Abstract
Abstract 1973
Mutations in the spliceosome machinery have recently been identified in myeloid neoplasms including acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), and MDS/MPN overlap syndromes. Somatic alterations in SF3B1, U2AF1 and SRSF2 are the most frequently affected spliceosome genes in myeloid malignancies with the frequencies of any of the 3 genes being 39% in low risk MDS, 11% in high risk MDS/AML (primarily U2AF1), and 24% in MDS/MPN, (primarily SRSF2). While SF3B1 mutations as well as ring sideroblasts (RS) appear to be associated with improved survival and lower risk of leukemic evolution, mutations of U2AF1 and SRSF2 appear to be associated with poorer outcomes. Allogeneic hematopoietic cell transplantation (HCT) is the only curative option for many myeloid malignancies, however, the prognostic role of SF3B1, U2AF1 or SRSF2 mutations in patients (pts) undergoing high intensity chemotherapy (HIC) and HCT has yet to be investigated.
We evaluated 404 pts with the diagnosis of AML (n=96), MDS and MDS/MPN (n=294), MPN (n=13), and congenital sideroblastic anemia (n=1). 35 pts received HIC defined as treatment with 7+3, high dose cytarabine, clofarabine, or other comparable chemotherapy in the context of a clinical trial. 97 pts underwent a myeloablative (n=69) or reduced intensity (n=28) allogeneic HCT from an HLA matched related (n=39), unrelated (n=50) or cord blood (n=8) donor from April 2003 until August 2011. Median age of the whole cohort was 69 and those who underwent BMT at time of transplant was 52 (range 19–70). We performed direct sequencing for the three most commonly mutated spliceosome genes, SF3B1 (exon 13–16), SRSF2 (exon 1 and 2) and U2AF1 (exon 2–6). Given the close association between SF3B1 mutations and RS, we analyzed the impact of the presence or absence of RS on the outcomes associated with the type of therapy received. We found a total of 24 SF3B1 mutations in MDS and MDS/MPN (primarily in pts with RS), 37 SRSF2 mutations in MDS and MDS/MPN (primarily CMML), and 13 U2AF1 mutations in MDS, MDS/MPN and secondary AML.
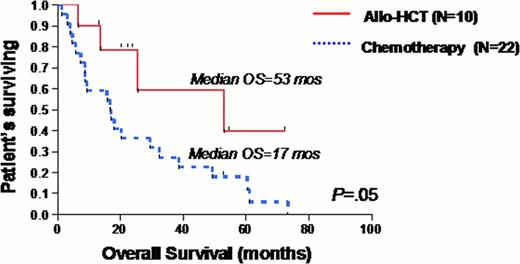
Among pts with MDS and MDS/MPN, significant overall survival (OS) differences were found between SF3B1 mutant and wild type (WT), (83 vs. 31 months, p=0.001) and U2AF1 or SRSF2 mutant (18 vs. 37 months, p=0.007). The U2AF1 mutation by itself was more strongly associated with poor outcome (8 vs. 37 months, p<0.0001), compared to an SRSF2 mutation (26 vs. 36 months, p=0.16). Analysis of survival according to treatment type revealed that no pts who underwent HIC or HCT carried an SF3B1 mutation. The presence of RS was also associated with improved survival (38 vs. 25 months, p=0.04), and this benefit remained if pts received HIC (18 vs. 9 months, p=0.04). However, this benefit became non-significant if pts underwent HCT. There were 19 pts who had either an U2AF1 or SRSF2 mutation that underwent HIC. Although it appeared that having either mutation was associated with worse survival, (9 vs. 21 months), it did not reach statistical significance (p=0.46). Among pts who underwent HCT, there were 10 pts who carried either an U2AF1 or SRSF2 mutation. Those pts with mutations appeared to have superior survival with HCT compared to WT, (53 vs. 34 months), but this did not reach statistical significance (p=0.54). Among pts with either an U2AF1 or SRSF2 mutation, those who underwent HCT had a superior survival (53 vs. 17 months, p=0.05) compared to those who received conventional chemotherapy, defined as HIC or low intensity chemotherapy (LIC), such as hypomethylating agents. There was no difference in survival between receiving HCT or conventional chemotherapy in pts who were WT for U2AF1 or SRSF2 (34 vs. 31 months, p=0.08).
Consistent with previous findings of improved survival and loss of SF3B1 with leukemic progression in MDS and MDS/MPN pts, we found no SF3B1 mutations in our cohort of pts receiving HIC or HCT. In contrast, we found poorer outcomes with SRSF2 or U2AF1 mutations. Although the incidence of these mutations was rare in our cohort of pts, we found statistically significant survival benefit for pts with these mutations if they underwent HCT compared to conventional chemotherapy. This is the first study evaluating the impact of spliceosomal mutations on the survival outcomes of pts undergoing HCT. Our preliminary findings of improved survival with HCT in poorer-risk spliceosome mutations is provoking and further molecular analysis is ongoing.
Maciejewski:NIH: Research Funding; Aplastic Anemia&MDS International Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal