Abstract
Abstract 4925
We have developed a method combining fluorescence in situ hybridization (FISH) and flow cytometry to detect monosomy 7 in interphase cells of patients with myelodysplastic syndrome. This method, Interphase Chromosome Flow-FISH (IC Flow-FISH), involves fixation of leukocytes cells, permeabilization of cellular membranes, DNA hybridization with peptide nucleic acid (PNA) probes, and analysis by flow cytometry. Samples of 10–20 mL of peripheral blood in patients with monosomy 7 yielded hundreds to thousands of monosomy 7 cells detected by flow cytometry.
Peripheral blood samples were obtained from patients with MDS who tested positive for monosomy 7 by conventional cytogenetics, and mononuclear cells were isolated by Ficoll density centrifugation and fixed with Carnoy's solution. Cell membranes were then permeabilized with a saponin-based detergent, and cellular DNA was denatured with heat and hybridized with denatured PNA probes while the cell membrane remained intact. Next cells were washed and analyzed by flow cytometry.
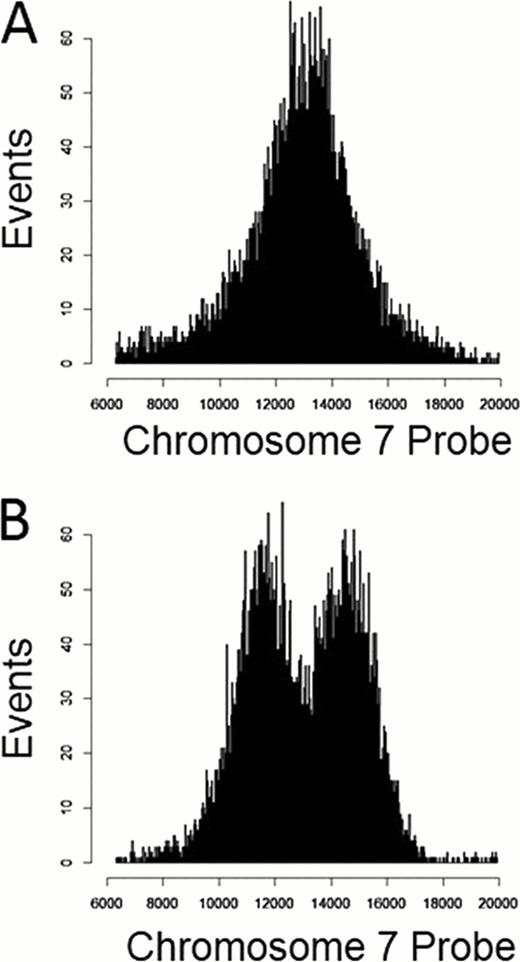
Healthy donor samples showed similar distributions of chromosome 7 probe fluorescence between monocytes and (unaffected) lymphocytes such that there was no clear distinction between these populations in a histogram of probe fluorescence (fig 1A). In samples from patients with monosomy 7, a population of monocytes exhibited distinctly lower probe fluorescence than did normal lymphocytes and monocytes (fig 1B). As MDS affects cells of the myeloid rather than the lymphoid lineage, a decrease in probe binding among monocytes rather than lymphocytes was consistent with disease characteristics. In 5 patient samples, there were no statistically significant differences between proportions of monocytes with monosomy 7 detected in IC Flow-FISH and proportions of cells with monosomy 7 detected in conventional cytogenetics.
Patient cells were sorted with fluorescence-activated cell sorting (FACS) to verify the population of cells with lower probe fluorescence corresponded to cells having monosomy 7 and that cells with higher fluorescence showed normal chromosome 7 copy number. Of patient cells gated for lower fluorescence, 45 of 50 (90%) cells analyzed by microscopy showed 1 distinct nuclear signal and 5 of 50 (10%) showed 2 signals. Of cells gated as having higher fluorescence, 39 of 42 (93%) cells showed 2 distinct signals and 3 of 42 (7%) showed only 1 distinct signal.
IC Flow-FISH provides the ability to detect monosomy 7 in MDS patients without bone marrow procurement or metaphase spreads. Because the number of monosomy 7 cells isolated by FACS may range in the thousands, the method offers a large amount of data while also allowing automated detection and isolation of cells with aneuploidy for further analysis and characterization. Application to other chromosome number abnormalities has the potential to expand the range of the technique to a variety of hematologic disease settings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal