Abstract
The Hedgehog (Hh) pathway is required for cell-fate determination during the embryonic life, as well as cell growth and differentiation in the adult organism, where the inappropriate activation has been implicated in several cancers. Here we demonstrate that Hh signaling plays a significant role in growth and survival of multiple myeloma (MM) cells. We observed that CD138+ MM cells express Hh genes and confirmed Smoothened (Smo)–dependent Hh signaling in MM using a novel synthetic Smo inhibitor, NVP-LDE225 (Novartis), which decreased MM cell viability by inducing specific down-regulation of Gli1 and Ptch1, hallmarks of Hh activity. In addition, we detected a nuclear localization of Gli1 in MM cells, which is completely abrogated by Forskolin, a Gli1-modulating compound, confirming Smo-independent mechanisms leading to Hh activation in MM. Finally, we identified that bone marrow stromal cells are a source of the Shh ligand, although they are resistant to the Hh inhibitor because of defective Smo expression and Ptch1 up-regulation. Further in vitro as well as in vivo studies showed antitumor efficacy of NVP-LDE225 in combination with bortezomib. Altogether, our data demonstrate activation of both canonical and noncanonical Hh pathway in MM, thus providing the rationale for testing Hh inhibitors in clinical trials to improve MM patient outcome.
Introduction
The Hedgehog (Hh) pathway regulates multiple processes involved in development and differentiation of tissues and organs during embryonic life.1 Recently, it has become evident that Hh signaling retains some activity even during adult life: in mature tissues, it regulates tissue homeostasis and repair and, in those tissues undergoing constant renewal, such as skin, colon, liver, and blood, it is also implicated in maintaining a stem/progenitor cell compartment,2,3 explaining how the Hh pathway deregulation may cause developmental defects during the embryonic life.1,4 Its abnormal activity can also lead to tumorigenesis during adult life either by stem cell pool expansion2,3 or mutations affecting the normal growth-regulatory mechanisms.5-7 An aberrant expression of developmental genes from Wnt and Hh pathways has been reported during malignant transformation of multiple myeloma (MM) cells.8 Several findings support a role of Hh signaling in regulating a stem cell niche also in MM9 and in modulating clinical response to conventional and novel therapeutic agents.10 Indeed, Hh ligands produced by murine bone marrow stromal cells (BMSCs) support growth and survival of human primary CD19+ lymphoma and CD138+ MM cells, demonstrating a role of the Hh pathway both in lymphoma and in terminally differentiated MM cells.11 Finally, we recently showed ciliary protein overexpression as a possible cause of constitutive and noncanonical Hh pathway activation, suggesting a cilia-dependent mode of Hh signaling in MM.12
Aberrant Hh signaling has been described in almost all tumors and is associated with 3 possible mechanisms: genetic alterations, autocrine and/or paracrine Hh activity, and alternative and synergistic pathways leading to Hh gene activation.13,14 Sonic (Shh), Desert (Dhh), and Indian (Ihh) hedgehog are the ligands for the pathway. The signaling is triggered by binding of endogenously or exogenously produced ligand to Patched1 (Ptch1) on target cells. This leads to inhibition of Ptch1 via cellular internalization and Smoothened (Smo) localization on the cell surface, both by a cilium-mediated mechanism.15 Smo activation leads to nuclear translocation of Glioma (Gli) transcription factors followed by expression of Gli target genes, including Ptch1 and Gli1, in a negative and positive feedback loop, respectively. The biologic effect is cell proliferation, with deregulation contributing to tumorigenesis. Abnormal Hh pathway activation occurs not only through ligand-dependent or receptor-induced signaling, also known as canonical Hh signaling, but also by mechanisms of activation downstream to Smo known as noncanonical or ligand-independent Hh signaling. Genetic alterations or ciliary protein overexpression leading to functional redundancy of Gli transcription factors, crosstalk between Hh signaling and unrelated pathways, are all causes of noncanonical Hh signaling activation.
In the present study, we first show Hh gene overexpression in CD138+ plasma cells (PCs) from persons with monoclonal gammopathy of undetermined significance (MGUS) compared with CD138+ PCs from healthy persons, MM and plasma cell leukemia (PCL) patients, suggesting that aberrant Hh activation is important in disease initiation. We further demonstrate that both canonical and Smo-dependent as well as noncanonical and Smo-independent mechanisms can contribute to Hh signaling activation in MM. Finally, we provide evidence that NVP-LDE225, a novel synthetic Smo antagonist currently in clinical development,16,17 decreases MM cell viability in vitro by inducing specific down-regulation of Gli1 and Ptch1, hallmarks of cell response to Hh pathway. Moreover, the combination of NVP-LDE225 with bortezomib in vitro has a modest additive effect compared with either drug alone, and the in vivo study shows an increased antitumor activity by the combination compared with bortezomib alone.
Methods
Microarray gene expression
The gene expression data were generated using our Affymetrix HG-U133A GeneChip Arrays (Affymetrix) from 4 healthy donors, 11 MGUS, 133 MM, and 9 PCL patients and from 23 human MM cell lines (HMCLs), obtained as previously described18 and deposited in National Center for Biotechnology Information's Gene Expression Omnibus (http://www.ncbi.nlm.mih.gov/geo) as accession no. GSE13591 and GSE6205, respectively. All samples were normalized and analyzed using the bioconductor function for Robust Multi-array Analysis19 in which perfect match intensities were background adjusted and normalized by means of quantile-quantile normalization. Gene expression levels were expressed as mean ± SEM. The statistical significance of differences among the groups was assessed using Kruskal-Wallis test. P values < .05 were considered significant.
Flow cytometry
Flow cytometry-based evaluation of Hh-protein expression was performed using a panel of MM cell lines and MM patient-derived BMSCs. U266, NCIH929, RPMI8226, and MM1R were obtained from ATCC. KMS12BM and KMS12PE were from German Collection of Microorganisms and Cell Cultures (DSMZ). MM1S was a gift from Steven Rosen (Northwestern University). OPM1 and OPM2 were provided by Dr P. Leif Bersagel (Mayo Clinic-Tucson). S6B45 and KMS11 were kindly provided by Dr T. Kishimoto (Osaka University) and the Kawasaki Medical School, respectively. All MM cells were cultured at 37°C in RPMI 1640 medium containing 10% heat inactivated FBS (Sigma-Aldrich), 2mM/L l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (Invitrogen). CD138− mononuclear cells obtained by microbead selection of primary MM samples were used to establish long-term BMSCs. To analyze the expression of Ptch1 and Smo receptors on MM cells, a single surface staining was performed by incubating MM cells in PBS/1% FBS in the presence of anti-Ptch1 or anti-Smo antibody (Ab) for 1 hour at 4°C. After washing, the cells were stained with specific secondary FITC-conjugated Ab for 45 minutes at 4°C and after final washes analyzed by a FACSCanto II flow cytometer (BD Biosciences). Cells stained with secondary Ab alone were used as negative controls to define background fluorescence and to gate the positive population. To confirm the specificity of Abs, we used 2 different primary Abs for each receptor: purified goat anti-Smo and rabbit anti-Ptch1 from Santa Cruz Biotechnology; and rabbit anti-Smo and anti-Ptch1 from Abcam. As secondary Abs, we used anti–rabbit FITC-conjugated (BD Biosciences) and antigoat AlexaFluor-488–conjugated (Invitrogen). To analyze Shh expression in the CD138+ population, we performed a double staining using anti-CD138 allophycocyanin (APC)–conjugated rat monoclonal Ab (R&D Systems) for surface staining followed after fixation with Cytofix/Cytoperm solution (BD Biosciences) for 30 minutes at 4°C, by intracellular staining with anti-Shh-PE–conjugated mouse monoclonal Ab (R&D System) in Perm/Wash buffer (BD Biosciences) for 1 hour at 4°C. After final washing, the cells were analyzed by flow cytometry. Isotype-matched control for both Abs (R&D Systems) was used as negative control. For the MM patient-derived BMSCs, single intracellular staining with anti-Shh PE-conjugated Ab was performed.
Immunoblot experiments
For Western blot analysis, MM cell lines and BMSCs from MM patients were harvested and lysed as previously described.12 For protein detection, we used anti-Smo and anti-Ptch1 Abs from Abcam and anti-Gli1Ab from Santa Cruz Biotechnology. Human heart lysate (Abcam) was used as positive control to detect Smo protein and lysate from HeLa and MCF-7 cell lines for detecting Ptch1 and Gli1, respectively. To study Ptch1 and Gli1 down-regulation, we treated cells in 1% FBS medium with 5μM NVP-LDE225 (Novartis) for 12, 24, and 36 hours. In addition, we assessed, by Western blotting analysis, time- and dose-dependent Hh-induced modifications of genes involved in cell cycle/apoptosis regulation and signal transduction, focusing on Hh-target genes in other cancers, such as Bcl-2, c-myc, cyclin-D1, caspase-3, ERK/pERK, and JNK/pJNK. The Abs used for immunoblotting included: rabbit anti-phosphop42/p44 MAPK (ERK1/2) and anti-p42/p44 MAPK (ERK1/2), anticaspase-3 from Cell Signaling Technology; rabbit anticyclin-D1, mouse anti-JNK, antiphospho JNK, anti–c-myc, anti-bcl2, and anti–α-tubulin as well as the secondary HRP-conjugated Abs were from Santa Cruz Biotechnology.
Immunofluorescence
MM cell lines (MM1S,U266) previously growth overnight in 1% FBS RPMI medium were cultured for 24 hours in the presence or absence of 10μM forskolin (Sigma-Aldrich) or 5μM NVP-LDE225. After cytospin, cells were fixed in acetone and methanol (1:1) for 15 minutes at room temperature; after 3 washes in PBS for 10 minutes each, they were blocked in PBS with 5% FBS for 1 hour at room temperature. After washing, the cells were cultured overnight at 4°C in a humidified chamber with the primary anti-Gli1Ab 1:50 in blocking solution. After 3 washes on the following day, cells were incubated for 1 hour at 4°C in a dark chamber with the secondary antibody (anti–rabbit FITC-conjugated, 1:800). After final washes, 4′-6′-diamidine-2phenylindole was used to stain nuclei followed by analysis using an epifluorescence microscope (Nikon Eclipse E800, Nikon) and a Photometrics Coolsnap CF color camera (Nikon).
Drug sensitivity assays
MM cell lines, BMSCs, and CD138+ PCs from MM patients, peripheral blood mononuclear cells (PBMCs) were treated for 24, 48, and 72 hours in RPMI medium supplemented with 1% FBS in the presence and absence of NVP-LDE225 in a range of doses from 1 to 20μM. The inhibitory effect of NVP-LDE225 on cell growth was assayed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) dye absorbance. In addition, we used annexin V–FITC assay for apoptosis qualification and flow cytometric analysis of DNA content of nuclei labeled with propidium iodide (PI) for evaluation of cell cycle changes, as described in the manufacturer's protocol (BD Biosciences PharMingen). MTT experiments have been performed in triplicate and repeated at least 3 times: data are presented as mean ± SD. Flow cytometry data are representative of 3 independent experiments. We also tested by MTT the effect on MM cell growth of 5μM NVP-LDE225 in combination with 5nM and 10nM bortezomib. Finally, to assess whether MM cell sensitivity to NVP-LDE225 is attenuated by coculture with cytokines and BMSCs, we used MTT assay to evaluate MM cell survival after adding BM supernatant and CFSE staining to quantify proliferation of MM cells cultured alone and cocultured with BMSCs, as previously described.20
In vivo combination study
CB-17 SCID mice were purchased from Charles River Laboratories and maintained and monitored in our Animal Research Facility. All animal studies were conducted according to protocols approved by the National Directorate of Veterinary Services. Animals were killed when their tumors reached 2 cm in diameter or when paralysis or major compromise in their life occurred. CB-17 SCID mice were subcutaneously inoculated in the interscapular area with 2 × 106 OPM1 cells in 100 μL RPMI medium. When tumors were measurable ∼ 3 weeks after the injection, animals were treated with vehicle alone (n = 6), NVP-LDE225 40 mg/kg (n = 7), NVP-LDE225 80 mg/kg (n = 6), bortezomib 1 mg/kg (n = 6), and bortezomib1 mg/kg + NVP-LDE225 80 mg/kg (n = 5). Vehicle and NVP-LDE225 alone were administered daily orally. Bortezomib was administered intraperitoneally on days 1, 4, 8, and 11. In the combination therapy group, NVP-LDE225 was added on day 8 and administered daily orally. All the drugs were given until the day of sacrifice or death. Tumor sizes were measured every 2 days in 2 dimensions using an electronic caliper, and tumor volume was calculated using the following formula: V = 0.5a × b2, where a and b was the long and short diameter of the tumor, respectively. The statistical significance of differences among the groups was assessed using the Student t test. P values < .05 were considered significant.
Results
CD138+PCs from MGUS and MM patients overexpress Hh components compared with CD138+ PCs from healthy persons
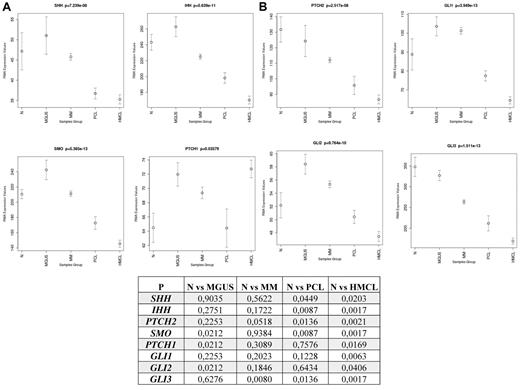
We first evaluated the Hh-gene expression in PCs isolated from BM aspirate of 4 healthy donors, as well as 11 MGUS, 133 MM, and 9 PCL patients and 23 MM cell lines using oligonucleotide microarray analysis. Compared with normal healthy donors (Figure 1), PCs from MGUS patients express higher levels of Ihh/Shh, Smo, and Ptch1 receptors, as well as Gli1 and Gli2 transcription factors, but lower levels of the repressor genes Ptch2 and Gli3, with statistically significant difference (P < .05) for Smo, Ptch1, and Gli2; suggesting that Hh pathway activation may play a role in malignant transformation of PCs and disease initiation. Up-regulation of Ptch1, Gli1, and Gli2 and down-regulation of Ptch2 and Gli3 with statistically significant difference for the last 2 genes (P < .05) have also been observed in PCs from MM patients supporting a Gli-dependent Hh activation in MM pathogenesis. Conversely, the relative down-regulation of Hh-gene expression observed in PCL, a more advanced and BM-independent disease, and in MM cell lines with statistically significant difference (P < .05) for the majority of the analyzed genes, suggests a role for stroma-derived Hh-signals in triggering paracrine Hh activity in MM.
Differential expression of Hh genes between CD138+ PCs from healthy persons, MGUS, MM, and PCL patients, and MM cell lines. Gene array analysis demonstrates the differences in expression of Hh pathway-related genes between normal (n = 4) and malignant PCs in MGUS (n = 11), MM (n = 133), and PCL (n = 9) patients, and MM cell lines (n = 23). Bars represent mean ± SEM. The statistical significance of differences among the groups was assessed using Kruskal-Wallis test. P < .05 was considered significant.
Differential expression of Hh genes between CD138+ PCs from healthy persons, MGUS, MM, and PCL patients, and MM cell lines. Gene array analysis demonstrates the differences in expression of Hh pathway-related genes between normal (n = 4) and malignant PCs in MGUS (n = 11), MM (n = 133), and PCL (n = 9) patients, and MM cell lines (n = 23). Bars represent mean ± SEM. The statistical significance of differences among the groups was assessed using Kruskal-Wallis test. P < .05 was considered significant.
MM cell lines are sources of Shh- ligand and coexpress Ptch1 and Smo receptors
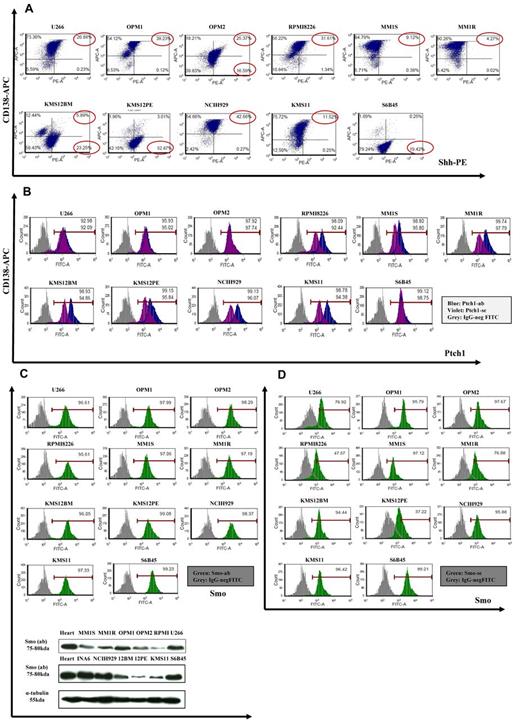
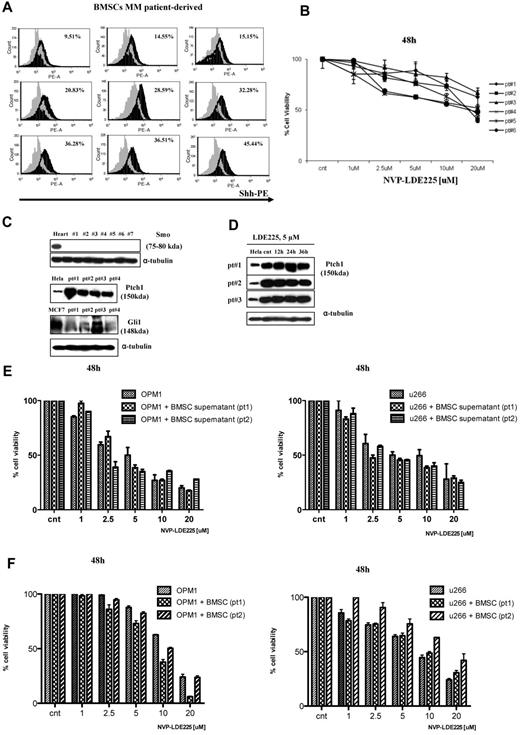
Although gene profiling showed a relatively lower Hh-gene expression in MM cell lines compared with normal PCs, we screened 11 MM cell lines for Hh protein. Because CD138 antigen is expressed, especially on terminally differentiated PCs, we used anti-CD138 APC-conjugated Ab for surface staining and anti-Shh PE-conjugated Ab for intracellular staining, followed by flow cytometric analysis for the percentage of double-positive cells. As seen in Figure 2A, Shh ligand was detected in both CD138+ as well as CD138− MM cell population. In addition, using anti-Ptch1 and anti-Smo mAb, we observed cell surface expression of Ptch1 and Smo receptors on MM cells showing similar staining patterns for these 2 proteins using 2 different Abs (Figure 2B-D). We have also confirmed basal levels of Smo expression by Western blot analysis (Figure 2C bottom panel). Because Ptch1 is the Shh binder and Smo is the Hh signal transducer of this receptor complex, their coexpression is indicative of cell responsiveness to Hh ligands.
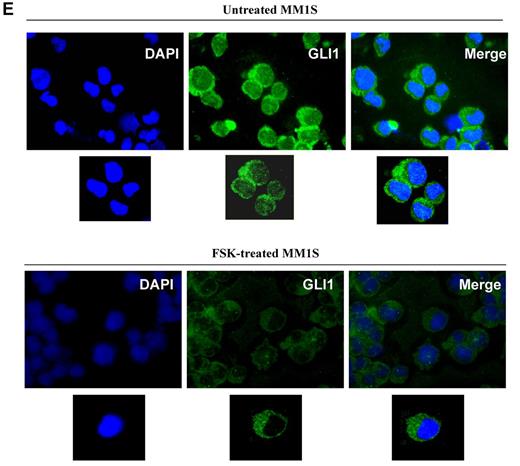
Expression of Hh components in MM cell lines. (A) MM cell lines were double-stained with anti-CD138–APC and anti-Shh–PE Abs, and the percentage of positive cells was analyzed by a FACSCanto II flow cytometer. Shh is expressed in CD138+ as well CD138− population. (B-D) MM cells were stained with 2 different anti-Ptch1 primary Abs (B) or anti-Smo primary Abs (C-D) followed by specific FITC-conjugated secondary Abs and analyzed with flow cytometry. For both Ptch1 and Smo, similar staining patterns were observed. In both experiments, background staining has been excluded using cells stained with secondary Ab FITC-conjugated as negative control. (C) Lower panel: Smo immunoblot of protein lysate from MM cell lines fractionated by electrophoresis and stained with anti-Smo Ab showing basal level of Smo expression in MM cell lines. (E) MM1S cells were cultured in control medium (top panel) or in the presence of forskolin (FSK; 10μM; bottom panel) for 24 hours. FSK inhibits the nuclear translocation of Gli1. Immunocytochemical analysis was assessed using anti-Gli1Ab, and 4,6-diamidino-2-phenylindole was used to stain nuclei. The cells were analyzed using an epifluorescence microscope (Nikon Eclipse E800, Nikon) and a Photometrics Coolsnap CF color camera (Nikon). (E) Original magnification ×100. Gli1 expression and its nuclear localization suggestive of Hh pathway activity were observed in MM cells. The inhibition by FSK suggests a Smo-independent activation of Gli1.
Expression of Hh components in MM cell lines. (A) MM cell lines were double-stained with anti-CD138–APC and anti-Shh–PE Abs, and the percentage of positive cells was analyzed by a FACSCanto II flow cytometer. Shh is expressed in CD138+ as well CD138− population. (B-D) MM cells were stained with 2 different anti-Ptch1 primary Abs (B) or anti-Smo primary Abs (C-D) followed by specific FITC-conjugated secondary Abs and analyzed with flow cytometry. For both Ptch1 and Smo, similar staining patterns were observed. In both experiments, background staining has been excluded using cells stained with secondary Ab FITC-conjugated as negative control. (C) Lower panel: Smo immunoblot of protein lysate from MM cell lines fractionated by electrophoresis and stained with anti-Smo Ab showing basal level of Smo expression in MM cell lines. (E) MM1S cells were cultured in control medium (top panel) or in the presence of forskolin (FSK; 10μM; bottom panel) for 24 hours. FSK inhibits the nuclear translocation of Gli1. Immunocytochemical analysis was assessed using anti-Gli1Ab, and 4,6-diamidino-2-phenylindole was used to stain nuclei. The cells were analyzed using an epifluorescence microscope (Nikon Eclipse E800, Nikon) and a Photometrics Coolsnap CF color camera (Nikon). (E) Original magnification ×100. Gli1 expression and its nuclear localization suggestive of Hh pathway activity were observed in MM cells. The inhibition by FSK suggests a Smo-independent activation of Gli1.
Hh pathway is constitutively activated by noncanonical and Smo-independent mechanisms in MM
In the absence of ligand, the Hh pathway is turned off and Gli transcription factors remain cytosolic. In the presence of ligand and after its binding to Ptch1, the pathway is turned on and Gli1 is activated and migrates to the nucleus, thereby inducing activation of a plethora of genes involved in signal transduction, apoptosis, and cell cycle regulation. In the MM1S cell line, we observed both cytosolic and nuclear Gli1 using immunofluorescence; the nuclear presence of Gli1 suggests constitutive Hh pathway activation (Figure 2E top panel). This is completely abrogated by 24-hour treatment with 10μM forskolin, which inactivates Gli1 via PKA (Figure 2E bottom panel), indicating that Smo-independent mechanisms might contribute to Hh activation in MM.
Canonical and Smo-dependent Hh activation is also present in MM
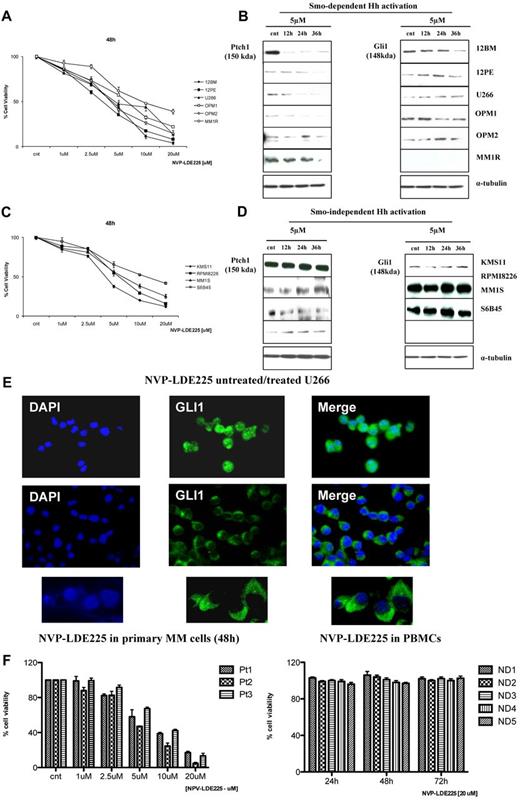
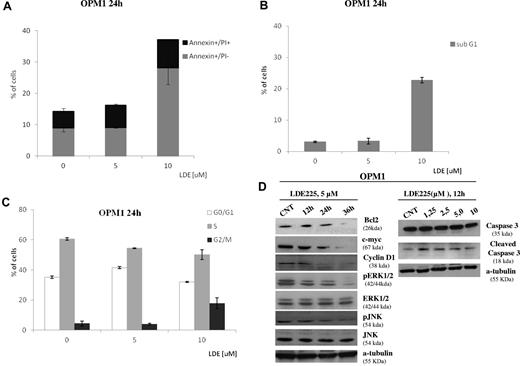
To further investigate additional mechanisms of Hh signaling in MM, we used NVP-LDE225, a novel synthetic Smo inhibitor currently in clinical development.16,17 At micro molar doses, NVP-LDE225 significantly reduced MM cell viability in vitro. In the majority of MM cell lines screened, IC50 was 3-5μM after 48 hours (Figure 3A); after treatment with 5μM NVP-LDE225 up to 36 hours, specific down-regulation of Gli1 and Ptch1 proteins was observed (Figure 3B), suggesting the existence of canonical and Smo-dependent Hh activation in MM and also a specific Hh inhibition by NVP-LDE225. The down-regulation of Ptch1 in MM cells was observed at 12 hours, whereas down-regulation of Gli1 was observed at 36 hours. Indeed, Ptch1 down-regulation also occurred in the MM1R cell line, which does not express Gli1, and in U266 cells where Gli1 down-regulation was not observed. This indicates that Gli1 is not the only transcription factor involved in Hh-signal transduction in MM and suggests Gli2 involvement, in agreement with previous data showing a critical role of Gli2 in infiltrating PCs and in human plasmacytoma tumors.21 Despite Smo expression in the remaining cell lines screened, the observed inhibition of survival (IC50 higher than 5μM) was not associated with Gli1 and/or Ptch1 down-regulation, suggesting that alternative, noncanonical, and Smo-independent mechanisms might contribute to aberrant expression of Hh components (Figure 3C-D). We did not find any correlation between Shh expression level and sensitivity to Smo inhibitor because cell lines highly positive for Shh (eg, RPMI8226 and S6B45; Figure 2A), were relatively less sensitive to NVP-LDE225 (Figure 3D), suggesting that some MM cell lines do not have autocrine Hh activity. Similarly, despite high expression levels of both receptors detected in all MM cell lines, not all MM cell lines were sensitive to Smo inhibitor, suggesting that protein expression levels do not reflect functional activity of the receptor in all cases. Indeed, in U266 cells with Smo-dependent activation, 5μM NVP-LDE225 treatment abrogated the nuclear localization of Gli1 at 24 hours (Figure 3E). PCs from MM patients also showed sensitivity to Smo inhibitor (Figure 3F left panel), whereas viability of PBMCs from 5 different healthy persons was not affected by treatment with 20μM NVP-LDE225 for 72 hours (Figure 3F right panel). Finally, we found that 24-hour treatment with 10μM NVP-LDE225 induces early as well as late apoptosis (Figure 4A), with accumulation of cells in G2/M phase, decreased S phase, and increased sub-G1 cell fractions (Figure 4B-C). Using NVP-LDE225 at dose and exposure time (5μM for 24 hours) that did not cause apoptosis and cell cycle modification (Figure 4A-C), we observed decreased c-myc, cyclin-D1, and Bcl-2 protein expression, ERK and JNK phosphorylation, and increased caspase-3 cleavage as earlier consequences of NVP-LDE225 treatment. These results suggest a potential involvement of these proteins in Hh-signal transduction in MM, as already described in other tumors.
Activity of Smo inhibitor NVP-LDE225 on MM cell lines. (A,C) MM cells were cultured for 48 hours in the presence of control medium or NVP-LDE225, and viability was measured by MTT assay. (B) Ptch1 and Gli1 immunoblot of protein lysate from MM cell lines treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with anti-Ptch1 and anti-Gli1 Abs showing down-regulation of Ptch1 and Gli1, suggesting Smo-dependent mechanisms of Hh activity in these MM cell lines. (D) Ptch1 and Gli1 immunoblot of protein lysate from MM cell lines treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with anti-Ptch1 and anti-Gli1 Abs showing no down-regulation of Hh genes after treatment, suggesting alternative Smo-independent mechanisms leading to Hh activation in MM. (E) U266 were cultured in the presence of control medium or NVP-LDE225 (5μM) for 24 hours. Immunocytochemical analysis was assessed using anti-Gli1 Ab, and 4,6-diamidino-2-phenylindole was used to stain nuclei. The cells were analyzed using an epifluorescence microscope. Top and middle panels: original magnification ×40. Bottom panel: original magnification ×100. NVP-LDE225 modulates Gli1 activity by inhibiting its nuclear localization. (F) Primary MM cells were cultured for 48 hours in the presence of control medium or NVP-LDE225 in a range of doses from 1 to 20μM (left panel). PBMCs from 5 healthy donors were treated with 20μM NVP-LDE225 up to 72 hours (right panel). Cell viability was evaluated by MTT assay.
Activity of Smo inhibitor NVP-LDE225 on MM cell lines. (A,C) MM cells were cultured for 48 hours in the presence of control medium or NVP-LDE225, and viability was measured by MTT assay. (B) Ptch1 and Gli1 immunoblot of protein lysate from MM cell lines treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with anti-Ptch1 and anti-Gli1 Abs showing down-regulation of Ptch1 and Gli1, suggesting Smo-dependent mechanisms of Hh activity in these MM cell lines. (D) Ptch1 and Gli1 immunoblot of protein lysate from MM cell lines treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with anti-Ptch1 and anti-Gli1 Abs showing no down-regulation of Hh genes after treatment, suggesting alternative Smo-independent mechanisms leading to Hh activation in MM. (E) U266 were cultured in the presence of control medium or NVP-LDE225 (5μM) for 24 hours. Immunocytochemical analysis was assessed using anti-Gli1 Ab, and 4,6-diamidino-2-phenylindole was used to stain nuclei. The cells were analyzed using an epifluorescence microscope. Top and middle panels: original magnification ×40. Bottom panel: original magnification ×100. NVP-LDE225 modulates Gli1 activity by inhibiting its nuclear localization. (F) Primary MM cells were cultured for 48 hours in the presence of control medium or NVP-LDE225 in a range of doses from 1 to 20μM (left panel). PBMCs from 5 healthy donors were treated with 20μM NVP-LDE225 up to 72 hours (right panel). Cell viability was evaluated by MTT assay.
NVP-LDE225 induces apoptosis and cell cycle modification in sensitive MM cell lines. (A) OPM1 cells were treated with 5μM and 10μM of compound for 24 hours, stained with annexin V–FITC and PI, and analyzed by a FACSCanto II flow cytometer. Percentages of early apoptotic (annexin V–FITC+/PI−) and late apoptotic/necrotic (annexin V–FITC+/PI+) cells are presented. (B-C) OPM1 cells were treated with 5μM and 10μM of compound for 24 hours, and the distribution of cells in G0/G1, S and G2/M phase was analyzed by flow cytometry and MultiCycle AV DNA analysis. Percentage of sub-G1 fraction was obtained from analysis of side scatter versus log FL3 dot plot using De Novo FCS Express Version 3 software. Data are from 3 independent experiments and presented as mean ± SD. *P < .05, control versus the different treatment. (D) Immunoblot experiment of protein lysate from OPM1 cells treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with different Abs as shown in figure (left). Cells were treated with different concentrations of NVP-LDE225 for 12 hours and cell lysate fractionated by electrophoresis and stained with anticaspase-3 Ab (right).
NVP-LDE225 induces apoptosis and cell cycle modification in sensitive MM cell lines. (A) OPM1 cells were treated with 5μM and 10μM of compound for 24 hours, stained with annexin V–FITC and PI, and analyzed by a FACSCanto II flow cytometer. Percentages of early apoptotic (annexin V–FITC+/PI−) and late apoptotic/necrotic (annexin V–FITC+/PI+) cells are presented. (B-C) OPM1 cells were treated with 5μM and 10μM of compound for 24 hours, and the distribution of cells in G0/G1, S and G2/M phase was analyzed by flow cytometry and MultiCycle AV DNA analysis. Percentage of sub-G1 fraction was obtained from analysis of side scatter versus log FL3 dot plot using De Novo FCS Express Version 3 software. Data are from 3 independent experiments and presented as mean ± SD. *P < .05, control versus the different treatment. (D) Immunoblot experiment of protein lysate from OPM1 cells treated with NVP-LDE225 (5μM) for 12, 24, and 36 hours and fractionated by electrophoresis and stained with different Abs as shown in figure (left). Cells were treated with different concentrations of NVP-LDE225 for 12 hours and cell lysate fractionated by electrophoresis and stained with anticaspase-3 Ab (right).
BMSC-derived Hh signaling
Intracellular staining of MM patient-derived BMSCs with anti-Shh Ab showed significantly higher Shh expression in BMSCs (Figure 5A). Moreover, NVP-LDE225 treatment at 20μM for up to 48 hours had minimal effect on BMSC survival (Figure 5B). Western blot analysis demonstrated that BMSCs are Smo-defective and have variable Gli1 expression (Figure 5C) and Ptch1 up-regulation, which did not change after treatment with 5μM NVP-LDE225 (Figure 5C-D). In the absence of Smo, which is required to activate Hh-target genes, Shh-producing BMSCs do not have autocrine Hh activity but provide Shh ligand in a paracrine fashion. Importantly, MM-BMSC interaction or MM cell growth in the presence of BMSC supernatant did not induce resistance to NVP-LDE225 treatment (Figure 5E-F).
Hh activity in MM patient-derived BMSCs. (A) BMSCs isolated from MM patients were stained with Shh-PE–conjugated Ab and analyzed by FACSCanto II flow cytometer; staining is relative to isotype-matched control. As seen, the Shh ligand is variably expressed by BMSCs. (B) MM patient-derived BMSCs were cultured in the presence of control medium or NVP-LDE225 for 48 hours, and proliferation was measured by MTT assay. (C) Immunoblot of protein lysate from MM patient-derived BMSCs fractionated by electrophoresis and stained with anti-Smo, anti-Ptch1, and anti-Gli1 Abs. (D) Immunoblot of protein lysate from MM patient-derived BMSCs fractionated by electrophoresis and stained with anti-Ptch1 Ab, showing no down-regulation of Ptch1 after NVP-LDE225 treatment. (E) MM cell lines were cultured for 48 hours in the presence of control medium or BMSC culture-derived supernatant and treated with NVP-LDE225 at indicated doses; cell viability was assessed by MTT assay. (F) MM cell lines were cultured for 48 hours in the absence or presence of patient-derived BMSCs and treated with NVP-LDE225 at indicated doses; MM cell viability was assessed by CFSE staining.
Hh activity in MM patient-derived BMSCs. (A) BMSCs isolated from MM patients were stained with Shh-PE–conjugated Ab and analyzed by FACSCanto II flow cytometer; staining is relative to isotype-matched control. As seen, the Shh ligand is variably expressed by BMSCs. (B) MM patient-derived BMSCs were cultured in the presence of control medium or NVP-LDE225 for 48 hours, and proliferation was measured by MTT assay. (C) Immunoblot of protein lysate from MM patient-derived BMSCs fractionated by electrophoresis and stained with anti-Smo, anti-Ptch1, and anti-Gli1 Abs. (D) Immunoblot of protein lysate from MM patient-derived BMSCs fractionated by electrophoresis and stained with anti-Ptch1 Ab, showing no down-regulation of Ptch1 after NVP-LDE225 treatment. (E) MM cell lines were cultured for 48 hours in the presence of control medium or BMSC culture-derived supernatant and treated with NVP-LDE225 at indicated doses; cell viability was assessed by MTT assay. (F) MM cell lines were cultured for 48 hours in the absence or presence of patient-derived BMSCs and treated with NVP-LDE225 at indicated doses; MM cell viability was assessed by CFSE staining.
In vitro and in vivo efficacy of NVP-LDE225 treatment in combination with bortezomib
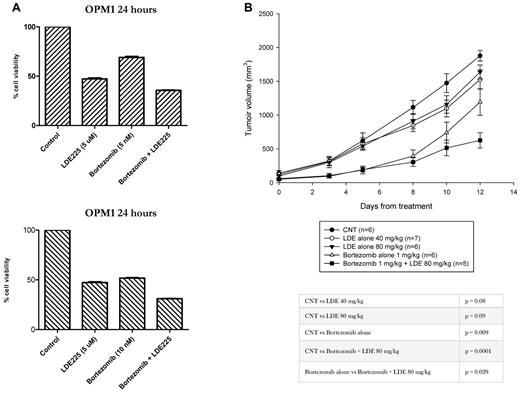
Recent data demonstrated that bortezomib exerts antitumor activity in medulloblastoma, by Ptch1 protein accumulation leading to down-regulation of Hh signaling.22 Therefore, we evaluated the in vitro and in vivo antitumor efficacy of NVP-LDE225 alone and in combination with bortezomib. In vitro combination of 5nM or 10nM bortezomib with 5μM NVP-LDE225 for 24 hours showed only modest effect on MM cell viability compared with either drug alone (Figure 6A). In a mouse subcutaneous xenograft model of MM, daily oral dosing of NVP-LDE225 at 40 mg/kg showed only modest antitumor efficacy, with 21% tumor growth inhibition by 12 days compared with the control mice (P = .08); whereas bortezomib as a single agent treatment produced 57% tumor reduction (P = .009). Importantly, the combination of NVP-LDE225 with bortezomib showed additive cytotoxicity with 78% tumor growth inhibition compared with control mice (P = .0001) and with bortezomib as a single agent (P = .029), suggesting that combination therapy with the Smo inhibitor can improve bortezomib response.
In vitro and in vivo activity of NVP-LDE225 alone and in combination with bortezomib. (A) OPM1 cells were treated for 24 hours with 5μM NVP-LDE225 as single agent or in combination with 5nM or 10nM of bortezomib. MM cell viability was assessed by MTT. (B) To evaluate the in vivo antitumor efficacy of NVP-LDE225, we used a mouse subcutaneous xenograft model of MM. CB-17 SCID mice were subcutaneously inoculated with OPM1 cells and treated with vehicle alone, NVP-LDE225, bortezomib, and the combination as detailed in “Methods.” In vivo study showed an increased antitumor activity by the combination compared to bortezomib alone.
In vitro and in vivo activity of NVP-LDE225 alone and in combination with bortezomib. (A) OPM1 cells were treated for 24 hours with 5μM NVP-LDE225 as single agent or in combination with 5nM or 10nM of bortezomib. MM cell viability was assessed by MTT. (B) To evaluate the in vivo antitumor efficacy of NVP-LDE225, we used a mouse subcutaneous xenograft model of MM. CB-17 SCID mice were subcutaneously inoculated with OPM1 cells and treated with vehicle alone, NVP-LDE225, bortezomib, and the combination as detailed in “Methods.” In vivo study showed an increased antitumor activity by the combination compared to bortezomib alone.
Discussion
The processes regulating self-renewal of both normal and cancer cells are poorly understood, but several pathways and genes, including Notch, Wingless (Wnt), and Hh, usually required for generation and maintenance of normal stem cells during embryonic development, have emerged as potential candidates.2,3 The Hh pathway is particularly attractive because several small molecules, acting as agonists and antagonists of Hh signaling, are currently under development23 and some of these have already showed clinical efficacy.17,24 These developmental pathways are generally silenced in most adult tissues, except during tissue homeostasis and repair, but are reactivated in many diseases1,4 and human cancers13 and therefore represent potential novel therapeutic targets. Here we provide evidence of the role of Hh signaling in MM pathogenesis and the therapeutic potential of targeting Hh pathway in MM.
To date, contrasting results, arising mostly from mice studies, are available about the role of Hh signaling in hematologic system and hematologic malignancies.25-34 Several data demonstrate a specific involvement of Hh signaling in hematologic malignancies both in supporting cancer stem cells and in promoting growth and survival of mature malignant cells. Specifically Hh gene overexpression during progenitor cell expansion is associated with malignant transformation in CD133+/CD34+ cells.35 The Hh pathway inhibition also induces apoptosis and reduces drug resistance in human acute myeloid and lymphoid leukemia.36,37 Indeed, in chronic myeloid leukemia, Hh signaling plays a role in maintaining bcr-abl+ leukemic stem cells38,39 through Smo up-regulation, as well as in the blastic transformation of CD34+ chronic myeloid leukemia cells by Ptch1 overexpression.40 Indeed, pharmacologic Smo inhibition reduces leukemic stem cells in vivo, enhances time to relapse, and impairs the growth of imatinib-resistant cells.41 Shh/Gli1 signaling is also active in some types of non-Hodgkin lymphoma,42,43 where perturbation of Hh pathway by Shh or cyclopamine influences cell proliferation/apoptosis and Gli1 down-regulation increases susceptibility to doxorubicin. Finally Smo-dependent as well Smo-independent Hh activation support B-CLL cell survival, correlates with adverse indicators of clinical outcome, and Gli1 inhibition increases susceptibility to fludarabine-induced cytotoxicity.44,45 Altogether, these data indicate that molecular targeting of Hh pathway is a potential therapeutic approach to improve treatment of hematologic malignancies.
In the present study, we provide data supporting a role of Hh pathway also in CD138+ PCs, and we show canonical as well as noncanonical mechanisms leading to its activation in MM. We previously demonstrated overexpression of ciliary proteins, such as OFD1, as one of the possible causes of constitutive, noncanonical, or Smo-independent Hh activation, and highlighted the important role of primary cilium in regulating cellular responses to Hh signaling in MM.12 Here we investigated whether the canonical Hh pathway was also active in MM. We first demonstrated that CD138+ PCs from MGUS patients have a significant up-regulation of Hh-activating genes, such as Smo, Ptch1, and Gli2 compared with CD138+ PCs from healthy persons. This supports a role of Hh pathway in malignant transformation of PCs and in the pathogenesis of the precursor condition MGUS. In contrast, we observed a significant down-regulation of Hh-repressor genes, such as Ptch2 and Gli3 in MM PCs compared with their normal cellular counterpart. In addition, MM tumor cells overexpress Gli1/Gli2 and Ptch1, which is itself a Gli1 target gene, suggesting a Gli-dependent Hh activation. These results are in agreement with our previous findings showing Gli1/Ptch1 down-regulation after OFD1 specific knockdown12 and also with recent evidence demonstrating that Gli1 overexpression increases MM cells resistance to the antiproliferative effects of the Smo inhibitor cyclopamine.9 These data suggest that Hh activity may be induced in MM cells in a Smo-independent manner. Finally, we found a relatively reduced Hh-gene expression in MM cell lines and PCL, a more advanced and BM-independent disease, suggesting a critical role for stroma-derived Hh signals consistent with a paracrine model of Hh pathway in MM. Despite the Hh-gene expression, Hh-protein analysis in MM cell lines revealed that Shh ligand is expressed at significant levels in both CD138+ as well as CD138− cells. The CD138+ MM cell population probably also includes the side population, which is a CD138low+ subpopulation with stem cell properties.46 Matsui et al47,48 have previously reported predominant effect and activity of Hh signaling in CD138− MM stem cells, although we observed that CD138+ cells are also susceptible to inhibition by NVP-LDE225. Various molecular explanations can be considered for this observation, including a lower level or differential expression of some Hh genes in CD138+ MM cells versus CD138low+ side population. Moreover, a wide spectrum of genetic alterations have been described in MM and are differently associated with MM disease initiation and progression; disease stage and previous treatment may also impact the biology of the cancer and hence Hh signaling in MM cells.48 Therefore, it is possible that MM represents a number of biologically distinct disease, each containing different initiating cells.48 These factors may all contribute to the reported differences.
We did not find correlation between Shh expression and Hh responsiveness, suggesting the absence of link among expression level and functional activity; therefore, autocrine and/or paracrine mechanisms can both contribute to Hh activation. Importantly, MM cell lines strongly coexpress Ptch1 and Smo receptors, suggesting their potential Hh responsiveness. We have confirmed Smo-dependent Hh signaling in MM using an Smo inhibitor NVP-LDE225,16,17 which decreased MM cell viability in a range of 3-5μM in the majority of MM cell lines tested. This was associated with specific down-regulation of Gli1 and/or Ptch1, hallmarks of cell response to the Hh pathway. In the remaining MM cell lines, despite Smo expression, Gli1 and/or Ptch1 down-regulation was not observed after treatment, suggesting lack of correlation between Smo expression level and functional protein activity. Importantly, in those MM cell lines not responding to Smo inhibitor, Gli1 nuclear localization indicates that the Hh pathway is constitutively activated, suggesting that alternative, noncanonical and Gli-dependent mechanisms may contribute to Hh signaling activation in MM. Therefore, the combination of Gli-modulating agents with Smo inhibitors may provide an alternative and more effective strategy for Hh inhibition.
NVP-LDE225 similarly inhibited Gli1 nuclear translocation in MM cells with Smo-dependent Hh activity. Cytotoxicity of NVP-LDE225 was also observed in primary MM cells, whereas no toxicity has been observed in PBMCs from healthy persons, suggesting a specific antitumor activity and a favorable therapeutic index. We observed that MM patient-derived BMSCs produce Shh ligand and overexpress Ptch1 but do not express Smo and rarely express Gli1. Recent reports show a role for Ptch1 in the BM compartment in inducing B-lymphocyte differentiation in mouse model,32 leading to the hypothesis that Ptch1 might mediate MM-BM microenvironment interactions and PC differentiation also in humans. Defective Smo expression, Ptch1 up-regulation, and absence of Gli1 may all contribute to Hh inhibitor resistance observed in MM patient-derived BMSCs. Importantly, Shh production by BMSCs suggests a paracrine role for stroma-derived Hh signals and also that Shh ligand is a novel cytokine supporting growth and survival of human PCs. Therefore, Shh-blocking agents may be useful to interrupt MM-BM interactions. MM-BMSC interaction does not induce resistance to NVP-LDE225. Finally, in vitro as well in vivo studies showed antitumor activity of NVP-LDE225 in combination with bortezomib, demonstrating that NVP-LDE225 can potentiate the efficacy of well-established anti-MM agents.
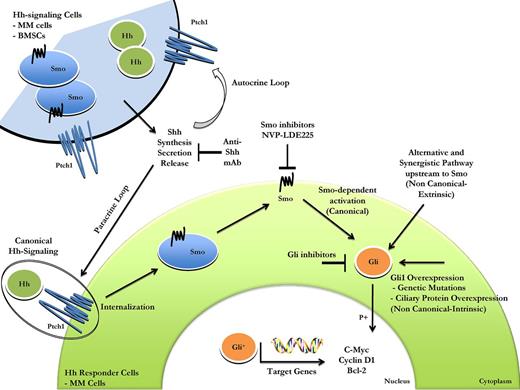
In conclusion, our findings suggest that both canonical (Smo-dependent) and noncanonical (Smo-independent) mechanisms are crucial regulators of Hh activation in MM cells. Canonical and noncanonical pathways probably work in parallel, with a possible crosstalk between them.49 Directed analyses of the noncanonical Hh signaling as well as a better understanding of all the mechanisms contributing to the noncanonical Hh activation, such as genetic mutations, ciliary protein overexpression, crosstalk between Hh signaling and unrelated pathways, and MM-BMSCs interactions, are therefore needed (Figure 7). Taken together, our findings provide the framework for novel therapeutic strategies targeting the Hh pathway to improve patient outcome in MM.
Schematic representation of canonical and noncanonical Hh signaling activation in MM. The signaling is triggered by binding of ligand, produced by Hh signaling cells, such as MM cells or BMSCs, to Ptch1 on target cells (canonical or ligand-dependent/ receptor-induced signaling). This leads to inhibition of Ptch1 via cellular internalization and Smo localization on the cell surface. Smo activation leads to nuclear translocation of Gli transcription factors followed by expression of Gli target genes, such as c-myc, cyclin-D1, and Bcl-2. The biologic effect is cell proliferation, with deregulation contributing to tumorigenesis. Abnormal Hh pathway activation occurs also by the mechanisms of activation downstream to Smo (noncanonical or ligand-independent Hh signaling). Genetic alterations or ciliary protein overexpression leading to functional redundancy of Gli transcription factors, crosstalk between Hh signaling, and unrelated pathway are all causes of noncanonical Hh signaling activation. Canonical and noncanonical Hh pathways probably work in parallel, and a multitargeted approach directed against Hh signaling may be a more effective therapeutic strategy.
Schematic representation of canonical and noncanonical Hh signaling activation in MM. The signaling is triggered by binding of ligand, produced by Hh signaling cells, such as MM cells or BMSCs, to Ptch1 on target cells (canonical or ligand-dependent/ receptor-induced signaling). This leads to inhibition of Ptch1 via cellular internalization and Smo localization on the cell surface. Smo activation leads to nuclear translocation of Gli transcription factors followed by expression of Gli target genes, such as c-myc, cyclin-D1, and Bcl-2. The biologic effect is cell proliferation, with deregulation contributing to tumorigenesis. Abnormal Hh pathway activation occurs also by the mechanisms of activation downstream to Smo (noncanonical or ligand-independent Hh signaling). Genetic alterations or ciliary protein overexpression leading to functional redundancy of Gli transcription factors, crosstalk between Hh signaling, and unrelated pathway are all causes of noncanonical Hh signaling activation. Canonical and noncanonical Hh pathways probably work in parallel, and a multitargeted approach directed against Hh signaling may be a more effective therapeutic strategy.
Presented in part as oral communication at the American Society of Hematology Annual Meeting, Atlanta, GA, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Novartis Institute for Bio-Medical Research (Cambridge, MA) for providing the NVP-LDE225 compound.
This work was supported by the Specialized Program of Research Excellence in Myeloma–National Institutes of Health (Career Development Award grant P50CA-100707, S.B.), Department of Veterans Affairs (Merit Review Award I01-BX001584), National Institutes of Health (grants RO1-124929 and PO1-155258, N.C.M.; grants P50-100007 and PO1-78378, N.C.M. and K.C.A.; and grant RO1-50947 to K.C.A. and C.S.M.), the American Cancer Society Clinical Research Professor (K.C.A.), and the Italian Association for Cancer Research (grant 9980, P.T.).
National Institutes of Health
Authorship
Contribution: S.B. conceived the project, designed and performed research, analyzed and interpreted the data, and wrote the manuscript; N.C.M. conceived and designed the project, analyzed and interpreted the data, supervised the research, and critically reviewed the manuscript; K.C.A. supervised the research and critically reviewed the manuscript; J.J. helped with the flow cytometry; T.C. helped with the animal study; A.M.R., N.A., A.K.A., and U.F.contributed to the research; C.S.M., M.R., P. Tagliaferri, and P. Tassone analyzed the data; K.T., S.M., F.M., and A.N. provided primary tumor specimens for the microarray experiment and performed the statistical analysis; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: K.C.A. is on the advisory board of Celgene, Millennium, Onyx, and Bristol-Myers Squibb and is Scientific Founder of Acetylon and Oncopep. N.C.M. is on the advisory board of Celgene, Millennium, Onyx, and Merck and is Scientific Founder of Oncopep. C.S.M. received consultant honoraria from Millennium, Celgene, Novartis, Bristol-Myers Squibb, Merck & Co, Centocor, and Arno Therapeutics; he received research funding from Amgen, AVEO Pharma, OSI, EMD Serono, Sunesis, Genzyme, and Johnson & Johnson; and he has an uncompensated role as scientific advisor for Axios Biosciences. The remaining authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, M1B28, Boston, MA 02115; e-mail: nikhil_munshi@dfci.harvard.edu.
References
Author notes
J.J. and T.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal