitor rFVIIa activity in clinical settings6 and laboratory investigations.24,33 Although clot time is often used as the response measurement, we opted to use thrombin response in the present study because we found that clot time was a less-sensitive measure at high TF concentrations.
Using the thrombin peak height readout, we demonstrated the existence of zymogen inhibition of the TF-dependent action of rFVIIa. rFVIIa in the absence of FVII zymogen expressed both TF-dependent and phospholipid-dependent (TF-independent) activities, but the addition of FVII delayed the onset and decreased TG induced by rFVIIa only in the presence of TF. Our results for the effect of zymogen inhibition were in good agreement with the work done by van't Veer et al,17 especially when low-density TF-bearing phospholipid vesicles were used. Mechanistically, we designed a simple, focused kinetic model relying only on FXa generation (which avoids many extraneous parts of the coagulation model such as prothrombinase and intrinsic Xase reactions), which predicted that zymogen inhibition depends on only 2 reactions: (1) competition between FVII and rFVIIa for TF and (2) the activation of FVII to rFVIIa. Both reactions were confirmed using in vitro TG experiments.
Overcoming FVII zymogen inhibition of rFVIIa action has been used to explain the need for high doses of rFVIIa to establish hemostasis. Indeed, we found that a 25nM concentration of rFVIIa was needed to completely overcome FVII inhibition, which is equivalent to the approximate plasma concentration reached with the current recommended dose of 90 μg/kg. The apparent lack of inhibitory effect at doses above 25nM can be explained by rapid activation of FVII to FVIIa, which was confirmed by our mathematical simulations and FVIIai experiments. Furthermore, increasing doses of rFVIIa beyond 25nM was shown previously to saturate TF-induced TG.18 In good agreement, we found that doses above 10nM rFVIIa in the presence of lipidated TF did not produce any added hemostatic benefit. This suggests that dose increases would not immediately benefit a patient if the TF-dependent mechanism is predominant. Furthermore, the action of rFVIIa across all concentrations from 0.1 to > 100nM was proportional to the concentration of TF, suggesting that TF is important for rFVIIa to express its pharmacologic activity even when FVII zymogen does not inhibit it or TF is saturated.
However, rFVIIa alone in our model was also found to express activity in a phospholipid-dependent, TF-independent manner, supporting the theory that TF-independent activity of rFVIIa on the phospholipid surface of activated platelets may be another mode of drug action.19,34 Indeed, procoagulant phospholipids provided by platelets,22,35 platelet microparticles,24,35 or synthetic vesicles20,24 promoted rFVIIa action. In the absence of TF, only doses above 50nM rFVIIa were able to generate phospholipid-dependent TG in our model. The dose needed to increase TG in our study differs greatly from the minimum 5nM rFVIIa concentration found in the Hoffman cell-based model activated with lipopolysaccharide-treated monocytes,22 but does agree with the whole-blood experiments,20 suggesting differences between synthetic and whole blood–derived plasma models. Moreover, the lack of zymogen inhibition observed in the Hoffman cell-based model was interpreted to reinforce the theory of predominant platelet-dependent action of rFVIIa.22 In contrast, we found in our model that zymogen inhibition can be present when TF-bearing cells are used to activate TG, but if the available concentration of TF is too high, which reduces the competition between FVII and rFVIIa for TF, then zymogen inhibition disappears. In fact, TG can actually be greater when FVII is present at high enough TF concentrations, which is in good agreement with the 2 reactions deemed important for rFVIIa TF-dependent action. The high levels of TF expression by lipopolysaccharide-treated monocytes32,36 may be a logical explanation for the lack of observed zymogen inhibition in the Hoffman model.
Problematic experimental design as a result of the use of (unjustified) high TF concentrations to study zymogen inhibition,22 and the use of TF-initiated clot time to study phospholipid action18 appear to be the reasons that previous models were unable to agree on the relative contributions of each rFVIIa mechanism. The addition of increasing concentrations of procoagulant phospholipids at low TF concentrations in our model allowed the promotion of rFVIIa-induced TG, but did not overcome the relative effect of zymogen inhibition, suggesting that the 2 mechanisms are independent. As a result, we were able to map the contributions of both TF-dependent and phospholipid-dependent mechanisms over the entire range of rFVIIa doses.
Understanding the contributions of the mechanisms that underlie the procoagulant activity of rFVIIa is important for the interpretation of the pharmacologic action of the drug and subsequent selection of dosing strategies.10,11 The results of the present study support the view that the rFVIIa mechanism of current pharmacologic action is more TF driven than is often described.4,5,22 In our experiments, the inhibitory effects of FVII were observed below a 15nM concentration of rFVIIa, which lies within the concentration range of 10-25nM expected after injection of a 90 μg/kg dose.37 The existence of FVII-dependent inhibition of rFVIIa implies that the efficacy of the drug may depend on the amount of FVII in circulation because of the current dose acting in the TF-dependent range, but only if TF is exposed at low density. In the extreme cases of FVII-deficient patients, lower doses of rFVIIa38 should prove to be effective. In the hemophiliac population, the basal level of FVII may be a variable that contributes to the lack of procoagulant action of low doses of rFVIIa. Moreover, the short half-life of rFVIIa would result in the procoagulant activity of rFVIIa quickly dropping below the FVII inhibition threshold if the amount of exposed TF is limited. Conversely, we speculate that increasing the dose along the range of saturation plateau is not wasteful because it may prolong the action of rFVIIa beyond the half-life of approximately 2.5 hours.37,39 Increasing the dose in this manner would extend the drug's action without increasing the maximal hemostatic response and any potentially associated thrombogenic risk.
In apparent agreement, several exploratory studies in hemophilic and nonhemophiliac patients found comparable hemostatic response to escalating doses between as low as 40 and up to 200 μg/kg.1,3,4,12,40 In one study, the comparable hemostasis was observed within 1 day of repeated dosing every 2-6 hours, whereas the tendency to rebleed emerged at the lower compared with the higher doses when the injection interval was increased on days 3-6.41 However, if the injected dose were to surpass the saturation plateau of TF-dependent action, the platelet-dependent mechanism would become the predominant mode of action, resulting in dramatic increases of drug action. Doses at this level could provide better efficacy, but could potentially pose new thrombogenic safety concerns if our analysis is correct.
In light of recent clinical and preclinical development of novel rFVIIa analogs with enhanced activities, careful study of the dose-response profiles of each analog must be undertaken. Two such molecules reportedly increase catalytic activity on platelets,42,,–45 whereas some may increase TF-dependent activity,46 and yet others have been found to prolong the lifetime in the circulation.47,48 Each of these changes can potentially alter the therapeutic window of TF- and phospholipid-dependent actions of rFVIIa, leading to drastically different efficacy and safety profiles. Our model is one tool that can be used to compare the dose-response profiles of NovoSeven and new rFVIIa analogs.
In conclusion, in the present study, we describe the independent TF- and phospholipid-mediated contributions of rFVIIa action. It is important to note that the work presented herein, while in good agreement with previous studies and detailed knowledge of the kinetics of blood coagulation, represents phenomena described in vitro only. Unfortunately, no formal dose-finding studies have yet been performed on NovoSeven.4 Therefore, the present work provides a basis for further investigation by animal and clinical studies to fully describe the hemostatic action of the drug.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Mark Weinstein, Charles Maplethorpe, Andrey G. Sarafanov, and Roman Drews for helpful discussions on the mechanisms of FVIIa action, and Dr Alireza R. Rezaie for providing us with soluble TF.
This study was supported in part by postgraduate and postbaccalaureate research fellowship awards to A.M.S. and S.A.W. from the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration.
A.M.S., S.A.W., T.K.L., and M.V.O. are employees of the US Food and Drug Administration (FDA). The findings and conclusions in this presentation have not been formally disseminated by the FDA and should not be construed to represent any agency determination or policy.
Authorship
Contribution: A.M.S. conducted most of the experimental and all of the theoretical studies and developed the mathematical model; S.A.W. and M.V.O. conducted the remaining thrombin-generation studies; A.M.S., S.A.W., and M.V.O. wrote the manuscript with assistance from T.K.L.; and M.V.O. supervised the project and the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mikhail V. Ovanesov, PhD, US Food and Drug Administration, 29 Lincoln Dr, N29/306, Bethesda, MD 20892; e-mail: mikhail.ovanesov@fda.hhs.gov.