Initial observations suggested that C-C motif chemokines exclusively mediate chemotaxis of mononuclear cells. In addition, recent studies also implicated these chemotactic cytokines in the recruitment of neutrophils. The underlying mechanisms remained largely unknown. Using in vivo microscopy on the mouse cremaster muscle, intravascular adherence and subsequent paracellular transmigration of neutrophils elicited by the chemokine (C-C motif) ligand 3 (CCL3, synonym MIP-1α) were significantly diminished in mice with a deficiency of the chemokine (C-C motif) receptor 1 (Ccr1−/−) or 5 (Ccr5−/−). Using cell-transfer techniques, neutrophil responses required leukocyte CCR1 and nonleukocyte CCR5. Furthermore, neutrophil extravasation elicited by CCL3 was almost completely abolished on inhibition of G protein–receptor coupling and PI3Kγ-dependent signaling, while neutrophil recruitment induced by the canonical neutrophil attractants chemokine (C-X-C motif) ligand 1 (CXCL1, synonym KC) or the lipid mediator platetelet-activating factor (PAF) was only partially reduced. Moreover, Ab blockade of β2 integrins, of α4 integrins, or of their putative counter receptors ICAM-1 and VCAM-1 significantly attenuated CCL3-, CXCL1-, or PAF-elicited intravascular adherence and paracellular transmigration of neutrophils. These data indicate that the C-C motif chemokine CCL3 and canonical neutrophil attractants exhibit both common and distinct mechanisms for the regulation of intravascular adherence and transmigration of neutrophils.
Introduction
Leukocyte recruitment from the microvasculature to the site of inflammation is a hallmark in the inflammatory response. This highly regulated multistep process requires the coordinated interplay of chemoattractants, signaling molecules, and adhesion receptors ultimately controlling intravascular rolling and firm adherence as well as transmigration of leukocytes to the inflamed tissue.1,,–4 Although the general principles of leukocyte extravasation have been studied in detail in the past decades, the mechanisms underlying subtype-specific leukocyte responses remain incompletely understood.
Chemokines are small molecules (8-14 kDa) which can be classified into C, C-C, C-X-C, and C-X3-C motif chemokines according to the arrangement of their N-terminal cysteine residues. Interaction of chemokines with specific G protein–coupled chemokine receptors is thought to activate intracellular signaling pathways ultimately mediating adhesion and directed migration of leukocytes.5,–7
According to our current knowledge, spatiotemporal expression patterns of chemokines, as well as expression of specific chemokine receptors on different leukocyte subtypes, are suggested to enable the guidance of defined leukocyte subsets to the site of inflammation. In this context, neutrophils are thought to be attracted by C-X-C motif chemokines (eg, CXCL1/KC, CXCL2/MIP-2), whereas C-C motif chemokines (eg, CCL3/MIP-1α) are supposed to primarily mediate the migration of mononuclear cells.3,5,–7 In addition to this well-known function, however, there is increasing evidence that C-C motif chemokines are also involved in the migration of granulocytes including eosinophils,8,9 basophils,10,11 and, in particular, neutrophils.12,,,–16 These reports reveal a previously unrecognized role of these chemotactic cytokines in the inflammatory response. The exact mechanisms underlying neutrophil recruitment by C-C motif chemokines are still unknown.
Recently, C-C motif chemokine receptors including CCR1 and CCR5 have been identified on the surface of neutrophils.17,,,,–22 Notably, these chemokine receptors are also present on a variety of resident cell populations known to promote extravasation of neutrophils such as endothelial cells,23,24 smooth muscle cells,25 or mast cells.26,27 The relative contribution of these receptors to the recruitment of neutrophils has not yet been investigated. Moreover, the relevant signaling and adhesion events involved in C-C motif chemokine-dependent neutrophil responses are largely unexplored.
Here, we demonstrate that CCL3-dependent intravascular firm adherence and subsequent paracellular transmigration of neutrophils require both C-C motif chemokine receptor CCR1 and CCR5. In this context, leukocyte CCR1 and nonleukocyte CCR5 are engaged regulating C-C motif chemokine-dependent neutrophil responses via G protein–receptor coupling as well as PI3Kγ-dependent signaling pathways. As a consequence, firm adherence to the vascular endothelium is particularly mediated by the β2 integrins lymphocyte function-associated Ag-1 (CD11a/LFA-1) and macrophage-1 Ag (CD11b/Mac-1), to a lesser degree by α4 integrins (α4β1 integrin/VLA-4 and/or α4β7 integrin) as well as by their (putative) counter receptors intercellular adhesion molecule-1 (CD54/ICAM-1) and vascular cell adhesion molecule-1 (CD106/VCAM-1), whereas subsequent paracellular transmigration of neutrophils is facilitated through platelet/endothelial cell adhesion molecule-1 (CD31/PECAM-1) and intercellular adhesion molecule-2 (CD102/ICAM-2).
Methods
Animals
BALB/cAnNCrl (briefly BALB/c) mice were obtained from Charles River. Ccr1-deficient mice (Ccr1tm1Gao) and Ccr5-deficient mice (Ccr5tm1Blck) have been generated as described28,29 and backcrossed for 10 (Ccr1) or 13 generations (Ccr5) to the BALB/c background. Experiments were performed exclusively with male mice at the age of 20 ± 5 weeks. Mice were raised in individually ventilated cages under specific pathogen-free conditions and housed during the experiments under conventional conditions with free access to food and water. All experiments were performed in compliance with the German legislation for the protection of animals and were approved by the Regierung von Oberbayern (government of Upper Bavaria).
Musculus cremaster assay
The surgical preparation of the cremaster muscle was performed as originally described by Baez with minor modifications.30,31 Mice were anesthetized using a ketamine/xylazine mixture (100 mg/kg ketamine and 10 mg/kg xylazine), administrated by IP injection. The left femoral artery was cannulated in a retrograde manner for administration of microspheres and drugs (see “Quantification of leukocyte kinetics and microhemodynamic parameters”). The right cremaster muscle was exposed through a ventral incision of the scrotum. The muscle was opened ventrally in an avascular zone, using careful electrocautery to stop any bleeding, and spread over the transparent pedestal of a custom-made microscopy stage. Epididymis and testicle were detached from the cremaster muscle and placed into the abdominal cavity. Throughout the procedure as well as after surgical preparation during in vivo microscopy, the muscle was superfused with warm-buffered saline.
In vivo microscopy
The setup for in vivo microscopy was centered around an Olympus BX 50 upright microscope (Olympus Microscopy), equipped for stroboscopic fluorescence epiillumination microscopy. Light from a 75-W xenon source was narrowed to a near-monochromatic beam of a wavelength of 700 nm by a galvanometric scanner (Polychrome II; TILL Photonics) and directed onto the specimen via a FITC filter cube equipped with dichroic and emission filters (DCLP 500, LP515; Olympus). Microscopy images were obtained with Olympus water immersion lenses (20×/numerical aperture [NA] 0.5 and 10×/NA 0.3) and recorded with an analog black-and-white charge-coupled device video camera (Cohu) and an analog video recorder (Panasonic). Oblique illumination was obtained by positioning a mirroring surface (reflector) directly below the specimen and tilting its angle relative to the horizontal plane. The reflector consisted of a round cover glass (thickness, 0.19-0.22 mm; diameter, 11.8 mm), which was coated with aluminum vapor (Freichel) and brought into direct contact with the overlying specimen as described previously.31 For measurement of centerline blood flow velocity, green fluorescent microspheres (2-μm diameter; Molecular Probes) were injected via the femoral artery catheter, and their passage through the vessels of interest was recorded using the FITC filter cube under appropriate stroboscopic illumination (exposure, 1 ms; cycle time, 10 ms; λ = 488 nm), integrating video images for sufficient time (> 80 ms) to allow for the recording of several images of the same bead on one frame. Beads that were flowing freely along the centerline of the vessels were used to determine blood flow velocity (see next section).
Quantification of leukocyte kinetics and microhemodynamic parameters
For offline analysis of parameters describing the sequential steps of leukocyte extravasation, we used the Cap-Image image analysis software (Dr Zeintl). Rolling leukocytes were defined as those moving slower than the associated blood flow and quantified as described previously. Firmly adherent cells were determined as those resting in the associated blood flow for > 30 seconds and related to the luminal surface per 100-μm vessel length. Transmigrated cells were counted in regions of interest (ROI), covering 75 μm on both sides of a vessel over 100-μm vessel length. By measuring the distance between several images of one fluorescent bead under stroboscopic illumination, centerline blood flow velocity was determined. From measured vessel diameters and centerline blood flow velocity, apparent wall shear rates were calculated, assuming a parabolic flow velocity profile over the vessel cross-section.
Quantification of fluorescent leukocyte responses
To investigate the contribution of leukocyte and nonleukocyte target proteins to CCL3-elicited leukocyte responses, a cell-transfer technique was used as described previously.32,33 Briefly, bone marrow leukocytes were isolated from donor mice by flushing the femur and tibia bones with PBS. Cells were then sieved and counted, resuspended in PBS containing BSA (0.25%), and incubated with calcein-AM (Molecular Probes; 10μM final concentration at 37°C for 30 minutes) as well as in separate experiments also with pertussis toxin (PTx), wortmannin, or drug vehicle. After 2 washes, the cells were injected intravenously into recipient mice via the right jugular vein (107 cells/mouse) 120 minutes before the surgical preparation. Fluorescent cells were counted in 175 high-power fields (HPF) per animal, this being equivalent to the total quantifiable area of an exteriorized cremaster muscle in the present studies. Results are shown as the number of adherent or transmigrated calcein-labeled cells/HPF.
Reagents
A nonblocking Alexa Fluor 488–conjugated anti–PECAM-1 mAb (clone 390; 40 μg/mL, applied into the superfusion solution; BioLegend) was used to delineate endothelial junctions. Clodronate liposomes (injection into the tail vein 48 hours [200 μL], 24 hours [100 μL], and 6 hours [100 μL] before the experiment; VUmc FdG) were prepared as described elsewhere and used to deplete monocytes and macrophages according to previous protocols.34,35 Recombinant murine CCL3, CXCL1 (300 ng in 0.4 mL of PBS; intrascrotally; R&D Systems), or platelet-activating factor (PAF; 0.4 mL of 10−6M solution, intrascrotally; Sigma-Aldrich) were used to induce leukocyte extravasation. Blocking anti–LFA-1 mAb (clone M17/4), anti–Mac-1 mAb (clone M1/70), anti-α4 integrin mAbs (blocking α4β1 integrin/VLA-4 and α4β7 integrin; clone R1-2 and clone PS/2), anti–PECAM-1 mAb (clone MEC 13.3), anti–ICAM-1 mAb (clone YN1), anti–ICAM-2 mAb (clone 3C4), and anti–VCAM-1 mAb (clone 429; 50 μg in 150 μL of saline, i.a.; BioLegend) were used to inhibit interactions with the respective adhesion molecules, and the nonblocking anti–PECAM-1 mAb (clone 390; 50 μg in 150 μL of saline, intra-arterially; BioLegend) was used as additional control. PTx (4 μg in 150 μL of saline, intra-arterially; Sigma-Aldrich) was used to inhibit G protein–receptor coupling. Wortmannin (irreversible pan-PI3K inhibitor; 1 mg/kg BW, intra-arterially; Sigma-Aldrich), PI103 (reversible pan-PI3K inhibitor; 3 × 5 mg/kg BW intraperitoneally), AS605240 (reversible PI3Kγ inhibitor; 3 × 5 mg/kg BW IP), and IC87114 (reversible PI3Kδ inhibitor; 3 × 5 mg/kg BW intraperitoneally) were used to inhibit PI3K activity.
Experimental groups
Animals were assigned randomly to the following groups: PBS-treated wild-type (WT) control mice as well as WT, Ccr1−/−, and Ccr5−/− mice undergoing intrascrotal stimulation with murine recombinant CCL3 (180 minutes; n = 6). Moreover, leukocyte responses were analyzed in the cremaster muscle of WT mice receiving fluorescent leukocytes from WT, Ccr1−/−, or Ccr5−/− donors as well of Ccr1−/− and of Ccr5−/− mice receiving fluorescent leukocytes from WT donors undergoing intrascrotal stimulation with CCL3 (180 minutes; n = 7 each group). Leukocyte responses were also analyzed in the cremaster muscle of WT mice treated with PTx, wortmannin, or drug vehicle receiving fluorescent leukocytes from WT mice coincubated with PTx, wortmannin, or drug vehicle undergoing intrascrotal stimulation with CCL3 (180 minutes; n = 4 each group).
In addition, experiments were conducted in PBS-treated WT control mice as well as in WT mice treated with PTx, with PI3K inhibitors PI103, AS605240, IC87114, with an anti–LFA-1 mAb, an anti–Mac-1 mAb, anti-α4 integrin mAbs (clone R1-2 and clone PS/2), anti–PECAM-1 mAbs (clone Mec13.3 and clone 390), an anti–ICAM-1 mAb, an anti–ICAM-2 mAb, an anti–VCAM-1 mAb, or with a respective isotype control Ab/drug vehicle undergoing intrascrotal stimulation with CCL3, CXCL1, or PAF (180 minutes; n = 4 each group). Furthermore, experiments were performed in WT animals receiving clodronate liposomes or control PBS liposomes undergoing intrascrotal stimulation with CCL3, CXCL1, or PAF (180 minutes; n = 4 each group). Finally, leukocyte transmigration routes were analyzed in the cremaster muscle of WT animals receiving a nonblocking Alexa Fluor 488–conjugated anti–PECAM-1 mAb (clone 390) undergoing intrascrotal stimulation with CCL3, CXCL1, or PAF (180 minutes; n = 3 each group).
Experimental protocols
For the analysis of CCL3-, CXCL1-, and PAF-dependent leukocyte responses, leukocyte recruitment to the cremaster muscle was induced by intrascrotal injection of recombinant murine CCL3, CXCL1, or PAF. After 180 minutes, 5 vessel segments were randomly chosen in a central area of the spread-out cremaster muscle among those that were at least 150 μm away from neighboring postcapillary venules and did not branch over a distance of at least 150 μm. Abs/inhibitors were applied 5 minutes before the intrascrotal injection of inflammatory mediators (see “Reagents”). After having obtained recordings of migration parameters, blood flow velocity was determined as described in “Quantification of leukocyte kinetics.” After in vivo microscopy, tissue samples of the cremaster muscle were taken for immunohistochemistry (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Blood samples were collected by cardiac puncture for the determination of systemic leukocyte counts using a Coulter ACT counter (Coulter Corp). Anesthetized animals were then killed by bleeding to death.
Statistics
Data analysis was performed with a statistical software package (SigmaStat for Windows; Jandel Scientific). The ANOVA on ranks test followed by the Student-Newman-Keuls test was used for the estimation of stochastic probability in intergroup comparisons. Mean values and SEM are given. P values < .05 were considered significant.
Mouse gene nomenclature
The officially approved gene symbols from the Mouse Genome Informatics Database are used throughout this report.
Results
Role of CCR1 and CCR5 in CCL3-elicited leukocyte responses
Using near-infrared transillumination in vivo microscopy, the role of the chemokine receptors CCR1 and CCR5 for chemokine-dependent rolling, firm adherence, and transmigration of leukocytes were analyzed in the mouse cremaster muscle 3 hours after intrascrotal injection of CCL3 (Figure 1A).
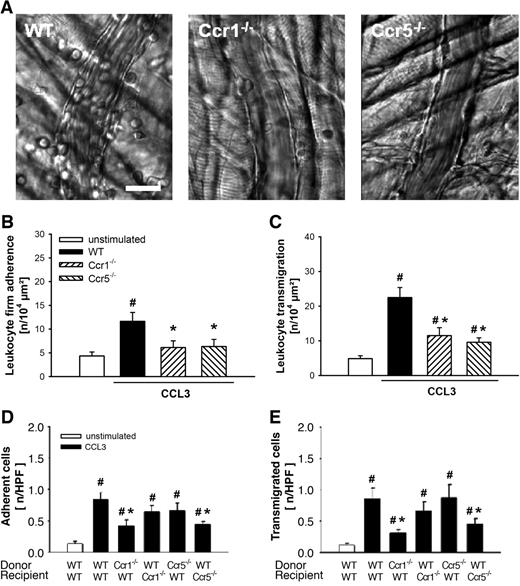
Role of CCR1 and CCR5 for CCL3-elicited leukocyte responses. (A) Representative RLOT in vivo microscopy images of postcapillary venules in WT, Ccr1-, and Ccr5-deficient mice after stimulation with CCL3 (scale bar: 20 μm). Leukocyte intravascular firm adherence and transmigration were quantified in postcapillary venules of the cremaster muscle as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” (B-C) Results for PBS-treated WT control mice as well as for WT, Ccr1-, and Ccr5-deficient mice after stimulation with CCL3 (mean ± SEM for n = 6 per group; #P < .05, vs unstimulated; *P < .05, vs WT). (D) Intravascular adherence and (E) transmigration of fluorescence-labeled bone marrow leukocytes were quantified in the cremaster muscle using in vivo fluorescence microscopy as detailed in “Quantification of florescent leukocyte responses.” Panels show results for WT mice receiving leukocytes from WT, Ccr1-, or Ccr5-deficient donors as well as for Ccr1- and Ccr5-deficient mice receiving leukocytes from WT donors after stimulation with CCL3 (mean ± SEM for n = 7 per group; #P < .05, vs unstimulated; *P < .05, vs WT→WT).
Role of CCR1 and CCR5 for CCL3-elicited leukocyte responses. (A) Representative RLOT in vivo microscopy images of postcapillary venules in WT, Ccr1-, and Ccr5-deficient mice after stimulation with CCL3 (scale bar: 20 μm). Leukocyte intravascular firm adherence and transmigration were quantified in postcapillary venules of the cremaster muscle as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” (B-C) Results for PBS-treated WT control mice as well as for WT, Ccr1-, and Ccr5-deficient mice after stimulation with CCL3 (mean ± SEM for n = 6 per group; #P < .05, vs unstimulated; *P < .05, vs WT). (D) Intravascular adherence and (E) transmigration of fluorescence-labeled bone marrow leukocytes were quantified in the cremaster muscle using in vivo fluorescence microscopy as detailed in “Quantification of florescent leukocyte responses.” Panels show results for WT mice receiving leukocytes from WT, Ccr1-, or Ccr5-deficient donors as well as for Ccr1- and Ccr5-deficient mice receiving leukocytes from WT donors after stimulation with CCL3 (mean ± SEM for n = 7 per group; #P < .05, vs unstimulated; *P < .05, vs WT→WT).
It is well known that surgical preparation of the cremaster muscle induced leukocyte rolling in postcapillary venules. No significant differences were observed in numbers of rolling leukocytes among all experimental groups (data not shown).
In contrast, the number of firmly adherent leukocytes (11.6 ± 1.9 μm2) was significantly increased on stimulation with CCL3 compared with PBS-treated control animals (4.3 ± 0.9 μm2). This increase was almost completely abolished in animals lacking the chemokine receptor Ccr1 (6.1 ± 1.4 μm2) or Ccr5 (6.3 ± 1.5 μm2; Figure 1B).
Moreover, there was a significant elevation in numbers of transmigrated leukocytes (22.5 ± 2.9 μm2) in response to CCL3 compared with controls (4.9 ± 0.8 μm2). This elevation was significantly attenuated in Ccr1−/− (11.5 ± 2.3 μm2) and Ccr5−/− (9.6 ± 1.3 μm2) animals (Figure 1C).
Role of leukocyte versus nonleukocyte CCR1 and CCR5 in CCL3-elicited leukocyte responses
To enable investigations into the contributions of leukocyte and nonleukocyte chemokine receptors in CCL3-elicited leukocyte responses, bone marrow leukocytes were isolated from WT, Ccr1−/−, or Ccr5−/− donor mice, fluorescently labeled with calcein AM, and injected intravenously into WT, Ccr1−/−, or Ccr5−/− recipient mice. Three hours after intrascrotal injection of CCL3, the number of fluorescent cells adherent or transmigrated was quantified in the cremaster muscle by in vivo fluorescence microscopy.
In unstimulated control mice, only a few fluorescent cells were found attached to the wall of the postcapillary venules (0.14 ± 0.03/HPF) or within the perivascular tissue (0.12 ± 0.02/HPF). In contrast, stimulation with CCL3 caused a significant elevation in numbers of adherent (0.84 ± 0.11/HPF; Figure 1D) and transmigrated (0.86 ± 0.17/HPF; Figure 1E) fluorescent cells. This elevation in the number of adherent and transmigrated fluorescent cells was significantly diminished in WT animals receiving Ccr1−/− cells (0.42 ± 0.09/HPF; 0.31 ± 0.06/HPF) as well as in Ccr5−/− animals receiving WT cells (0.44 ± 0.06/HPF; 0.45 ± 0.10/HPF), respectively.
Role of G protein–receptor coupling and PI3K activation for CCL3-elicited leukocyte responses
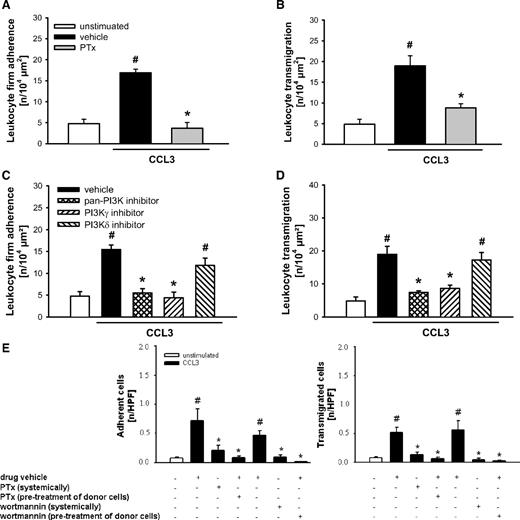
Interaction of chemokines to their cognate receptors is supposed to activate intracellular G proteins. To explore the functional relevance of these processes for CCL3-elicited leukocyte responses, another series of experiments was performed. Using reflected light oblique transillumination (RLOT) in vivo microscopy, CCL3-elicited intravascular firm adherence and (subsequent) transmigration of leukocytes were nearly abolished in animals treated with PTx (a compound blocking G protein– receptor coupling; Figure 2A-B), whereas leukocyte rolling was not significantly altered (data not shown). Furthermore, treatment with a reversible pan-PI3K inhibitor or a specific PI3Kγ inhibitor, but not with a PI3Kδ inhibitor, almost completely abrogated CCL3-elicited leukocyte responses (Figure 2C-D). Using a cell transfer technique, we found that stimulation with CCL3 caused a significant increase in the numbers of adherent and transmigrated fluorescent cells compared with controls (Figure 2E). This increase was almost completely abolished in animals treated systemically with PTx or the irreversible pan-PI3K inhibitor wortmannin as well as in animals receiving WT donor cells pretreated with PTx or wortmannin.
Role of G protein–receptor coupling and PI3K for CCL3-elicited leukocyte responses. (A,C) Leukocyte firm adherence, and (B,D) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving PTx, the PI3K inhibitors PI103 (pan-PI3K), AS605240 (PI3Kγ), and IC87114 (PI3Kδ), or respective drug vehicle after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs vehicle). (E) Intravascular adherence and transmigration of fluorescence-labeled bone marrow leukocytes were quantified in the cremaster muscle using in vivo fluorescence microscopy as detailed in “Quantification of florescent leukocyte responses.” Panels show results for WT mice treated with PTx, the irreversible PI3K inhibitor wortmannin, or drug vehicle receiving leukocytes from WT donors pretreated with PTx, wortmannin, or drug vehicle after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs WT→WT + drug vehicle).
Role of G protein–receptor coupling and PI3K for CCL3-elicited leukocyte responses. (A,C) Leukocyte firm adherence, and (B,D) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving PTx, the PI3K inhibitors PI103 (pan-PI3K), AS605240 (PI3Kγ), and IC87114 (PI3Kδ), or respective drug vehicle after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs vehicle). (E) Intravascular adherence and transmigration of fluorescence-labeled bone marrow leukocytes were quantified in the cremaster muscle using in vivo fluorescence microscopy as detailed in “Quantification of florescent leukocyte responses.” Panels show results for WT mice treated with PTx, the irreversible PI3K inhibitor wortmannin, or drug vehicle receiving leukocytes from WT donors pretreated with PTx, wortmannin, or drug vehicle after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs WT→WT + drug vehicle).
Role of β2 and α4 integrins as well as of ICAM-1, ICAM-2, VCAM-1, and PECAM-1 in CCL3-elicited leukocyte responses
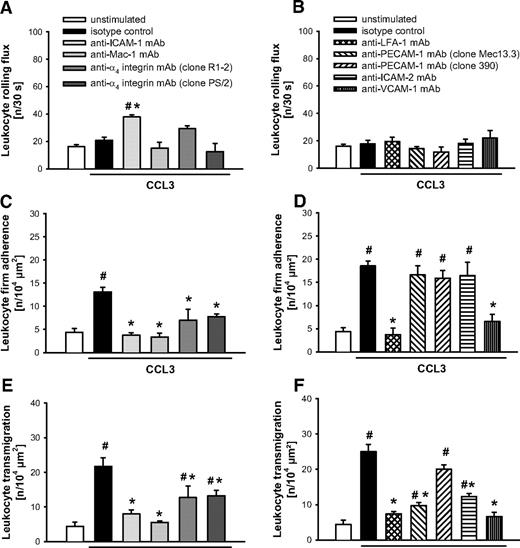
In a next step, candidate adhesion molecules involved in the leukocyte recruitment process were analyzed for their functional relevance in CCL3-dependent leukocyte responses. In our experiments, intravascular firm adherence (Figure 3C-D) and subsequent transmigration of leukocytes (Figure 3E-F) elicited by CCL3 were almost completely abolished on Ab blockade of the β2 integrins LFA-1 or Mac-1 compared with animals receiving isotype control or the nonblocking anti–PECAM-1 mAb “clone390,” whereas leukocyte rolling remained unaffected (Figure 3A-B). It is noteworthy that blockade of their putative counter receptor ICAM-1 nearly abolished intravascular firm adherence and transmigration of leukocytes and significantly enhanced the number of rolling leukocytes. In contrast, blockade of α4 integrins only slightly attenuated CCL3-elicited leukocyte responses, while blockade of its putative counter receptor VCAM-1 significantly diminished intravascular adherence and subsequent transmigration of leukocytes. Finally, blockade of PECAM-1 or of ICAM-2 selectively reduced transmigration of leukocytes on stimulation with CCL3 without affecting intravascular rolling or firm adherence of leukocytes.
Role of β2 and α4 integrins as well as PECAM-1, ICAM-1, ICAM-2, and VCAM-1 for CCL3-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against LFA-1, Mac-1, α4 integrins, PECAM-1 (clone Mec13.3), ICAM-1, ICAM-2, and VCAM-1 or isotype control and a nonblocking anti-PECAM-1 mAb (clone 390) after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Role of β2 and α4 integrins as well as PECAM-1, ICAM-1, ICAM-2, and VCAM-1 for CCL3-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against LFA-1, Mac-1, α4 integrins, PECAM-1 (clone Mec13.3), ICAM-1, ICAM-2, and VCAM-1 or isotype control and a nonblocking anti-PECAM-1 mAb (clone 390) after stimulation with CCL3 (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Role of G protein–receptor coupling and PI3K activation for CXCL1- or PAF-elicited leukocyte responses
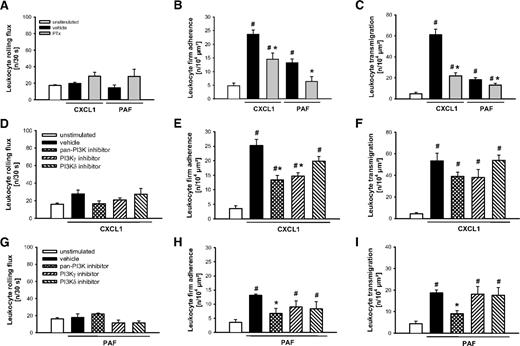
In further experiments, the mechanisms underlying neutrophil responses elicited by canonical neutrophil attractants were analyzed. On stimulation with the C-X-C motif chemokine CXCL1 or with the lipid mediator PAF, application of PTx only partially reduced intravascular adherence (Figure 4B) and (subsequent) transmigration of leukocytes (Figure 4C), while leukocyte rolling remained unaltered (Figure 4A). In addition, CXCL1-elicited intravascular adherence of leukocytes was only slightly attenuated in animals treated with the pan-PI3K inhibitor or the specific PI3Kγ inhibitor, but not with the PI3Kδ inhibitor, while leukocyte rolling and transmigration in response to CXCL1 were not significantly altered on treatment with the PI3K inhibitors (Figure 4D-F). Moreover, treatment with the pan-PI3K inhibitor, but not with the PI3Kγ or PI3Kδ inhibitor significantly reduced PAF-elicited leukocyte responses (Figure 4G-I).
Role of G protein–receptor coupling and PI3K for CXCL1- and PAF-elicited leukocyte responses. (A,D,G) Leukocyte rolling, (B,E,H) firm adherence, and (C,F,I) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving PTx, the PI3K inhibitors PI103 (pan-PI3K), AS605240 (PI3Kγ), and IC87114 (PI3Kδ), or respective vehicle after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs vehicle).
Role of G protein–receptor coupling and PI3K for CXCL1- and PAF-elicited leukocyte responses. (A,D,G) Leukocyte rolling, (B,E,H) firm adherence, and (C,F,I) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving PTx, the PI3K inhibitors PI103 (pan-PI3K), AS605240 (PI3Kγ), and IC87114 (PI3Kδ), or respective vehicle after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs vehicle).
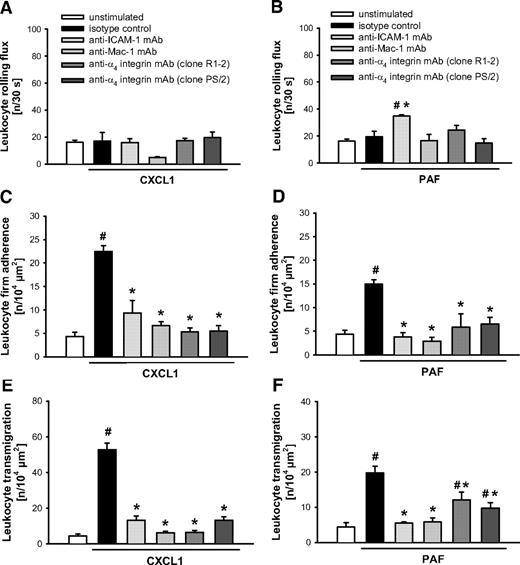
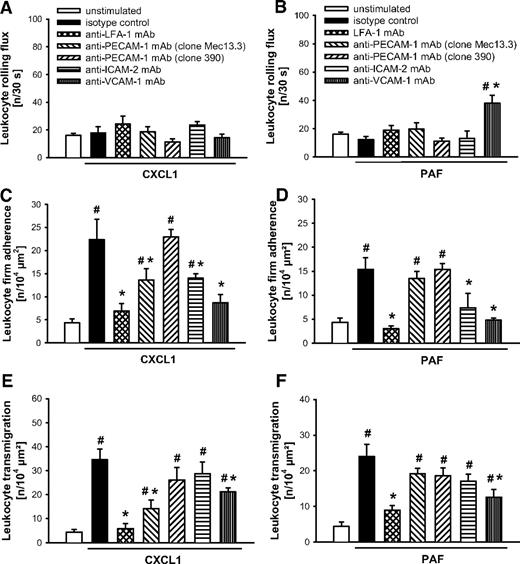
Role of β2 and α4 integrins as well as of ICAM-1, ICAM-2, VCAM-1, and PECAM-1 in CXCL1- or PAF-elicited leukocyte responses
On stimulation with CXCL1 (Figures 5A,C,E and 6A,C,E) or PAF (Figures 5B,D,F and 6B,D,F), intravascular firm adherence and subsequent transmigration of leukocytes were almost completely abolished on Ab blockade of the β2 integrins Mac-1 or LFA-1 compared with animals receiving isotype control or the nonblocking anti–PECAM-1 mAb “clone390,” whereas leukocyte rolling remained unchanged in these experiments. Interestingly, on stimulation with CXCL1 or PAF, blockade of ICAM-1 also almost completely abrogated firm adherence and transmigration of leukocytes, while only in PAF-elicited inflammation the number of rolling leukocytes was additionally enhanced. Furthermore, blockade of α4 integrins (α4β1 integrin/VLA-4 and/or α4β7 integrin) or of their putative counter receptor VCAM-1 attenuated CXCL1- and PAF-elicited leukocyte responses. Finally, blockade of PECAM-1 partially reduced intravascular adherence and transmigration of leukocytes on stimulation with CXCL1, but not in response to PAF. Interestingly, blockade of ICAM-2 significantly diminished intravascular firm adherence of leukocytes elicited by CXCL1 or PAF, but did not significantly alter leukocyte transmigration to the inflamed tissue.
Role of Mac-1, α4 integrins, and ICAM-1 for CXCL1- or PAF-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against Mac-1, α4 integrins, ICAM-1, or isotype control after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Role of Mac-1, α4 integrins, and ICAM-1 for CXCL1- or PAF-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against Mac-1, α4 integrins, ICAM-1, or isotype control after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Role of LFA, PECAM-1, ICAM-2, and VCAM-1 for CXCL1- or PAF-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against LFA-1, PECAM-1 (clone Mec13.3), ICAM-2, and VCAM-1 or isotype control and a nonblocking anti–PECAM-1 mAb (clone 390) after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Role of LFA, PECAM-1, ICAM-2, and VCAM-1 for CXCL1- or PAF-elicited leukocyte responses. (A-B) Leukocyte rolling, (C-D) firm adherence, and (E-F) transmigration were quantified in postcapillary venules of the cremaster muscle using RLOT in vivo microscopy as detailed in “Quantification of leukocyte kinetics and microhemodynamic parameters.” Panels show results for PBS-treated WT control mice as well as for WT mice receiving blocking mAbs directed against LFA-1, PECAM-1 (clone Mec13.3), ICAM-2, and VCAM-1 or isotype control and a nonblocking anti–PECAM-1 mAb (clone 390) after stimulation with CXCL1 or PAF (mean ± SEM for n = 4 per group; #P < .05, vs unstimulated; *P < .05, vs isotype control).
Transmigration routes of leukocytes in response to CCL3, CXCL1, or PAF
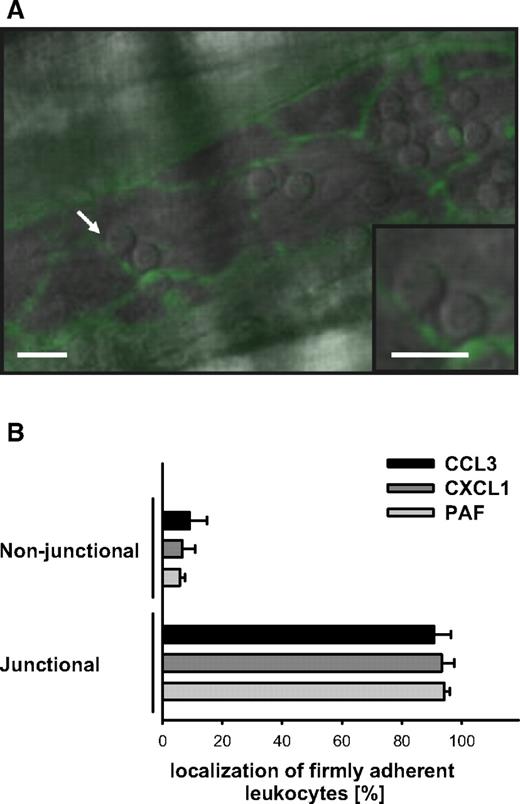
Transmigration of leukocytes occurs via the paracellular or the transcellular transmigration route. To characterize the transmigration route of extravasating leukocytes in CCL3-, CXCL1-, or PAF-elicited inflammation, endothelial boundaries in postcapillary venules of the cremaster muscle were visualized by fluorescence in vivo microscopy and, concomitantly, the localization of firmly arrested leukocytes was traced by using near-infrared RLOT in vivo microscopy (Figure 7A). Three hours after intrascrotal injection of CCL3 (90.8% ± 5.7%), CXCL1 (93.3% ± 4.2%), or PAF (94.2% ± 1.8%), firmly adherent leukocytes predominantly colocalized with PECAM-1–immunoreactive endothelial junctions (Figure 7B).
Transmigration routes in CCL3-, CXCL1-, and PAF-elicited leukocyte responses. (A) Representative in vivo microscopy image of a postcapillary venule in the inflamed cremaster muscle, immunostained for PECAM-1 (scale bars: 20 μm). Arrow indicates a firmly adherent leukocyte colocalizing with PECAM-1–immunoreactive endothelial junctions (green). (B) Results for the relative localization of firmly adherent leukocytes to PECAM-1–immunoreactive endothelial junctions in postcapillary venules of WT mice undergoing stimulation with CCL3, CXCL1, or PAF (mean ± SEM for n = 3 per group).
Transmigration routes in CCL3-, CXCL1-, and PAF-elicited leukocyte responses. (A) Representative in vivo microscopy image of a postcapillary venule in the inflamed cremaster muscle, immunostained for PECAM-1 (scale bars: 20 μm). Arrow indicates a firmly adherent leukocyte colocalizing with PECAM-1–immunoreactive endothelial junctions (green). (B) Results for the relative localization of firmly adherent leukocytes to PECAM-1–immunoreactive endothelial junctions in postcapillary venules of WT mice undergoing stimulation with CCL3, CXCL1, or PAF (mean ± SEM for n = 3 per group).
Phenotyping of transmigrated leukocytes
Phenotyping of transmigrated leukocytes was performed by immunostaining of paraffin-embedded tissue sections of the cremaster muscle. In response to CCL3, CXCL1, or PAF, > 80% of transmigrated CD45+ leukocytes were Ly-6G+ neutrophils. The remaining 10%-20% were F4/80+ monocytes/macrophages.
Effect of monocyte/macrophage depletion on CCL3-, CXCL1-, or PAF-elicited neutrophil responses
To characterize the role of monocytes/macrophages for CCL3-, CXCL1-, or PAF-elicited neutrophil responses, experiments were performed in animals receiving monocyte/macrophage-depleting clodronate liposomes. On stimulation with CCL3, CXCL1, or PAF, no significant differences in numbers of rolling, firmly adherent, or transmigrated leukocytes were detected among animals receiving clodronate liposomes or control PBS liposomes (supplemental Figure 1).
Effect of CCL3, CXCL1, or PAF on activation of endothelial cells
To further dissect the mechanisms underlying CCL3-, CXCL1-, or PAF-elicited neutrophil responses, the effect of inflammatory mediators on activation of endothelial cells was characterized. To measure endothelial cell activation, RNA expression of E-selectin was determined in cremaster muscle samples by using RT-PCR. On stimulation with CCL3, there was a strong increase in RNA expression of E-selectin (149.4% ± 15.4%) compared with PBS-treated controls. By contrast, RNA levels of E-selectin were not significantly altered on stimulation with CXCL1 (−4.3% ± 33.6%) and only slightly enhanced in response to PAF (10.0% ± 1.3%; supplemental Figure 2).
Systemic leukocyte counts and microhemodynamic parameters
To assure intergroup comparability, systemic leukocyte counts and microhemodynamic parameters including inner vessel diameter, blood flow velocity, and wall shear rate were analyzed in each experiment. No significant differences were detected among all experimental groups (supplemental Table 1).
Discussion
Leukocyte migration to the site of inflammation is a key event in innate and adaptive immunity.1,,–4 In this context, it is widely accepted that C-C motif chemokines, which represent 1 of the 4 major classes of chemokines, primarily govern the migration of mononuclear cells.3,5,–7 It is noteworthy that this assumption appears to be no longer valid as recent studies have clearly indicated that chemokines belonging to the C-C motif subfamily do also play a crucial role for the recruitment of eosinophils,8,9 basophils,10,11 and, in particular, neutrophils.12,,,–16 CCL3 binds to 2 closely related chemokine receptors designated CCR1 and CCR5. Recently, the chemokine receptors CCR1 and CCR5 have been detected on neutrophils.17,19,21,22 The relative contribution of both chemokine receptors to discrete steps of the CCL3-mediated extravasation of neutrophils is still unclear.
In a first approach, we therefore sought to evaluate the functional relevance of CCR1 and CCR5 for CCL3-dependent neutrophil responses. Using in vivo microscopy on the mouse cremaster muscle, we observed that CCL3-induced intravascular firm adherence followed by transmigration of neutrophils were significantly diminished in Ccr1- or Ccr5-deficient animals, whereas leukocyte rolling remained unaltered. Our findings extend previous studies demonstrating that deficiency/blockade of CCR1 or CCR5 was associated with reduced neutrophil recruitment in animal models of ischemia-reperfusion injury13,14,16 or in experimental aspergillosis,29 and convincingly demonstrate that both chemokine receptors play a significant role during the extravasation of neutrophils.
Interestingly, CCR1 and CCR5 are not only expressed on neutrophils and mononuclear cells as these chemokine receptors have previously been detected on the surface of resident cells (eg, endothelial cells,23,24 smooth muscle cells,25 or mast cells26,27 ). Binding of C-C motif chemokines to leukocyte chemokine receptors is supposed to elicit immediate affinity changes of surface-expressed integrins,3,5,–7 while in resident cells these chemotactic cytokines are known to primarily induce the generation of further leukocyte-attracting mediators.36,37 To which extent leukocyte and nonleukocyte chemokine receptors contribute to CCL3-mediated neutrophil recruitment remained to be determined. Using a cell-transfer technique, we found that leukocyte CCR1 and nonleukocyte CCR5 mediate CCL3-elicited intravascular adherence and subsequent transmigration of neutrophils to inflamed tissue. Moreover, we show that monocytes and macrophages are not involved in CCL3-dependent neutrophil responses. Collectively, our observations point to a complex interplay between chemokine receptors CCR1 in neutrophils and CCR5 in resident cells for the regulation of CCL3-dependent neutrophil recruitment.
Inflammatory stimuli individually use specific mechanisms for the control of the single steps of the leukocyte recruitment process. Which signaling and adhesion molecules C-C motif chemokines engage for the single steps of the neutrophil recruitment process, however, has not yet been investigated. Previous in vitro data suggest that ligand binding to a chemokine receptor on the cell surface causes allosteric changes of the receptor which, in turn, activates intracellular proteins of the G-protein family. These processes subsequently allow interaction of G proteins with different target proteins.3,5,–7 Here, we show that CCL3-induced intravascular adherence and subsequent transmigration of neutrophils strictly require the activation of PTx-sensitive G proteins. Of note, on stimulation with canonical neutrophil attractants such as the C-X-C motif chemokine CXCL1 or the lipid mediator PAF, treatment with PTx only partially inhibited neutrophil extravasation.
Activated G proteins interact with intracellular target proteins such as the catalytic subunit isoforms p110γ and p110δ of PI3K. These lipid kinases, in turn, produce phosphatidylinositol-3,4,5-trisphosphate (PIP3), an intracellular second messenger which is involved in a variety of cellular functions.38,39 In our experiments, we found that signaling through the catalytic subunit isoform p110γ, but not through the catalytic subunit isoform p110δ of PI3K controls CCL3-induced intravascular firm adherence and subsequent transmigration of neutrophils. Interestingly, neutrophil extravasation elicited by CXCL1 did not require PI3K, and might therefore be regulated via different signaling pathways. In contrast, PAF-elicited neutrophil recruitment was significantly attenuated on broad-spectrum blockade of PI3K, but not after specific inhibition of the catalytic subunit isoforms p110γ or p110δ. Consequently, neutrophil responses induced by PAF might primarily be regulated via PI3K catalytic subunit isoforms different from PI3Kγ or PI3Kδ (eg, by PI3Kα or PI3Kβ), which are supposed to be activated independently of G proteins.38,39 Collectively, these data lead us to the conclusion that receptor coupling of PTx-sensitive G proteins and activation of PI3Kγ are essential events for the initiation of the CCL3-elicited inflammatory response, while this signaling pathway is not critical for neutrophil recruitment mediated by the canonical neutrophil attractants CXCL1 or PAF.
Activation of neutrophils results in immediate affinity changes of surface-expressed integrins thereby regulating adhesion and migration of these inflammatory cells.1,–3 Which integrins C-C motif chemokines use for the recruitment of neutrophils is still unknown. In our experiments, we observed that, particularly, the β2 integrins Mac-1 and LFA-1 as well as their putative counter receptor ICAM-1 are critically involved in the regulation of CCL3-dependent intravascular adherence and subsequent transmigration of neutrophils. Moreover, neutrophil responses elicited by CXCL1 or PAF were also found to require Mac-1, LFA-1, and ICAM-1 underlining the elementary role of these proteins for the neutrophil recruitment process. Interestingly, blockade of ICAM-1 significantly enhanced leukocyte rolling on stimulation with CCL3 or PAF, while in CXCL1-elicited inflammation, leukocyte rolling was not significantly altered on blockade of Mac-1. These observations point to a stimulus-specific control of the initial steps in the leukocyte recruitment cascade. In this context, it should be noted that at present we have no clear answer to the question of whether ICAM-1 serves as a binding partner of Mac-1 and LFA-1 under these inflammatory conditions or whether these promiscuous integrins interact with one of their multiple other ligands (eg, ICAM-2, RAGE, or components of the complement system as well as of the fibrinolytic system for Mac-1; ICAM-2, ICAM-3, or JAM-A for LFA-1) in CCL3-, CXCL1-, or PAF-elicited neutrophil responses.
Astonishingly, we found that α4 integrins (α4β1 integrin/VLA-4 and/or α4β7 integrin, which are highly expressed in lymphocytes, but only weakly in neutrophils) participate in CCL3-, CXCL1-, and PAF-induced neutrophil extravasation. Moreover, it is interesting that blockade of their putative counter receptor VCAM-1 was also associated with significantly diminished intravascular adherence and subsequent transmigration of neutrophils elicited by CCL3, CXCL1, or PAF. Our results extend previously published observations. VLA-4 has been shown to mediate neutrophil recruitment in experimental peritonitis40 or vasculitis41 and VCAM-1 has been reported to control neutrophil infiltration in a model of myocardial ischemia reperfusion (I/R).42 These data highlight a formerly neglected role of these molecules in the innate immune response.
After their adhesion to endothelial cells, leukocytes either squeeze between adjacent endothelial cells or directly penetrate endothelial cells (paracellular vs transcellular transmigration route) to enter the perivascular space.1,2 In this report, we demonstrate that on stimulation with CCL3, CXCL1, or PAF, > 90% of firmly adherent leukocytes colocalized with endothelial junctions. These observations strongly suggest that under these inflammatory conditions (CCL3-, CXCL1-, or PAF-induced) neutrophils predominantly take the paracellular transmigration route to overcome the endothelial barrier. Our data agree with previous reports as the leukocyte transmigration route was not dependent on the inflammatory stimulus applied.43,44 Moreover, we found that PECAM-1 and ICAM-2 selectively mediate CCL3-elicited transmigration of neutrophils to the inflamed tissue extending previous results under different inflammatory conditions. In this context, ICAM-2 is suggested to act in an early stage of leukocyte transmigration whereas PECAM-1 (and other molecules such as JAM-A) are thought to be involved later in this sequential process.45 Interestingly, we found that PECAM-1 was engaged in the extravasation process of neutrophils already on the level of intravascular adherence on stimulation with CXCL1 while this molecule was not required for PAF-elicited neutrophil recruitment. Furthermore, ICAM-2 was dispensable for leukocyte transmigration in response to CXCL1 or PAF. These results are in line with previous observations demonstrating that PECAM-1 and ICAM-2 facilitate leukocyte transmigration elicited by the cytokine IL-1β, but not by TNF-α.45 A possible explanation for the stimulus-specific control of leukocyte recruitment might be that individual inflammatory mediators differentially activate distinct target cell populations involved in the leukocyte extravasation process thereby engaging different molecular mechanisms: while nonleukocyte cells (eg, endothelial cells, mast cells) strongly respond to stimulation with C-C motif chemokines, canonical neutrophil attractants such as C-X-C motif chemokines and lipid mediators serve as potent activators of neutrophils.37 From this perspective, the entirety of our experimental data also reveals that C-C motif chemokines, C-X-C motif chemokines, and lipid mediators exhibit both common and distinct mechanisms for the regulation of the neutrophil recruitment process.
In conclusion, our in vivo findings demonstrate that both CCR1 and CCR5 are critically involved in CCL3-dependent intravascular firm adherence and subsequent paracellular transmigration of neutrophils. In this context, leukocyte CCR1 and nonleukocyte CCR5 are engaged, initiating intracellular signal transduction through G protein–receptor coupling and activation of PI3Kγ. Subsequently, intravascular adherence is particularly mediated by the β2 integrins LFA-1 and Mac-1, to a lesser degree by α4 integrins as well as by their putative counter receptors ICAM-1 and VCAM-1, whereas paracellular transmigration of neutrophils is facilitated through PECAM-1 and ICAM-2. These findings corroborate the significance of C-C motif chemokines for neutrophil recruitment, provide a rationale of how these inflammatory mediators exert their effects, and identify these chemotactic cytokines as well as their receptors as potential therapeutic targets for the treatment of neutrophil-driven inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Schropp and G. Adams for technical assistance. Data presented in this manuscript are part of the doctoral thesis of D.P.-W.
This work was supported by Deutsche Forschungsgemeinschaft (DFG; RE 2885-1/1 [C.A.R.]; LU612/4-3 [B.L.]; SFB 914 [C.A.R., F.K.]) and Friedrich-Baur-Stiftung (C.A.R.).
Authorship
Contribution: C.A.R. designed and performed experiments, and contributed to data analysis, interpretation, and the writing of the manuscript; D.P.-W., G.Z., B.U., N.B., and S.Z. performed experiments and contributed to data analysis, interpretation, and the writing of the manuscript; M.P.W. and B.L. provided key reagents and contributed to data analysis, interpretation, and the writing of the manuscript; and F.K. contributed to data analysis, interpretation, and the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Christoph A. Reichel, Walter Brendel Centre of Experimental Medicine, Marchioninistr 15, D-81366 München, Germany; e-mail: christoph.reichel@med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal