Abstract
Relapse after allogeneic stem cell transplant (SCT) is a major cause of morbidity and mortality of acute myeloblastic leukemia (AML) patients. Most relapses primarily involve bone marrow whereas extramedullary (EM) relapses are relatively uncommon. Data on natural history, optimal management and treatment outcome in EM relapse following allogeneic SCT are limited.
We retrospectively reviewed 130 AML patients who underwent allogeneic SCT at the University of Iowa Hospitals and Clinics between January 2003 and December 2010. Pre-transplant baseline characteristics and post-transplant variables were abstracted. Patterns of relapse after allotransplant, management and outcome were reported.
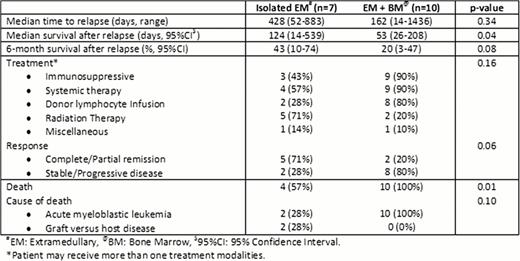
Among 130 AML patients who underwent allogeneic SCT, we identified 61 (47%) relapsed AML after allotransplant. Of 61 relapsed cases, there were 7 (12%) isolated EM, 44 (72%) isolated bone marrow (BM) and 10 (16%) concurrent BM + EM relapses. Among 17 EM relapsed cases, there was a higher prevalence of acute monocytic leukemia subtype (41.5% VS 2.3%, odd ratio [OR] = 4.1, p=0.03), moderate to severe chronic graft versus host disease (GVHD) (35.3% VS 4.6%, OR=2.3, p=0.01) compared to non-EM relapsed patients. Median time to relapse in EM cases was significantly longer than non-EM group (428 days, 95% confidence interval [CI] 203–652 VS 162 days, 95%CI 100–231, p=0.003). EM relapse sites included 1 pulmonary (6%), 3 (18%) genitourinary, 7 (41%) skin/soft tissue, 2 (12%) gastrointestinal and 4 (23%) multisystem involvement. Of 17 EM relapsed cases, 12 (71%) had immunosuppression withdrawn, 13 (76%) received systemic chemotherapy with/without donor lymphocytic infusion and 7 (41%) had radiation therapy to EM relapse sites. The 6-month survival after relapse of EM patients was significantly higher than non-EM group (29.4%, 95%CI 10.7–51.2 VS 6.8%, 95%CI 1.8–16.7, p=0.014). Further subgroup analysis showed that isolated EM relapsed group had significantly higher 6-month survival after relapse than systemic relapse group (isolated BM or concurrent EM + BM) (42.9%, 95%CI 9.8–73.4 VS 9.3%, 95%CI 3.4–18.7, p=0.008). Table 1 compares the management and outcome between isolated EM relapse and concurrent BM + EM relapse. At the time of analysis, three (18%) of 17 EM relapsed cases (all of which were isolated EM patients) achieved disease controlled/remission and remained alive at 10, 13 and 19 months following relapse respectively. Cox proportional hazards analysis identified low hematopoietic cell transplant comorbidity index (HR 0.86, 95%CI 0.74–0.99, p=0.038), relapse after 180 days (HR 0.05, 95%CI 0.01–0.16, p<0.001) and presence of chronic GVHD (HR 3.57, 95%CI 1.02–12.45, p=0.04) to be associated with favorable survival after relapse.
EM relapse of AML after alloSCT presents later than bone marrow relapse and occurs despite the presence of GVHD, especially chronic GVHD. As transplant survival has improved, increasing EM relapses have been observed. Although appropriate management is unknown, prognosis of EM relapse is better than systemic relapse and durable remission can be achieved.
Kaplan Meier analysis demonstrates survival after relapse stratified by patterns of relapse.
Kaplan Meier analysis demonstrates survival after relapse stratified by patterns of relapse.
Outcome of extramedullary relapse of acute myeloblastic leukemia after allogeneic stem cell transplantation.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal