Abstract
Abstract 1802
Hairy cell leukemia (HCL) is an indolent B-cell malignancy characterized by marked splenomegaly, progressive pancytopenia, and BRAF V600E mutation as a disease-defining genetic event. Currently, treatment with purine nucleoside analogues lead to remission in nearly all patients, however most patients eventually progress and may became purine analogue-resistant after multiple courses; therefore, new targeted therapy approaches for HCL are needed. B cell receptor (BCR) signaling is involved in pathogenesis and progression of several B-cell malignancies. Upon antigen stimulation, the BCR initiates activation of a signaling cascade including the kinases Lyn and Syk, causing downstream BTK activation. BTK is also known to be involved in signaling of chemokine receptors (CXCR4, CXCR5) and adhesion molecules that are important for B-cell migration and tissue homing. Ibrutinib (PCI-32765) is a selective, irreversible BTK inhibitor (IC50= 0.5 nM) that has demonstrated clinical activity in phase I and II studies in patients with non-Hodgkin lymphoma, particularly in patients with chronic lymphocytic leukemia and mantle cell lymphoma.
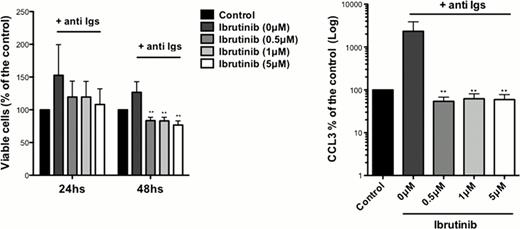
The importance of BTK in BCR signaling in hairy cell leukemia has not yet been characterized. Here, we explored the role of BTK and the activity of Ibrutinib in BCR signaling in HCL cell lines and primary HCL cells. We found BTK expression at the protein level in HCL cell lines (ESKOL and HC-1) and RAMOS control B cells. In cell growth and XTT assays, we found a significantly inhibition of HCL growth and proliferation with Ibrutinib at 0.5 μM, 1 μM and 5 μM concentrations in a dose-dependent manner. In accordance with these findings, we noticed a dose dependent S phase reduction by Ibrutinib, assessed by BrdU incorporation. Ibrutinib had no effects on HCL cell line viability in apoptosis assays after 24 or 48hs of incubation with Ibrutinib. Next, we tested effects of Ibrutinib on signaling after BCR triggering with anti-IgM or anti-IgA. Treatment with 1μM Ibrutinib reduced levels of BCR-induced phospho-ERK and phospho-AKT. Also, we demonstrated significant reductions in levels of BCR-induced chemokines (CCL3, CCL4) in HCL supernatants, which function as a surrogate marker for BCR activation. Primary HCL cells from 8 patients were tested for Ibrutinib-induced apoptosis. Here, we found a significantly reduction in HCL viability after 48 h of incubation, as shown in the figure. Similar results were obtained with the XTT assay, which was performed in parallel. To study effects of Ibrutinib on BCR signaling and activation in primary HCL cells, we performed immunoblot analysis after BCR triggering and measured CCL3 and CCL4 levels in HCL supernatants. Ibrutinib inhibited activation of BTK, ERK and ATK and significantly reduced levels of CCL3 and CCL4 (see Figure). Collectively, our studies demonstrate that Ibrutinib inhibits BCR signaling in primary HCL cell and HCL cell lines, and significantly diminishes HCL survival and proliferation. These data provide a strong rationale for testing the clinical activity of this new, targeted and well-tolerated agent in patients with HCL.
Buggy:Pharmacyclics: Employment, Equity Ownership. Burger:Pharmacyclics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal