Abstract
Abstract 2396
Despite recent advances in understanding the key molecular mechanisms of leukemogenesis, the outcome for patients with Acute Myeloid Leukemia, particularly with a normal karyotype, remains poor. For this large group of patients, genetic alterations in genes such as FLT3, NPM1, CEBPA, IDH1/2, and DNMT3A provide useful prognostic information. However, risk stratification of this group remains only partially resolved and markers of response that can be therapeutically targeted would likely improve outcome for these patients. GADD45A is a tumor suppressor gene that plays cell-type dependent roles in cellular stress coordinating DNA repair and de-methylation, cell cycle arrest, and pro-apoptotic or pro-survival responses (Cancer Ther. 2009;7:268). Methylation of four discrete CpG residues in the proximal promoter of GADD45A is a hallmark of many solid tumours and has been associated with impaired cell stress signalling and reduced drug response (Cancer Res. 2009;69:1527; Oncogene. 2005;24:2705). In AML, GADD45A expression is broadly down-regulated both in normal karyotype and other cytogenetic classes. Down-regulation of GADD45A in AML has been associated with FLT3-ITD (Leukemia. 2009;23:729) and RUNX1 mutations (Satoh et al, Leukemia. 2011;Epub). For those patients without these mutations, the mechanism of GADD45A down-regulation and its prognostic significance remains unknown. We hypothesised that the promoter of GADD45A is methylated in AML and that this methylation is functionally important in patient response.
Using the Sequenom MassARRAY methodology we screened for methylation of four GADD45A promoter CpG dinucleotides (CpG1–4) previously shown to be associated with silencing of GADD45A in breast and prostate cancer, in a retrospective cohort of 222 AML patients collected at diagnosis from the Royal Adelaide Hospital. We then determined association of CpG methylation with outcome and mutation status in our treated patient cohort of 167 patients. In AML cell lines and in primary patient samples we also determined the response to cytotoxic agents in vitro in the presence and absence of demethylating agents.
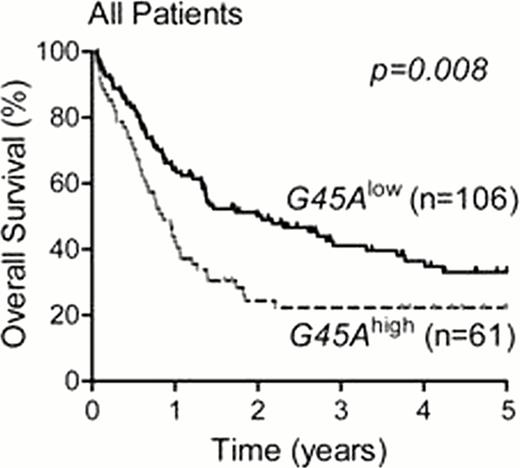
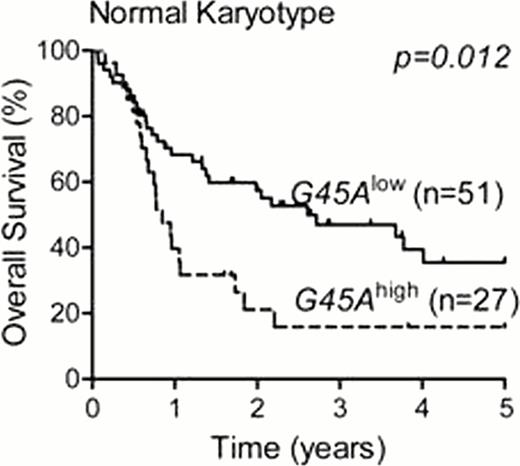
We observed hypermethylation of the CpG1–4 in the proximal promoter of GADD45A in 93 of 222 (42%) of AML patients and in 6 AML cell lines. In the 167 patients treated with standard induction chemotherapy regimes, 61 patients showed methylation of the GADD45A proximal promoter. Of the four CpG residues, methylation of CpG1 was associated with poor overall and event-free survival, in AML overall (Figure 1A) and in normal karyotype AML (Figure 1B). GADD45A CpG1 methylation was significantly associated with IDH1 and IDH2 mutations (p<0.001), but was not associated with FLT3-ITD or other high risk cytogenetic groups. Multivariate analysis (including age, wcc, FLT3-ITD, IDH1/2) revealed that methylation of GADD45A CpG1 is an independent predictor of poor survival in AML, overall (OS; HR 2.17, p=0.006: EFS; HR 2.43, p=0.001), and in normal karyotype AML (OS; HR 2.86, p=0.014: EFS; 5.25, p<0.001). Additionally, treatment of AML cell lines and patient blasts with decitabine (5-Aza-deoxycitidine) resulted in induction of GADD45A mRNA selectively in samples with GADD45A hyper-methylation, and this was associated with increased sensitivity to daunorubicin.
DNA methylation of the GADD45A proximal promoter marks a large percentage AML patients, including those with with IDH1/2 mutations, and is an independent predictor of poor outcome, particularly in AML patients with normal karyotype. Our biological data shows that induction of GADD45A mRNA expression with decitabine in methylated samples is associated with increased response to cytotoxic agents. Thus GADD45A proximal promoter methylation represents a new biomarker that may provide prognostic information in the heterogeneous normal karyotype AML group. Given that recent clinical trials are combining Azacitidine with chemotherapy and other agents for induction therapy in AML (Blood;118:1472), this may represent a marker to help define patients that will benefit from this approach.
Kaplan-Meier survival curves showing 5-year overall survival of patients with GADD45A CpG1 high (G45Ahigh) versus low (G45Alow) methylation in AML overall, and in normal karyotype AML.
Kaplan-Meier survival curves showing 5-year overall survival of patients with GADD45A CpG1 high (G45Ahigh) versus low (G45Alow) methylation in AML overall, and in normal karyotype AML.
Wei:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal