Abstract
Abstract 32
Since the introduction of tyrosine kinase inhibitor (TKI) therapy overall survival and complete molecular response rates in chronic phase chronic myeloid leukemia (CP-CML) patients have significantly improved. However, leukemic stem cells (LSCs) and progenitor cells persist and are thought to be responsible for disease progression, development of TKI resistance and disease recurrence after stopping TKI therapy. Protection by cytokines, such as IL-3 and GM-CSF, provides a potential mechanism of LSC resistance. While in acute myeloid leukemia (AML) monoclonal antibody (mAb) targeting of IL-3 receptor α (CD123), a recognized marker for AML LSCs, has been studied in vitro and in vivo, similar investigations have not been undertaken in CML to date. CSL362 is a genetically-engineered form of the specific blocking mAb 7G3 optimized for Fc receptor binding to achieve maximal antibody-dependent cell-mediated cytotoxicity (ADCC) capacity. Here we investigate the expression of CD123 in CD34+ progenitors and CD34+CD38− LSCs, isolated from CP- and blast crisis (BC) - CML patients, and study the benefits of targeting those cells by CSL362 alone and in combination with TKIs.
Flow cytometry analysis established significantly elevated expression of CD123 on CD34+CD38− cells from CP-CML (53.0 ± 5.8 %, n=16, p=0.003) and BC-CML (73.2 ± 6.7 %, n=18, p<0.001) patients compared to normal donors (20.3 ± 4.2 %, n=8), with clear increases in CD123 expression with disease progression in matched samples (n=2). Subsequent assessment of apoptosis, colony forming unit (CFU-GM) and long-term culture-initiating cell (LTC-IC) potential confirmed the ability of CSL362 to block IL-3-mediated rescue of TKI-induced cell death. However, in the presence of other cytokines, likely found in the physiological bone marrow microenvironment, this effect was lost.
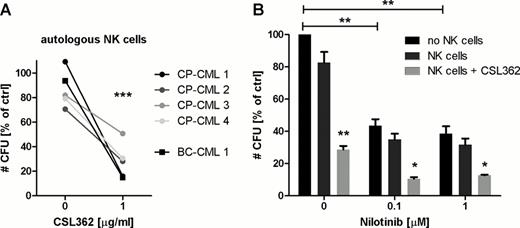
We also demonstrate by lactate dehyrogenase release and clonogenic assays that CML CD34+ cell numbers are significantly reduced, in a dose-dependent manner, by CSL362-induced ADCC employing NK cells from healthy donors (42.4 ± 8.1 % lysis, n=3, and CFU-GM decreased to 30 ± 10.8 % of controls, n=5, p=0.003). In keeping with this, flow cytometry analysis revealed specific elimination of CP- and BC-CML CD123+ CD34+CD38− cells (from 42.9 % to 18.6 %, n=5, p=0.004, and from 71 % to 35.3 %, n=3, p=0.044, respectively). Importantly, autologous CML patient NK cells, collected after achievement of major molecular response, also mediate CSL362-dependent cytotoxicity similar to allogeneic healthy donor NK cells as indicated by equivalent numbers of remaining CFUs (28 ± 6.7 % vs. 34.9 ± 3.4 %, n=5, Fig. A). We further have evidence to suggest preferential elimination of CML over normal LTC-ICs (30.3 ± 9.9 % vs. 62.6 ± 11.2 % remaining, n=3, p=0.096) in the autologous setting. Of clinical importance, the combination of Nilotinib and CSL362 resulted in a significantly greater reduction in CFUs (additive effect) when compared to either agent alone (Fig. B).
Taken together these data suggest that selective ADCC-mediated lysis, likely the major mode of action of CSL362 in vivo, efficiently eliminates CML progenitor and stem cells. Promising results evaluating CSL362/TKI combination treatments, with the expectation to further enhance specificity for leukemic while sparing normal progenitor and stem cells as indicated from preliminary experiments, warrant further studies.
A: Autologous NK cells are able to confer CSL362-induced ADCC against CML CD34+ cells. Cells were co-cultured at an effector to target cell ratio (E:T) of 10:1 in the absence and presence of CSL362 as indicated for 4 h and remaining CFU-GM were enumerated. Data is normalized to target cells alone (*** p<0.001).
B: CSL362-mediated ADCC and TKI treatment show additive effects. CP-CML CD34+ cells were cultured with nilotinib at varying concentrations as indicated for 48 h before overnight exposure to CSL362 (1 μg/ml) with or without allogeneic NK cells (E:T 1:1). Mean ± SE of CFU-GM colony numbers is shown (n=3, * p<0.05, ** p<0.01).
A: Autologous NK cells are able to confer CSL362-induced ADCC against CML CD34+ cells. Cells were co-cultured at an effector to target cell ratio (E:T) of 10:1 in the absence and presence of CSL362 as indicated for 4 h and remaining CFU-GM were enumerated. Data is normalized to target cells alone (*** p<0.001).
B: CSL362-mediated ADCC and TKI treatment show additive effects. CP-CML CD34+ cells were cultured with nilotinib at varying concentrations as indicated for 48 h before overnight exposure to CSL362 (1 μg/ml) with or without allogeneic NK cells (E:T 1:1). Mean ± SE of CFU-GM colony numbers is shown (n=3, * p<0.05, ** p<0.01).
Nievergall:CSL Ltd: Research Funding. White:BMS: Research Funding; CSL Ltd: Research Funding; Novartis Oncology: Honoraria, Research Funding. Ramshaw:CSL Ltd: Research Funding. Busfield:CSL Ltd: Employment. Vairo:CSL Ltd: Employment. Lopez:CSL Ltd: Research Funding. Hughes:Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Ltd: Research Funding. Hiwase:CSL Ltd: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal