Abstract
Abstract 2124
Without effective iron chelation therapy (ICT), patients with transfusional iron overload are at risk of excess iron-related cardiac complications. Cardiac iron accumulation can be measured using T2* magnetic resonance (normal >20 ms, high risk <10 ms). There are few randomized controlled trials assessing ICT for cardiac iron removal. CORDELIA is a Phase II, multinational, randomized comparison of efficacy and safety of 1-yr treatment with deferasirox or deferoxamine (DFO). Primary objective was non-inferiority of deferasirox vs DFO for cardiac iron removal after 1 yr.
Patients with β-thalassemia major, cardiac T2* 6–20 ms, no clinical symptoms of cardiac dysfunction, aged ≥10 yrs, history of ≥50 transfusions, left ventricular ejection fraction (LVEF) ≥56% and liver iron concentration (LIC) ≥3 mg Fe/g dry weight (dw) were recruited. Patients were randomized to an intensified DFO regimen with a target dose of 50–60 mg/kg/d sc for 8–12 hrs, 5–7 d/wk, or deferasirox with a target daily oral dose of 40 mg/kg/d. Dose adjustment recommendations were based on continuous assessment of efficacy and safety markers.
Efficacy was assessed in the per-protocol analysis population. Primary efficacy endpoint was change after 1-yr treatment (using last available value ≥150 d after randomization) in cardiac T2* expressed as the ratio of geometric means (Gmean) at end of study (EOS) over baseline (BL) for deferasirox divided by the ratio of Gmeans for DFO. Non-inferiority was pre-defined if the lower limit of the 2-sided repeated 95% confidence interval (CI) for ratio of Gmeans was >0.9.
From 925 screened patients, 197 patients (mean age 19.8 ± 6.4 yrs) were randomized. Mean time since start of transfusions was 19.3 and 18.4 yrs in deferasirox and DFO patients, respectively. All patients had received previous ICT. At BL, Gmean cardiac T2* was 11.4 ms; mean ± SD LIC was 29.8 ± 17.5 mg Fe/g dw in deferasirox patients and 30.3 ± 17.9 mg Fe/g dw in DFO patients; median (range) serum ferritin level was 5062 (613–15331) and 4684 (677–13342) ng/mL, respectively. 160 (81.2%) patients completed 1 yr. Mean actual dose of deferasirox was 36.7 ± 4.2 mg/kg/d and DFO was 41.5 ± 8.7 mg/kg/d for 7 d/wk.
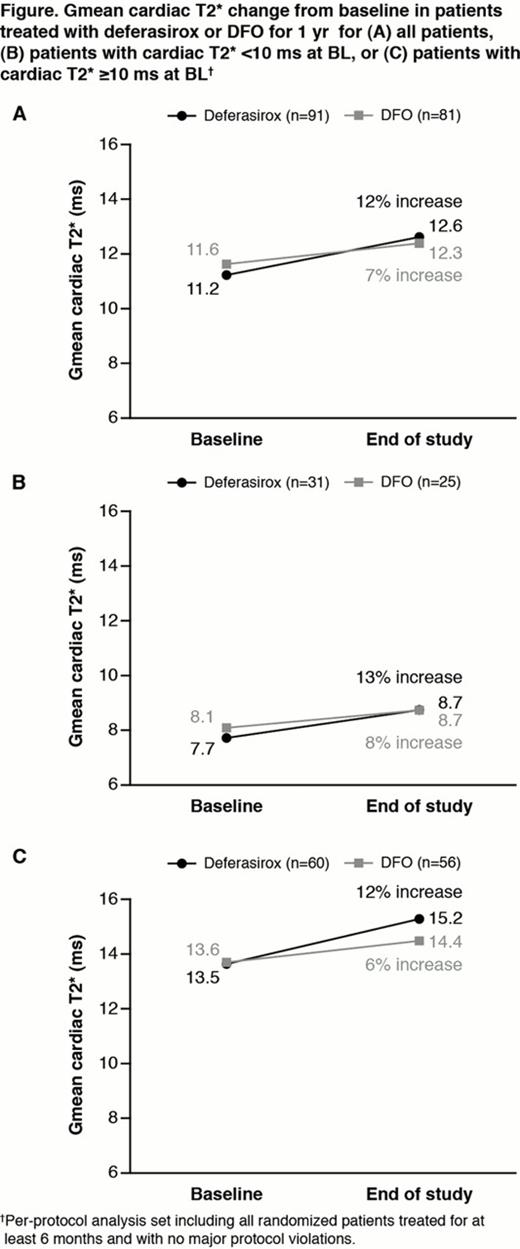
Overall, Gmean cardiac T2* increased by 12% with deferasirox and 7% with DFO after 1 yr (Fig A). The Gmean ratio between the two arms was 1.0557 (95% CI 0.9981, 1.1331). Lower limit of the 95% CI was >0.9, demonstrating non-inferiority of deferasirox vs DFO, with a trend towards superiority (P=0.0567). Trends toward increases were observed in patients with severe or mild/moderate cardiac iron (Fig B, C). In patients with BL LIC <7 mg Fe/g dw, increase in cardiac T2* was 30% for deferasirox (n=11) and 10% for DFO (n=8), for BL LIC 7–<15 mg Fe/g dw increase was 19% (n=14) for deferasirox and 13% (n=14) for DFO, and in patients with BL LIC ≥15 mg Fe/g dw increase was 9% (n=66) and 5% (n=59), respectively.
LVEF was stable with deferasirox (BL 66.9 ± 5.61%; EOS 66.3 ± 5.8%) and DFO (BL 66.4 ± 5.2%; EOS 66.4 ± 5.8%). LIC absolute change from BL was –8.9 ± 11.4 (95% CI –11.5, –6.4) mg Fe/g dw for deferasirox and –12.7 ± 11.4 (–15.3, –10.1) mg Fe/g dw for DFO.
Overall adverse event (AE) rates were 67.7% in deferasirox patients and 75.8% in DFO patients. In deferasirox patients, most common AEs were diarrhea (12.5%), proteinuria (11.5%) and influenza (10.4%). Most common AEs in DFO patients were proteinuria (8.8%), upper respiratory tract infection (8.8%) and influenza (6.6%). Serious AEs occurred in 10.7% patients overall (10.4% deferasirox; 11.0% DFO), with many related to the underlying disease.
3 deferasirox patients and 1 DFO patient had 2 consecutive serum creatinine increases >33% above BL and >upper limit of normal (ULN). Overall, 14.6% of deferasirox patients and 3.3% of DFO patients had ALT levels >5xULN and >2xBL. One death (arrhythmia) in the deferasirox arm was considered unrelated to study drug. One death (meningitis) in a DFO patient was suspected to be related to DFO.
CORDELIA, the first randomized controlled trial comparing deferasirox with DFO for cardiac iron removal, met its primary endpoint in demonstrating non-inferiority of deferasirox vs DFO, with a trend for superiority. There was a trend toward more pronounced improvements in cardiac T2* with deferasirox vs DFO in patients with BL LIC <15 mg Fe/g dw. The frequency of AEs was similar between treatment groups and the deferasirox safety profile was comparable to previous reports.
Pennell:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Siemens: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Apotex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CVIS: Equity Ownership. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Piga:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lawniczek:Novartis: Employment. Habr:Novartis: Employment. Weisskopf:Novartis: Employment. Zhang:Novartis: Employment. Aydinok:Ferrokin: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal