Pioneering work by Janet Rowley and colleagues conclusively demonstrated that recurring chromosomal translocations are not merely the byproduct of cancer, but rather are initiating events. In particular, the (8;21) translocation, which was described as the first translocation in acute myeloid leukemia (AML) by Rowley in 1973,3 leads to expression of RUNX1-RUNX1T1 and suppression of endogenous RUNX1 function. t(8;21) is present in 5% to 8% of AML and in 15% to 20% of M2 AML cases and is now recognized by the World Health Organization classification scheme as its own subtype of AML. Previous studies have demonstrated that RUNX1-RUNX1T1 promotes self-renewal, disrupts terminal differentiation of myeloid cells, and increases DNA damage.4 However, expression of the fusion protein alone is not sufficient to promote leukemia from either human or murine hematopoietic progenitors and the pathways that it activates have been incompletely defined.
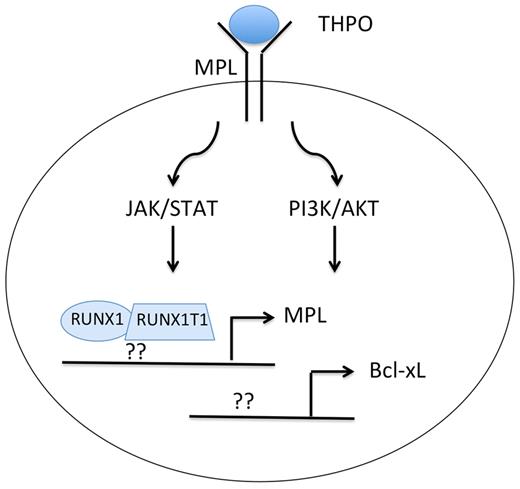
In an effort to better characterize the activity of RUNX1-RUNX1T1, Chou and colleagues expressed the fusion protein in human CD34+ cord blood progenitors, evaluated changes in gene expression, and then assayed self-renewal and survival of cells in vitro and in vivo.1 In the gene expression comparison, they noticed that RUNX1-RUNX1T1 led to increased expression of the antiapoptotic gene Bcl-xL. By knocking down Bcl-xL with shRNA, they discovered that persistent elevated expression is required for growth and survival of RUNX1-RUNX1T1 cells both ex vivo and in vivo. They also found that Bcl-xL is needed for sustaining the long-term culture potential of primitive clonogenic cells. Next, in the course of studying how Bcl-xL is up-regulated by RUNX1-RUNX1T1, they made the crucial observation that the fusion protein directly up-regulates expression of the thrombopoietin receptor MPL (see figure): RUNX1-RUNX1T1 expression led to a 15-fold increase in MPL mRNA and to noticeable increases in MPL and Bcl-xL protein levels. Consistent with a role for THPO signaling in survival and clonogenic expansion of RUNX1-RUNX1T1 cells, THPO withdrawal, MPL knockdown, or treatment with MPL-neutralizing antibodies led to increased apoptosis and decreased self-renewal. Furthermore, although RUNX1-RUNX1T1 cells engineered to overexpress Bcl-2 survived in the absence of THPO, they failed to expand in culture.
Expression of the RUNX1-RUNX1T1 fusion protein leads to up-regulation of MPL and Bcl-xL in AML with t(8;21). Whether the fusion protein directly binds and up-regulates expression of MPL and Bcl-xL or whether it suppresses expression of transcriptional repressors is unclear. Note that t(8;21) AML, unlike myeloproliferative neoplasms, requires THPO for enhanced JAK/STAT and PI3K/AKT signaling through wild-type MPL.
Expression of the RUNX1-RUNX1T1 fusion protein leads to up-regulation of MPL and Bcl-xL in AML with t(8;21). Whether the fusion protein directly binds and up-regulates expression of MPL and Bcl-xL or whether it suppresses expression of transcriptional repressors is unclear. Note that t(8;21) AML, unlike myeloproliferative neoplasms, requires THPO for enhanced JAK/STAT and PI3K/AKT signaling through wild-type MPL.
How do these results translate to human leukemia? Importantly, Chou et al found that t(8;21) leukemic blasts display significant up-regulation of MPL compared with non-t(8;21) blasts. They also witnessed higher MPL levels in blasts with t(8;21) than in those with a normal cytogenetic pattern. Finally, there was a positive correlation between MPL and Bcl-xL expression in t(8;21) blasts, but no correlation in blasts with normal karyotype. Overall, this study demonstrates that THPO/MPL/Bcl-xL signaling is required for RUNX1-RUNX1T1 cells to maintain a steady growth rate.
In a closely related paper, Pulikkan and colleagues identified THPO/MPL signaling as a key cooperating pathway in RUNX1-RUNX1T1–mediated leukemia.2 Beginning with the observation that MPL is up-regulated in human AML cells that harbor the RUNX1-RUNX1T1 fusion, they assessed the effects of overexpression of MPL alone or MPL with RUNX1-RUNX1T1 in murine hematopoietic progenitor cells. While MPL alone caused a transient expansion in hematopoietic progenitors and expression of RUNX1-RUNX1T1 alone led to leukemia in recipient mice with a median latency of 140 days, co-expression of the 2 genes induced a fully penetrant myeloid leukemia with a mean latency of 50 days. Leukemia cells were found to be hypersensitive to THPO and were characterized by enhanced PI3K/AKT, ERK, and JAK/STAT signaling. Subsequent experiments revealed that JAK2 and PI3K activities are required for survival of the cells in vitro, and that the PI3K pathway in particular is crucial for leukemia progression in vivo. Thus, this study demonstrates that wild-type MPL cooperates with RUNX1-RUNX1T1 to increase survival and tumorigenesis of hematopoietic cells and that the JAK2/PI3K/AKT signaling axis is critical for this effect.
In comparing the 2 studies, there is an interesting difference. Chou and colleagues observed coordinate expression of RUNX1-RUNX1T1 and MPL in both human umbilical cord progenitors and murine fetal liver cells, whereas Pulikkan et al did not detect up-regulation of MPL gene expression on RUNX1-RUNX1T1 expression in hematopoietic progenitor cells. Nevertheless, human AML cells with the t(8;21) indeed appear to express higher levels of MPL compared with other subtypes. Precisely how RUNX1-RUNX1T1 leads to increased MPL is thus an open question. Does the fusion protein directly bind to the MPL gene and up-regulate expression or does it repress a critical negative regulator of MPL transcription (see figure)? Or are there both transcriptional and posttranscriptional mechanisms at work?
Together, these exciting papers demonstrate that increased signaling through wild-type MPL is a crucial cooperating event in RUNX1-RUNX1T1–induced leukemogenesis. Although the MPL/THPO axis is primarily associated with megakaryopoiesis, MPL is expressed on hematopoietic stem cells and these cells may thus depend on JAK/STAT signaling.5,6 Activation of the JAK/STAT pathway through mutagenesis of JAK2, MPL, or LNK is associated with several hematologic malignancies, most notably the myeloproliferative neoplasms.7 Although activation of STAT3 is common in de novo AML, mutations in JAK2, MPL, and other genes in the pathway are rare.8 The observations provided in these reports by Chou et al and Pulikkan et al provide a mechanism by which increased expression of wild-type MPL can increase self-renewal and proliferation along with enhanced downstream signaling. These studies therefore provide incentives for rational testing of JAK, Bcl-xL, and PI3K/AKT inhibitors in AML cases with the t(8;21). These reports also beg the question of whether increased expression of wild-type MPL may contribute to myeloproliferative neoplasms that lack activating mutations in JAK2 or MPL.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal