Abstract
Mixed lineage leukemia (MLL) fusion genes arise from chromosomal translocations and induce acute myeloid leukemia through a mechanism involving transcriptional deregulation of differentiation and self-renewal programs. Progression of MLL-rearranged acute myeloid leukemia is associated with increased activation of Rac GTPases. Here, we demonstrate that MLL fusion oncogenes maintain leukemia-associated Rac activity by regulating Frat gene expression, specifically Frat2. Modulation of FRAT2 leads to concomitant changes in Rac activity, and transformation of Frat knockout hematopoietic progenitor cells by MLL fusions results in leukemias displaying reduced Rac activation and increased sensitivity to chemotherapeutic drugs. FRAT2 activates Rac through a signaling mechanism that requires glycogen synthase kinase 3 and DVL. Disruption of this pathway abrogates the leukemogenic activity of MLL fusions. This suggests a rationale for the paradoxical requirement of canonical Wnt signaling and glycogen synthase kinase 3 activity for MLL fusion oncogenicity and identifies novel therapeutic targets for this disease.
Introduction
Translocations affecting the mixed lineage leukemia (MLL) gene on chromosome band 11q23 are the most common chromosomal aberrations in pediatric acute myeloid leukemia (AML).1 This cytogenetic subgroup is associated with an intermediate risk,2 intensive chemotherapy having improved overall survival, although MLL-rearranged AML is still unfavorable in older patients.3 Taken together, these data suggest that novel therapeutic interventions based on targeting MLL fusion function would benefit a significant number of patients.
The MLL fusion genes resulting from these translocations are potent oncogenes.4-6 They are sufficient to induce aberrant self-renewal programs in mouse hematopoietic progenitor cells (HPCs) leading to transformation and leukemic progression.7,8 Recent studies have demonstrated that this is also true for human HPCs.9,10 We and others have previously shown that inhibition of MLL fusion expression reverses HPC immortalization11-13 and abrogates established leukemia in vivo.14,15 This suggests that the transcriptional and signaling pathways controlled by MLL fusions are necessary for maintenance as well as initiation of leukemia. Recent studies have demonstrated that progression of preleukemic to leukemic MLL fusion cells involves increased canonical Wnt signaling16 and Rac GTPase activation17 ; both of these signaling pathways play critical roles in leukemia progression.10,16,18-20
The sensitivity of MLL-rearranged AML cells to Rac inhibition suggests that targeting Rac activity or signaling pathways leading to Rac activation may represent novel therapeutic opportunities. In support of this, pharmacologic Rac inhibition was found to interfere with leukemic engraftment of MLL-rearranged human AML cell lines.19 The available evidence indicates that MLL fusions can induce Rac activity. Thus, transformation of human cord blood-derived HPCs by MLL-AF9 was accompanied by increased Rac activation.10,19 However, little is known about the molecular details of how this is achieved. In the present study, we have used conditionally immortalized preleukemic and leukemic cells to address the dependency of Rac GTPase activity on continued MLL fusion expression and to establish components of the signaling pathway linking the 2. These experiments identify multiple points potentially suitable for therapeutic targeting of MLL-rearranged AML.
Methods
Mice
All mice were maintained in the animal facilities of the UCL Institute of Child Health and experiments were performed according to United Kingdom Home Office regulations. The generation and characterization of Frat1/2/3 triple-knockout (TKO) mice have been reported previously.21 These mice have been backcrossed onto the C57BL/6 background 20 times. Age- and sex-matched C57BL/6 J mice were used as controls.
Retrovirus and lentivirus cloning and production
The pMSCV-MLL-ENL-pgk-neo retroviral construct has been previously described.14 Oligonucleotides for shRNAs targeting Frat1, Frat2, and Dvl1 were designed using RNAi central (http://katahdin.cshl.org/siRNA/RNAi.cgi?type = shRNA). The following sense target sequences were used: F1a-AGGAAACAAGAGTGGACTTAAT; F1b-CCGGCTCAGCCTGCTATGGAAC; F2a-CGGGCTTCTAACAATACTTGAA; F2b-CCCTGATTTATAGGATTCATAA; DVL1a-ACAGGTGAACGATGTCAACTTT; and DVL1b-ATGCTACTATGTCTTTGGCGAC. These were cloned into the LMP vector containing the miR30 sequence flanking the shRNA.22 The Scrambled (Sc) shRNA sequence (TCTCGCTTGGGCGAGAGTAAG) does not match any known mammalian gene and was obtained from Thermo Scientific. IMAGE clones containing full-length mouse Frat1 and Frat2 cDNAs were obtained from the Source BioScience. Frat1, Frat2, and FratN (encoding amino acids 1-185)23 were generated by PCR using the high-fidelity thermostable DNA polymerase Pfu (Promega). HA or myc epitope tags were introduced to the N-termini of the full-length and mutant molecules and subcloned to the pMSCV-IRES-hCD2T retroviral vector.24 The active β-catenin cDNA25 was obtained from Addgene and cloned into the pMSCV-IRES-hCD4T vector, expressing the human “tailless” CD4 molecule (hCD4T). Retrovirus was produced by transfecting LinXE packaging cells26 with retroviral vectors. Retroviral supernatant was harvested after 48 hours, cleared of cell debris, and was concentrated 10-fold in some experiments by centrifugation for 1 hour at 16 000g.
The FratN cDNA was cloned into the pCSGW-PIG vector, made by replacing the GFP cDNA from pCSGW27 with a puro-IRES-GFP cassette, and the resulting vector used to transduce human leukemia cell lines. The 293FT packaging cells (Invitrogen) were transiently cotransfected with the lentiviral expression vectors, the pCMV-PAX2 construct and the pVSV-G envelope construct (kind gifts of D. Trono, Lausanne, Switzerland). Human leukemia cells were transduced with lentiviral supernatant by spinoculation at 700g, 25°C for 45 minutes in the presence of 5 μg/mL polybrene. Proliferation of transduced cells was measured by plating 5 × 104 cells per well in culture and adding MTS (CellTiter 96 Aqueous One Solution Reagent; Promega) 48 hours later. Cell viability determined by measuring the absorbance at 490 nm using a 550 Bio-Rad plate-reader (Bio-Rad).
Generation, transduction, and transplantation of MLL fusion cells
The generation of preleukemic and leukemic cells with constitutive and conditional expression of MLL-ENL has been described previously.14 shRNA-mediated knockdown was achieved by retroviral transduction of immortalized and leukemic cell lines, as for HPCs. At 48 hours after transduction, the cells were selected with 1 μg/mL puromycin for 72 hours. For overexpression experiments, cells transduced with the pMSCV-IRES-hCD2T vector, containing various cDNAs, were either used directly for experiments or purified by MACS using anti-hCD2 antibodies (Miltenyi Biotec). Cells were cultured in methylcellulose for 5-7 days or transplanted into sublethally (6.5 Gy) γ-irradiated mice by lateral tail vein injection of 5 × 104 cells per mouse. Morphology of colonies formed in methylcellulose was analyzed as described in the literature.28 Cells were treated with NSC23766 (Cambridge Bioscience), BIO acetoxime (R&D Systems), CT99021 (Cambridge Bioscience), and SB216763 (R&D Systems) as indicated.
Quantitative RT-PCR analysis
Total RNA was isolated from cells using RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. RNA was converted into cDNA using a cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. Samples were treated with DNase I (Invitrogen) before reverse transcription using the Moloney murine leukemia virus reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed on isolated mRNA using TaqMan probe-based chemistry and an ABI Prism 7900HT fast Sequence Detection System (Invitrogen). All primer/probe sets were from Applied Biosystems and Invitrogen.
Rac pull-down and Western blot analysis
Rac GTP pull down was performed with the Active Rac1 Pull-Down and Detection Kit (Thermo Scientific) and immunoprecipitates subjected to electrophoresis using NuPAGE Novex 4012% Bis-Tris Midi Gels (Invitrogen) in MES SDS running buffer (Invitrogen). Western blot transfer was performed in 0.025M Tris, 0.192M glycine (pH 8.5). Blocking and antibody staining were performed according to the Active Rac1 Pull-Down and Detection Kit (Thermo Scientific) instructions. Anti-Rac2 (C11) and anti-MCL1 (S-19) antibodies (Santa Cruz Biotechnology), anti-glycogen synthase kinase 3α/β (GSK3α/β; D75D3) antibody (Cell Signaling), and antitubulin antibody (Serotec) were used to stain the respective proteins. Identical exposure conditions were used for all blots that are grouped together (apart from Figure 6A), as stated in the figure legends.
Flow cytometry
Cells were resuspended in PBS, 0.5% BSA, and 0.05% sodium azide, and preincubated with unlabeled anti-Fcγ III/II receptor mAb (2.4G2; BD Biosciences) before staining. Flow cytometry was performed using a BD LSRII (BD Biosciences) flow cytometer and data analyzed using Summit Version 4.3 software (Beckman Coulter). Flow cytometry antibodies and the Annexin V Apoptosis detection kit were from eBioscience.
Statistical analysis
Statistical analysis of survival curves was performed using the Mantel-Haenszel log-rank test. Statistical analysis of means was performed using the Student t test, 2-sided P values of < .05 being considered statistically significant.
Results
Rac activity is MLL fusion dependent
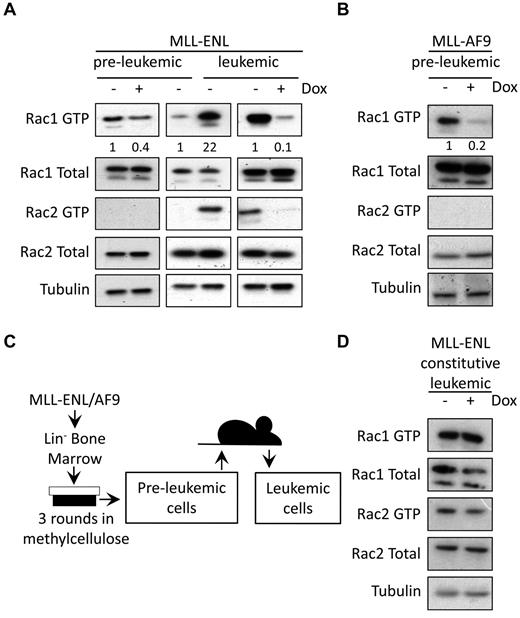
To determine whether elevated Rac GTPase activity observed in MLL fusion-transformed cells is dependent on and maintained by fusion protein expression, total Rac expression and Rac activity were assessed in conditionally immortalized preleukemic cells,13,14 treated with doxycycline to turn off fusion gene expression. Rac pull-down assays showed that Rac1 activity decreased significantly on loss of MLL-ENL (Figure 1A; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and MLL-AF9 (Figure 1B) expression. Active Rac2 was not detectable in preleukemic cells (Figure 1A-B). Total levels of Rac1 and Rac2 protein expression were not affected by doxycycline treatment (Figure 1A-B). Preleukemic MLL-ENL cells were then transplanted into sublethally irradiated recipient mice and their leukemic progeny harvested (Figure 1C). In agreement with previous studies, leukemic progression was accompanied by increased Rac activity,17 evident for both Rac1 and Rac2 (Figure 1A; supplemental Figure 1). Interestingly, increased Rac activity in leukemic cells was still found to be MLL fusion dependent and was substantially reduced after doxycycline treatment (Figure 1A; supplemental Figure 1). Doxycycline did not alter Rac activity in constitutive leukemic cells (Figure 1D). Thus, leukemic cells express increased levels of active Rac1 and Rac2 compared with preleukemic cells, but Rac activity in both is dependent on MLL fusion expression.
MLL fusions regulate the activity of Rac GTPases. (A-B) Western blot analysis of active Rac pull-down assays. Rac1 GTP and Rac2 GTP levels, and total Rac protein expression are shown in (A) preleukemic and leukemic conditional MLL-ENL cells (data are representative of 3 independent experiments) and (B) preleukemic conditional MLL-AF9 cells (data are representative of 2 independent experiments). Numbers represent densitometric quantitation of Rac1 GTP levels normalized to total Rac1 protein bands. α-tubulin served as a loading control. (C) Model for generation of preleukemic and leukemic MLL fusion cells. (D) Rac1 GTP and Rac2 GTP levels, and total Rac protein expression, in leukemic constitutive MLL-ENL cells, treated with and without doxycycline for 72 hours. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 2 independent experiments.
MLL fusions regulate the activity of Rac GTPases. (A-B) Western blot analysis of active Rac pull-down assays. Rac1 GTP and Rac2 GTP levels, and total Rac protein expression are shown in (A) preleukemic and leukemic conditional MLL-ENL cells (data are representative of 3 independent experiments) and (B) preleukemic conditional MLL-AF9 cells (data are representative of 2 independent experiments). Numbers represent densitometric quantitation of Rac1 GTP levels normalized to total Rac1 protein bands. α-tubulin served as a loading control. (C) Model for generation of preleukemic and leukemic MLL fusion cells. (D) Rac1 GTP and Rac2 GTP levels, and total Rac protein expression, in leukemic constitutive MLL-ENL cells, treated with and without doxycycline for 72 hours. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 2 independent experiments.
FRAT oncoproteins mediate Rac activation by MLL fusions
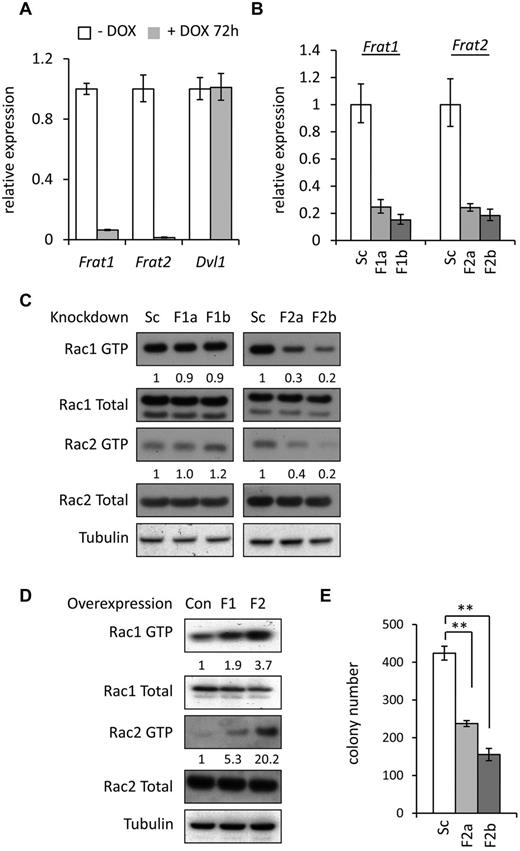
Recent evidence from a number of different systems suggests that Rac can be activated through noncanonical Wnt signaling,29 potentially mediated via complexes containing the disheveled homologue (DVL).30,31 The FRAT oncoproteins32,33 can interact with DVL complexes23,34 and can potentiate both canonical and noncanonical Wnt signaling.35 Interestingly, elevated expression of FRAT1 has previously been shown to be associated with MLL-rearranged leukemia,36 whereas Frat2 was found to be a transcriptional target of wild-type MLL.37 Furthermore, a recent study demonstrated reduction in the expression of Frat2 after shRNA-mediated silencing of the MLL fusion target gene Myb, in leukemic mouse MLL-AF9 cells.15 For these reasons, we decided to examine whether Dvl and Frat expression was regulated by MLL fusions and whether they had any impact on Rac activity in our MLL-ENL cells. First, quantitative PCR experiments showed that Frat1 and Frat2 expression is down-regulated on loss of MLL ENL in preleukemic cells (Figure 2A). Furthermore, expression of both genes was up-regulated after transduction of mouse HPCs with MLL-ENL (supplemental Figure 2). The Frat3 gene, present in mice but not humans,38,39 was not expressed in MLL-ENL–immortalized cells (data not shown). In contrast to the Frat genes, no change was detected in levels of Dvl1 mRNA on loss of MLL-ENL expression (Figure 2A). To determine whether Rac activation was mediated by FRAT, we used retroviral vectors to deliver shRNA targeting Frat1 or Frat2 in leukemic MLL-ENL cells (Figure 2B), which have higher levels of Rac activity compared with preleukemic cells. Significantly reduced activity of Rac1 and Rac2 was detected in leukemic MLL-ENL cells expressing Frat2 shRNA compared with scramble-control shRNA-transduced cells (Figure 2C). However, expression of Frat1 shRNA did not interfere with the activity of Rac1 or Rac2 (Figure 2C).
FRAT proteins mediate MLL fusion-induced Rac activity. (A) Expression of Frat1, Frat2, and Dvl1 mRNA in preleukemic MLL-ENL cells treated with and without doxycycline for 72 hours, as determined by quantitative PCR. Bars represent means of quadruplicate values; and error bars, the SD. Data are representative of 3 independent experiments. (B) Quantitative PCR analysis of Frat1 and Frat2 mRNA expression in leukemic MLL-ENL cells transduced with Scrambled (Sc), Frat1 (F1a and F1b), and Frat2 (F2a and F2b) shRNA retroviral vectors. Bars represent means of quadruplicate values; and error bars, the SD. Data are representative of 3 independent experiments. (C-D) Western blot analysis of Rac1 GTP and Rac2 GTP levels, and total Rac protein expression (C) in leukemic MLL-ENL cells transduced with Scrambled (Sc), Frat1 (F1a and F1b), and Frat2 (F2a and F2b) shRNA retroviral vectors, and (D) in preleukemic MLL-ENL cells transduced with control (Con), Frat1 (F1), and Frat2 (F2) cDNA expressing retroviral vectors. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data are representative of 2 independent experiments. (E) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells transduced with Scrambled (Sc) and Frat2 (F2a and F2b) shRNA retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. Data are representative of 3 independent experiments. (B-C,E) At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then harvested for analysis or plated into methylcellulose. (D) At 48 hours after transduction, hCD2T+ transduced cells were positively selected by MACS and then harvested for protein lysates 5 days later.
FRAT proteins mediate MLL fusion-induced Rac activity. (A) Expression of Frat1, Frat2, and Dvl1 mRNA in preleukemic MLL-ENL cells treated with and without doxycycline for 72 hours, as determined by quantitative PCR. Bars represent means of quadruplicate values; and error bars, the SD. Data are representative of 3 independent experiments. (B) Quantitative PCR analysis of Frat1 and Frat2 mRNA expression in leukemic MLL-ENL cells transduced with Scrambled (Sc), Frat1 (F1a and F1b), and Frat2 (F2a and F2b) shRNA retroviral vectors. Bars represent means of quadruplicate values; and error bars, the SD. Data are representative of 3 independent experiments. (C-D) Western blot analysis of Rac1 GTP and Rac2 GTP levels, and total Rac protein expression (C) in leukemic MLL-ENL cells transduced with Scrambled (Sc), Frat1 (F1a and F1b), and Frat2 (F2a and F2b) shRNA retroviral vectors, and (D) in preleukemic MLL-ENL cells transduced with control (Con), Frat1 (F1), and Frat2 (F2) cDNA expressing retroviral vectors. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data are representative of 2 independent experiments. (E) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells transduced with Scrambled (Sc) and Frat2 (F2a and F2b) shRNA retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. Data are representative of 3 independent experiments. (B-C,E) At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then harvested for analysis or plated into methylcellulose. (D) At 48 hours after transduction, hCD2T+ transduced cells were positively selected by MACS and then harvested for protein lysates 5 days later.
To establish whether FRAT proteins could potentiate Rac activity, Frat1 and Frat2 were overexpressed in preleukemic MLL-ENL cells, which showed lower levels of Rac activity compared with leukemic cells. Rac1 activity increased on overexpression of either Frat1 or Frat2, with Frat2 inducing significantly greater activation (Figure 2D). Levels of total Rac1 and Rac2 protein expression remained unchanged. The effect of Frat inhibition on colony formation by MLL-ENL cells was then examined. Knockdown of Frat2, but not Frat1, expression resulted in a significant decrease in the number of colonies formed by leukemic cells (Figure 2E; supplemental Figure 3). Furthermore, Rac activation was no longer reduced in cells from the Frat2-silenced colonies that did form (supplemental Figure 4A). It was also apparent that Frat2 knockdown was not as efficient in these cells as in the cell originally plated out (supplemental Figure 4B), suggesting selection against cells with low Frat2 expression and low Rac activity. On the other hand, no such selection was evident in Frat1-silenced colonies (supplemental Figure 4B). Taken together, these data suggest that FRAT2 mediates Rac activation in preleukemic and leukemic MLL-ENL cells, with consequences for the oncogenic activity of MLL fusions.
FRAT proteins have been shown to take part in the formation of complexes between DVL, GSK3β, and Axin during canonical Wnt signaling.23 Because GSK3β can regulate Rac activity,40-42 we reasoned that FRAT2 may require DVL and GSK3β to induce activation of Rac in MLL-ENL cells. Thus, we decided to examine directly the requirement of DVL and GSK3 for MLL fusion-mediated Rac activation. Knockdown of Dvl1 expression resulted in decreased Rac1 activity in preleukemic cells (Figure 3A-B), reduced colony formation (Figure 3C; supplemental Figure 5A), and selection against colony-forming cells with low Dvl1 expression levels (supplemental Figure 5B). Similarly, pharmacologic inhibition of GSK3 also decreased Rac activity and colony formation (Figure 3D-E; supplemental Figure 6). Taken together, these data suggest that FRAT, DVL, and GSK3 are all required for Rac activation by MLL fusions.
DVL and GSK3 are required for Rac activation. (A) Quantitative PCR analysis of Dvl1 mRNA expression in leukemic MLL-ENL cells transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. Bars represent means of quadruplicate values; and error bars, the SD. (B) Western blot analysis of Rac1 and Rac2 GTP and total Rac in leukemic MLL-ENL cells, transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then harvested for protein lysates. Data are representative of 3 independent experiments. (C) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. (D) Western blot analysis of Rac1 and Rac2 GTP and total Rac in leukemic MLL-ENL cells, 24 hours after treatment with 2.5μM BIO acetoxime, 5μM CT99021, and 5μM SB216763. Data are representative of 6 (BIO acetoxime) and 3 (CT99021 and SB216763) independent experiments. (B,D) Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. (E) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells treated with 2.5μM BIO acetoxime, 5μM CT99021, and 5μM SB216763. Bars represent means of duplicate values; and error bars, the SD. *P < .05, versus control. **P < .01 versus control.
DVL and GSK3 are required for Rac activation. (A) Quantitative PCR analysis of Dvl1 mRNA expression in leukemic MLL-ENL cells transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. Bars represent means of quadruplicate values; and error bars, the SD. (B) Western blot analysis of Rac1 and Rac2 GTP and total Rac in leukemic MLL-ENL cells, transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then harvested for protein lysates. Data are representative of 3 independent experiments. (C) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells transduced with Scrambled (Sc) and Dvl1 (Dvl1a and Dvl1b) shRNA retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. (D) Western blot analysis of Rac1 and Rac2 GTP and total Rac in leukemic MLL-ENL cells, 24 hours after treatment with 2.5μM BIO acetoxime, 5μM CT99021, and 5μM SB216763. Data are representative of 6 (BIO acetoxime) and 3 (CT99021 and SB216763) independent experiments. (B,D) Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. (E) Number of colonies formed in methylcellulose by leukemic MLL-ENL cells treated with 2.5μM BIO acetoxime, 5μM CT99021, and 5μM SB216763. Bars represent means of duplicate values; and error bars, the SD. *P < .05, versus control. **P < .01 versus control.
FRAT oncoproteins confer chemoresistance to leukemic MLL fusion cells
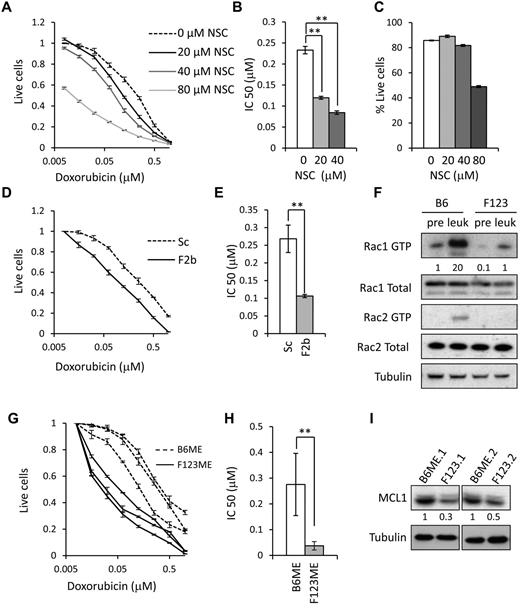
MLL-rearranged AML has a generally poor response to therapy.43 Recently, this has been recapitulated in a mouse model using a chemotherapeutic regime similar to that used in human patients, consisting of the anthracycline doxorubicin together with cytarabine.44 We reasoned that the poor response to doxorubicin might be explained by hyperactivation of Rac in the leukemic MLL-ENL cells because Rac has previously been implicated in the sensitivity of cancer cells to doxorubicin and other topoisomerase II inhibitors.45-47 To test this, leukemic MLL-ENL cells were exposed to a titration of doxorubicin concentrations, with or without the pharmacologic Rac inhibitor NSC23766 (NSC).48 This compound has previously been shown to effectively inhibit Rac activity in MLL fusion-expressing cells.10,20 Rac inhibition was found to synergize with the response to doxorubicin (Figure 4A), with 40μM NSC causing a > 2-fold reduction in the IC50 of doxorubicin (Figure 4B) without inducing significant loss of viability on its own (Figure 4C). Furthermore, knockdown of Frat2 in leukemic MLL-ENL cells was also found to significantly reduce the IC50 of doxorubicin (Figure 4D-E).
FRAT2 confers chemoresistance through Rac activation. (A) The proportion of leukemic MLL-ENL cells surviving exposure to the indicated concentrations of doxorubicin and NSC23766 (NSC) after 24 hours, normalized to cells treated with vehicle alone. Error bars represent SD of mean values (n = 3). (B) Bar chart showing IC50 values for graph in panel A. Error bars represent SD of mean values (n = 3). **P < .01 versus control. (C) Percentage of viable leukemic MLL-ENL cells after 24 hours of exposure to the indicated concentrations of NSC. Error bars represent SD of mean values (n = 3). (D) The proportion of leukemic MLL-ENL cells transduced with Scrambled (Sc) or Frat2 (F2b) shRNA retroviral vectors surviving exposure to the indicated concentrations of doxorubicin. Error bars represent SD of mean values (n = 3). At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then used in the analysis. (E) Bar chart showing IC50 values for graph in panel D. Error bars represent SD of mean values (n = 3). **P < .01 versus control. (F) Western blot analysis of Rac1 and Rac2 GTP and total Rac in preleukemic and leukemic wild-type B6ME (B6) and F123ME (F123) cells. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 3 independent experiments. (G) Graph showing the proportion of leukemic wild-type B6ME and F123ME cells surviving exposure to the indicated concentrations of doxorubicin after 24 hours, normalized to cells treated with vehicle alone. Responses for 3 independent B6ME and F123ME leukemic cell lines are shown. Error bars represent SD of mean values (n = 3). Data are representative of 4 independent experiments. (H) Bar chart showing IC50 values for graph in panel G. Error bars represent SD of mean values (n = 3 independent cell lines). **P < .01 versus control. (I) Western blot analysis of MCL expression in 2 independently generated leukemic B6ME and 2 F123ME cell lines. α-tubulin served as a loading control. Numbers represent densitometric quantitation of MCL1 levels normalized to α-tubulin.
FRAT2 confers chemoresistance through Rac activation. (A) The proportion of leukemic MLL-ENL cells surviving exposure to the indicated concentrations of doxorubicin and NSC23766 (NSC) after 24 hours, normalized to cells treated with vehicle alone. Error bars represent SD of mean values (n = 3). (B) Bar chart showing IC50 values for graph in panel A. Error bars represent SD of mean values (n = 3). **P < .01 versus control. (C) Percentage of viable leukemic MLL-ENL cells after 24 hours of exposure to the indicated concentrations of NSC. Error bars represent SD of mean values (n = 3). (D) The proportion of leukemic MLL-ENL cells transduced with Scrambled (Sc) or Frat2 (F2b) shRNA retroviral vectors surviving exposure to the indicated concentrations of doxorubicin. Error bars represent SD of mean values (n = 3). At 48 hours after shRNA transduction, cells were selected with puromycin for 72 hours and then used in the analysis. (E) Bar chart showing IC50 values for graph in panel D. Error bars represent SD of mean values (n = 3). **P < .01 versus control. (F) Western blot analysis of Rac1 and Rac2 GTP and total Rac in preleukemic and leukemic wild-type B6ME (B6) and F123ME (F123) cells. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 3 independent experiments. (G) Graph showing the proportion of leukemic wild-type B6ME and F123ME cells surviving exposure to the indicated concentrations of doxorubicin after 24 hours, normalized to cells treated with vehicle alone. Responses for 3 independent B6ME and F123ME leukemic cell lines are shown. Error bars represent SD of mean values (n = 3). Data are representative of 4 independent experiments. (H) Bar chart showing IC50 values for graph in panel G. Error bars represent SD of mean values (n = 3 independent cell lines). **P < .01 versus control. (I) Western blot analysis of MCL expression in 2 independently generated leukemic B6ME and 2 F123ME cell lines. α-tubulin served as a loading control. Numbers represent densitometric quantitation of MCL1 levels normalized to α-tubulin.
To confirm that FRAT oncoproteins mediate chemoresistance of leukemic MLL-ENL cells via activation of Rac, we immortalized bone marrow HPCs from Frat1/2/3 triple-knockout mice21 with MLL-ENL (F123ME cells) and generated leukemic cells by injecting these into sublethally irradiated syngeneic mice (supplemental Figure 7A-B). Rac1 activity was substantially reduced in preleukemic F123ME cells (Figure 4F). Although Rac1 activity increased in F123ME leukemic cells, compared with F123ME preleukemic cells, the final levels were only equivalent to those observed in wild-type preleukemic (B6ME) cells (Figure 4F). Active Rac2 was only detected in leukemic B6ME cells, being undetectable in both preleukemic and leukemic F123ME cells (Figure 4F). These data suggest that FRATs are required for efficient Rac activation by MLL fusions, although moderate Rac activation and the increase in Rac activity associated with leukemic progression can nevertheless occur in their absence. Interestingly, leukemic F123ME cells induced leukemia in secondary transplants with equivalent latency to that produced by leukemic B6ME cells (supplemental Figure 7C). Next, we compared the doxorubicin sensitivity of leukemic F123ME cells with that of wild-type leukemic B6ME cells. Consistent with the knockdown data, leukemic F123ME cells were found to be significantly more sensitive to doxorubicin (Figure 4G), the IC50 being reduced > 5-fold (Figure 4H). Inhibition of Rac has recently been shown to result in decreased expression of members of the antiapoptotic Bcl2 family, including MCL1.20 Consistent with this, MCL1 expression levels were considerably reduced in leukemic F123ME cells compared with those in leukemic B6ME cells (Figure 4I).
FRATN blocks Rac activation and induction of leukemia by MLL-ENL
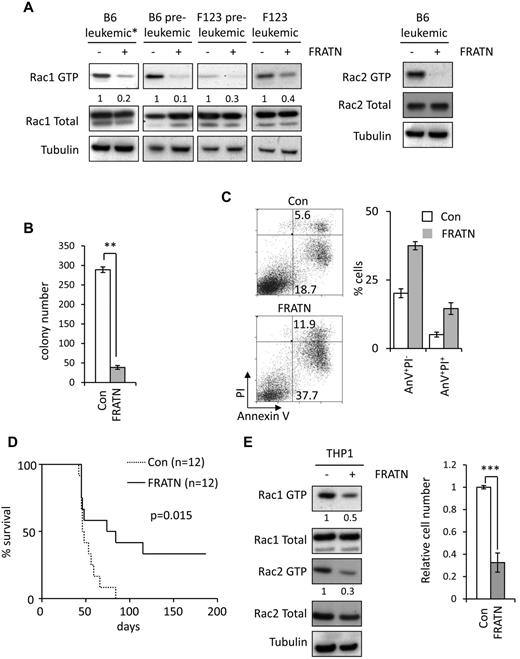
Our data suggest that FRAT2 mediates activation of Rac by MLL fusions. However, a degree of Rac activation can nevertheless be induced in the absence of FRAT proteins, in the F123ME cells. One explanation for this is that the signaling pathway involving DVL and GSK3 still operates in these cells. If this hypothesis is correct, blocking this pathway would inhibit Rac activation in wild-type and F123ME cells. A potential way of achieving this block would be to sequester DVL from GSK3 using the FRATN deletion mutant, which is unable to bind GSK3β. FRATN has been previously shown to disrupt the FRAT/DVL/GSK3 complex and inhibit Wnt signaling.23 To test this possibility, leukemic MLL-ENL cells were transduced with retroviral FRATN or control vectors, expressing the human “tailless” CD2 molecule (hCD2T),24 and the percentage of hCD2T+ cells was monitored in the culture over time. FRATN-transduced hCD2T+ MLL-ENL (Figure 5A) and MLL-AF9 (Figure 5B) cells were rapidly lost from culture compared with control cells. FRATN-expressing F123ME cells were also lost from culture (data not shown). Coexpression of constitutively active β-catenin did not rescue loss of FRATN-expressing cells (Figure 5C), indicating that inhibition of canonical Wnt signaling was not involved.
FRATN-expressing cells are selectively lost from culture. (A-B) Graphs showing the percentage of (A) hCD2T+ leukemic MLL-ENL cells (data are representative of 2 independent experiments) and (B) hCD2T+ preleukemic MLL-AF9 cells, at indicated time points in liquid culture, after transduction with control and FRATN-expressing retroviral vectors. Data are representative of 3 independent experiments. Error bars represent SD of mean values (n = 3). (C) Leukemic MLL-ENL cells were transduced with active β-catenin or control vectors; and 48 hours later, hCD4T+ cells were purified by MACS. hCD4T+ cells were then transduced with the FRATN-expressing or control pMSCV-IRES-hCD2T retroviral vectors. Graph showing the percentage of control or FRATN-transduced hCD2T+ leukemic MLL-ENL cells, with or without active β-catenin, at indicated time points in liquid culture. Error bars represent SD of mean values (n = 3). Data are representative of 2 independent experiments.
FRATN-expressing cells are selectively lost from culture. (A-B) Graphs showing the percentage of (A) hCD2T+ leukemic MLL-ENL cells (data are representative of 2 independent experiments) and (B) hCD2T+ preleukemic MLL-AF9 cells, at indicated time points in liquid culture, after transduction with control and FRATN-expressing retroviral vectors. Data are representative of 3 independent experiments. Error bars represent SD of mean values (n = 3). (C) Leukemic MLL-ENL cells were transduced with active β-catenin or control vectors; and 48 hours later, hCD4T+ cells were purified by MACS. hCD4T+ cells were then transduced with the FRATN-expressing or control pMSCV-IRES-hCD2T retroviral vectors. Graph showing the percentage of control or FRATN-transduced hCD2T+ leukemic MLL-ENL cells, with or without active β-catenin, at indicated time points in liquid culture. Error bars represent SD of mean values (n = 3). Data are representative of 2 independent experiments.
In subsequent experiments, hCD2T+ FRATN and control leukemic cells were purified by MACS. FRATN expression resulted in a marked reduction in Rac GTP levels in both preleukemic and leukemic wild-type B6ME cells (Figure 6A). We next examined whether FRATN was able to inhibit Rac activation in F123ME cells. FRATN expression also caused significant reduction in Rac activity in both preleukemic and leukemic F123ME cells (Figure 6A). These data indicate that the modest Rac activation, induced by MLL fusions in the absence of FRAT, is still mediated via noncanonical Wnt signaling, and blocking this pathway may be an effective means of inhibiting MLL fusion-induced leukemia. Indeed, expression of FRATN in wild-type leukemic MLL-ENL cells resulted in inhibition of colony formation, selection against FRATN expression on serial replating, and increased apoptosis (Figure 6B-C; supplemental Figure 8). Furthermore, FRATN expression was found to significantly reduce the incidence of leukemia induced by wild-type leukemic MLL-ENL cells after transplantation into sublethally irradiated syngeneic recipient mice (Figure 6D). Surprisingly, leukemic bone marrow from FRATN transfers that did develop disease exhibited a profound selection against hCD2T+ FRATN-expressing cells, despite these cells composing > 98% of the inoculum (supplemental Figure 9). In contrast, leukemic bone marrow cells from mice receiving control cells were uniformly hCD2T+. Similar data were obtained by expression of FRATN in leukemic F123ME cells (supplemental Figure 10). This indicates that FRATN expression is strongly detrimental to outgrowth of MLL-ENL–induced leukemia. To extend our analyses to human MLL-rearranged leukemia, we expressed FRATN in MLL-AF9+ THP1 cells. Consistent with our results in mouse cells, FRATN expression resulted in decreased Rac activity and growth of THP1 cells (Figure 6E).
FRATN blocks Rac activation and inhibits leukemia induction by MLL-ENL. (A) Western blot analysis of Rac1 and GTP and total Rac1 in preleukemic and leukemic wild-type B6ME (B6) and F123ME (F123) cells, transduced with control and FRATN-expressing retroviral vectors. Rac2 GTP and total Rac2 are also shown in leukemic B6ME cells. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 3 independent experiments. *The leukemic B6ME Rac1 blot shown is a shorter exposure compared with the other lanes. (B) Number of colonies formed in methylcellulose by purified hCD2T+ leukemic wild-type MLL-ENL cells transduced with control (Con) and FRATN-expressing (FRATN) retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. Data are representative of 4 independent experiments. (C) Plots represent apoptosis in leukemic wild-type MLL-ENL cells 5 days after transduction with control and FRATN-expressing retroviral vectors. Bar chart represents the mean percentages of early-stage (annexin V+PI−) and late-stage (annexin V+PI+) apoptotic cells in triplicate cultures; and error bars, the SD. Data are representative of 4 independent experiments. (D) Kaplan-Meier survival curves for mice transplanted with wild-type MLL-ENL leukemic cells, after transduction with control and FRATN-expressing retroviral vectors. Group numbers are shown. These data are representative of 2 independent experiments. (E) Western blot analysis of Rac1 and Rac2 GTP and total Rac in human THP1 cells transduced with control (−) and FRATN-expressing (+) lentiviral vectors (left panel). Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Graph represents relative cell number-transduced THP1 cells as measured by MTS assay. Bars represent mean values obtained from 3 independently transduced cultures; and error bars, the SD. ***P < .001 versus control. (A-D) At 48 hours after transduction, hCD2T+ transduced cells were positively selected by MACS and either used directly for the experiments (A-B,D) or equivalent numbers plated into culture and analyzed 3 days later (C).
FRATN blocks Rac activation and inhibits leukemia induction by MLL-ENL. (A) Western blot analysis of Rac1 and GTP and total Rac1 in preleukemic and leukemic wild-type B6ME (B6) and F123ME (F123) cells, transduced with control and FRATN-expressing retroviral vectors. Rac2 GTP and total Rac2 are also shown in leukemic B6ME cells. Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Data were obtained from individual preleukemic and leukemic cell lines in each case and are representative of 3 independent experiments. *The leukemic B6ME Rac1 blot shown is a shorter exposure compared with the other lanes. (B) Number of colonies formed in methylcellulose by purified hCD2T+ leukemic wild-type MLL-ENL cells transduced with control (Con) and FRATN-expressing (FRATN) retroviral vectors. Bars represent means of duplicate values; and error bars, the SD. **P < .01 versus control. Data are representative of 4 independent experiments. (C) Plots represent apoptosis in leukemic wild-type MLL-ENL cells 5 days after transduction with control and FRATN-expressing retroviral vectors. Bar chart represents the mean percentages of early-stage (annexin V+PI−) and late-stage (annexin V+PI+) apoptotic cells in triplicate cultures; and error bars, the SD. Data are representative of 4 independent experiments. (D) Kaplan-Meier survival curves for mice transplanted with wild-type MLL-ENL leukemic cells, after transduction with control and FRATN-expressing retroviral vectors. Group numbers are shown. These data are representative of 2 independent experiments. (E) Western blot analysis of Rac1 and Rac2 GTP and total Rac in human THP1 cells transduced with control (−) and FRATN-expressing (+) lentiviral vectors (left panel). Numbers represent densitometric quantitation of Rac GTP levels normalized to total Rac protein bands. α-tubulin served as a loading control. Graph represents relative cell number-transduced THP1 cells as measured by MTS assay. Bars represent mean values obtained from 3 independently transduced cultures; and error bars, the SD. ***P < .001 versus control. (A-D) At 48 hours after transduction, hCD2T+ transduced cells were positively selected by MACS and either used directly for the experiments (A-B,D) or equivalent numbers plated into culture and analyzed 3 days later (C).
Discussion
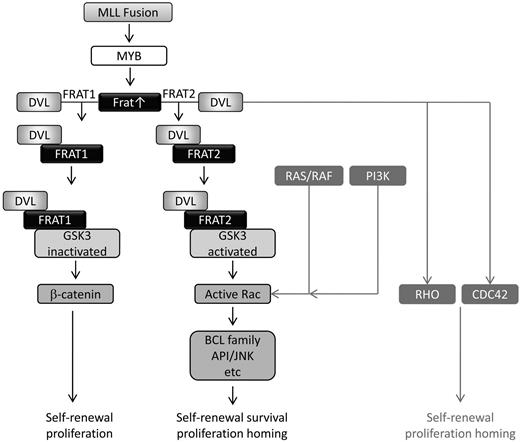
We present evidence for a mechanism to explain how MLL fusions induce Rac activation. MLL fusions up-regulate expression of Frat1 and Frat2, encoding oncoproteins that differentially promote canonical and noncanonical Wnt signaling, respectively.35 Inhibition of Frat2 expression results in reduced Rac activity, whereas Frat2 overexpression leads to enhanced activity. These effects are mediated via DVL1 and GSK3, both of which can modulate Rac activation, and sequestration of DVL from GSK3 inhibits Rac activation and the leukemogenic activity of MLL-ENL (Figure 7).
Model for MLL fusion-mediated noncanonical Wnt signaling leading to Rac activation. MLL fusions induce Frat1 and Frat2 gene expression. The FRAT1 protein promotes canonical Wnt signaling by binding and inactivating GSK3 and disrupting the β-catenin destruction complex. FRAT2 promotes activation of Rac GTPases, in a pathway requiring DVL expression and GSK3 activity. Activation of RHO and CDC42 by DVL and Rac by the RAS/RAF and PI3K pathways is also indicated. FRAT proteins thereby coordinate the differential requirement of GSK3 in canonical and noncanonical signaling pathways that are essential for the oncogenicity of MLL fusion proteins in AML.
Model for MLL fusion-mediated noncanonical Wnt signaling leading to Rac activation. MLL fusions induce Frat1 and Frat2 gene expression. The FRAT1 protein promotes canonical Wnt signaling by binding and inactivating GSK3 and disrupting the β-catenin destruction complex. FRAT2 promotes activation of Rac GTPases, in a pathway requiring DVL expression and GSK3 activity. Activation of RHO and CDC42 by DVL and Rac by the RAS/RAF and PI3K pathways is also indicated. FRAT proteins thereby coordinate the differential requirement of GSK3 in canonical and noncanonical signaling pathways that are essential for the oncogenicity of MLL fusion proteins in AML.
The up-regulation of Frat1 and Frat2 expression by MLL fusions and consequent induction of both canonical and noncanonical Wnt signaling35 may go some way toward explaining the apparently contradictory role of GSK3 in MLL-rearranged leukemia. Recent studies have suggested that canonical Wnt signaling plays a critical role in this disease.16,18 Blocking the expression or function of β-catenin, the key mediator of canonical Wnt signaling, impaired induction of leukemia by MLL-AF9.16,18 Furthermore, up-regulation of canonical Wnt signaling was shown to be essential for preleukemic progression into overt leukemia.16 Despite its well-characterized negative regulation of Wnt signaling, other studies have demonstrated a central role for GSK3 activity in mediating MLL fusion leukemogenesis. Thus, GSK3 enforces the negative regulation of p27 protein expression and promotes CREB/MEIS1 association to maintain an aberrant self-renewal program.49,50 At first glance, these data present an apparent contradiction. On the one hand, inhibition of GSK3β is required to protect transcriptionally active β-catenin from degradation and in order for canonical Wnt signaling to proceed.51 On the other hand, inhibition of GSK3β blocks key oncogenic activities of MLL fusions. Our data suggest a potential solution to this paradox. Through regulation of Frat expression, MLL fusions promote both canonical and noncanonical Wnt signaling, GSK3β playing distinct and opposing roles in each of these processes (Figure 7).
Although FRAT1 is not essential for Wnt/β-catenin signaling, it amplifies canonical Wnt signaling by competing with AXIN for GSK3β binding and disrupting the β-catenin destruction complex.23 FRAT2 was found to be less effective at inducing canonical Wnt pathway but did promote noncanonical Wnt signaling.35 Thus, although FRAT1 and FRAT2 can each activate canonical and noncanonical Wnt signaling to some extent, they appear to have different specificities.35 This is supported by studies demonstrating that FRAT1 binds and inhibits GSK3β, whereas FRAT2 stimulates GSK3β phosphorylation of certain substrates.52 In some circumstances, active GSK3β can in turn lead to activation of Rac.41,42 Because FRAT1 has previously been shown to bridge DVL to GSK3β in canonical Wnt signaling,23 it is possible that in noncanonical Wnt signaling FRAT2 bridges DVL to GSK3β to promote Rac activation. Our data would be consistent with this scenario. This would explain why FRATN-mediated sequestration of DVL from GSK3β, knockdown of Dvl expression, or pharmacologic inhibition of GSK3β results in inhibition of Rac activation.
The ability of MLL-ENL to immortalize Frat TKO HPCs is somewhat surprising, given the effect of Frat2 silencing on colony formation by leukemic MLL-ENL cells. However, because Frat TKO HPCs develop in the absence of FRAT expression, they may use compensatory mechanisms to activate noncanonical Wnt signaling. In this case, MLL-ENL may be able to immortalize Frat TKO cells that have bypassed a requirement for FRAT2 expression, using alternative signaling pathways to induce the modest Rac activation observed in these cells. On the other hand, wild-type HPCs immortalized by MLL-ENL may be more dependent on continued FRAT2 expression. A precedent for such a scenario is provided by a study demonstrating a requirement for Stat3 activity in NPM-ALK–mediated lymphomagenesis.53 Thus, this study found that, although there was no difference in the ability of NPM-ALK to induce lymphomas in wild-type and Stat3-deficient T-cells, NPM-ALK–transformed lymphomas with wild-type Stat3 were absolutely dependent on continued Stat3 expression. The generation of a conditional Frat TKO model, in which the Frat genes could be deleted in leukemic cells after immortalization with MLL-ENL, would go some way to addressing this matter.
Although there was no significant difference in the ability of MLL-ENL to immortalize and induce leukemia in wild-type and Frat TKO HPCs, it was only able to induce weak Rac activation in the latter. The mechanism underlying such activation in the absence of FRAT proteins is unclear. A recent study demonstrated that HOXA9 is able to induce Rac1 activity via transactivation of the Vav2 gene.54 Because Hoxa9 is a downstream target of MLL fusions, this may explain part of the residual Rac activity in the Frat TKO cells. However, it is important to note that, even in Frat TKO cells, a large component of Rac activity is sensitive to DVL sequestration by FRATN. This suggests that, by sequestering DVL from GSK3β, FRATN acts as a dominant negative mutant, inhibiting Rac activation, even in the absence of FRAT protein expression.
Previous studies have implicated Rac in the progression of many solid cancers55,56 and hematologic malignancies.57,58 In MLL-rearranged AML, the importance of Rac activation to the leukemogenic function of MLL fusions has also been recently demonstrated.10,19,20 By identifying the mediators of MLL fusion-induced Rac activation, the present study offers new candidates for novel therapeutic targeting. The inhibition of leukemic progression and Rac activation by FRATN suggests that targeting the FRAT2/DVL/GSK3β axis would be an effective means of interfering with MLL fusion activity. This is further supported by our data showing that leukemic cells lacking FRAT proteins are more sensitive to chemotherapeutic drugs. Interestingly, active Rac has recently been shown to enhance canonical Wnt signaling, possibly by promoting nuclear localization of β-catenin.29 Thus, as well as regulating proliferation and survival of leukemic cells, Rac activation may also serve to reinforce canonical Wnt signaling. This suggests the possibility that MLL fusions promote integrated canonical and noncanonical Wnt signaling by regulating FRAT expression. Interfering with the function of FRAT proteins and their downstream partners may represent a new approach to developing novel therapies for MLL-rearranged AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank D. Trono (Lausanne) for providing the lentiviral packaging constructs, Ayad Eddaoudi (UCL Institute of Child Health Flow Cytometry Facility) for providing assistance with flow cytometry, and all the staff of the UCL Institute of Child Health Western Laboratories for their help and support.
This work was supported by Leukemia and Lymphoma Research (grant 10032; J.d.B. and O.W.) and the Great Ormond Street Hospital Children's Charity (grant W1055; V.W.-V. and O.W.).
Authorship
Contribution: V.W.-V. and J.d.B. performed research, analyzed and interpreted data, and wrote the manuscript; S.J.H. performed research and analyzed and interpreted data; R.v.A., N.P., and A.B. contributed vital reagents and revised the manuscript; and O.W. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.J.H. is Department of Haematology, University of Cambridge, Cambridge Institute for Medical Research, Cambridge, United Kingdom.
Correspondence: Owen Williams, Molecular Haematology and Cancer Biology Unit, UCL Institute of Child Health and Great Ormond Street Hospital, 30 Guilford Street, London WC1N 1EH, United Kingdom; e-mail: owen.williams@ucl.ac.uk.
References
Author notes
V.W.-V. and J.d.B. contributed equally to this study.