Abstract
Abstract 2870
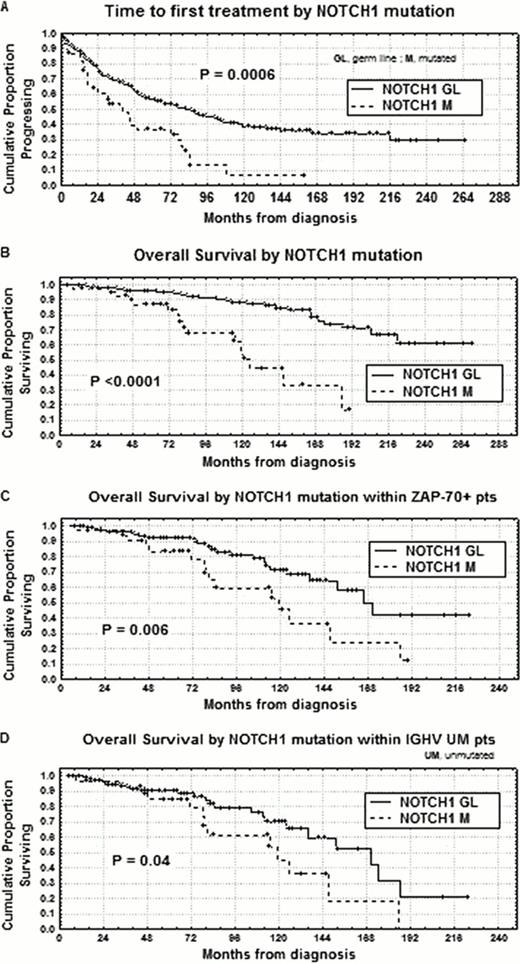
Chronic lymphocytic leukemia (CLL) is a very heterogeneous disease ranging from rapid disease progression leading to death to a nearly normal life expectancy and therefore it is mandatory to find valid prognostic markers. Recent studies showed that activating mutations of NOTCH1 proto-oncogene occur in about 10% CLL at diagnosis and are associated with an unfavorable clinical outcome (Rossi et al, 2012). About 85% of NOTCH1 mutated CLL cases displayed a DCT7544–7545 frameshift deletion (hereafter NOTCH1 mutation), that has been demonstrated to predict NOTCH1 degradation impairment through the truncation of the C-terminal PEST domain. Given the possibility of targeting NOTCH1 with drugs currently under development, the primary endpoints of our research were: 1) to correlate NOTCH1 mutation with other clinical and biological prognostic factors; 2) to determine time to first treatment (TTFT) and overall survival (OS) upon NOTCH1 mutation in univariate analysis; 3) to validate NOTCH1 mutation as an independent prognostic factor. We investigated 463 pts, median age 65 years (range 33–89), 256 males and 207 females. With regard to modified Rai stages at diagnosis, 159 had a low stage, 290 an intermediate stage and 14 a high stage. NOTCH1 mutation was investigated by amplification refractory mutation system (ARMS) PCR at diagnosis or before any chemotherapeutic approach. The ARMS PCR approach was set up in order to identify NOTCH1 mutation when present in at least 10% of the alleles. Using this approach, NOTCH1 mutated pts were 45/463 (9.7%). Considering the association with markers of tumor burden and proliferation, NOTCH1 mutation correlated with intermediate/high Rai stages (37/45; P=0.002), multiple thoracic/abdominal lymphadenopathies and/or splenomegaly (26/45, P=0.003), beta-2 microglobulin >2.2 mg/ml (27/45; P=0.02), lymphocyte doubling time <12 months (19/45; P=0.0006) and soluble CD23>70 U/ml (26/39; P=0.00001). Significant associations were also found with the main biologic prognostic markers in CLL. In this regard, NOTCH1 mutation was associated with an unmutated IGHV status (available for 446 total cases, 30/43; P<0.0001), CD38>30% (26/45, P<0.0001), ZAP-70>20% (33/45; P<0.0001), and CD49d>30% (22/34; P=0.009). Finally, considering associations with specific chromosomal aberrations defined by FISH cytogenetics (available in 417 cases), significant correlations (P=0.003) were found between NOTCH1 mutation and trisomy 12 (14/41; 25%), and del11q (7/41;16% ), whereas only 2/43 NOTCH1 mutated cases presented 17p deletion. With regard to clinical outcome, 30/45 (67%) NOTCH1 mutated pts received chemotherapy vs 193/418 (46%) among NOTCH1 germ line CLL (P=0.01), with 15/45 (33%) vs 48/418 (11%) cases, belonging to the same subgroups, undergoing at least two lines of treatment (P=0.001). Moreover, both significant shorter TTFT and OS were observed in NOTCH1 mutated pts (7% vs 35% at 12 years, P=0.0006 and 34% vs 78% at 14 years, P<0.0001; Figures A, B). To further test the clinical value of NOTCH1 mutation in CLL, we investigated its prognostic impact in bivariate analyses with the main clinical/biological prognosticators. According to these analyses, pts with NOTCH1 mutation showed shorter OS both within the ZAP-70 positive (>20%, 188 pts) and unmutated IGHV (<2%, 144 pts) subsets (24% vs 50% at 14 years; P=0.006, and 18% vs 52% at 14 years, P=0.04, respectively; Figures C, D). In multivariate analysis of OS, NOTCH1 mutation (P=0.02) together with age (P=0.001), FISH cytogenetics (P=0.001) and CD38 (P=0.002) was confirmed to be an independent prognostic factor, after correcting for colinearity with IGHV status. Therefore, NOTCH1 mutation, as determined by ARMS PCR, is a novel important prognostic parameter in CLL to be considered in drawing prognostic scores. In addition, NOTCH1 might represent a commendable therapeutic target for specific inhibitors to be employed especially in NOTCH1 mutated CLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal