Abstract
Abstract 1801
Chronic lymphocytic leukemia (CLL) is the most common leukemia seen in Western countries, primarily in the elderly, with a median age of diagnosis of 72 years. CLL is characterized by the aggressive accumulation of monoclonal peripheral (mature) CD5+ B cells in primary and secondary lymphoid tissues. Several classes of drugs currently exist to treat CLL and these include - nitrogen mustard alkylating agents, purine analogs, monoclonal antibodies, cyclin dependent kinase inhibitors, BTK and PI3K inhibitors. CLL can become resistant to existing therapies necessitating the need for identification of new targets and therapeutic strategies. Unique metabolic dependencies of cancer cells have been identified, further investigation of which could provide tumor cell specific targeting modalities. A myriad of tumor cells exhibit increased glucose uptake and metabolism of glucose via the in-efficient glycolytic pathway, a phenomenon first described by Warburg in the 1900's. Restriction of glucose utilization and metabolism has been shown to chemosensitize and/or elicit toxicity in a wide range of cancers.
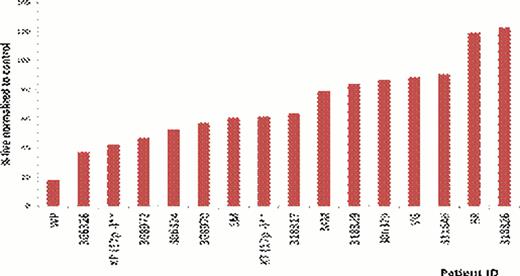
CLL metabolism is a relatively unexplored area. We sought to determine the dependency of CLL on glucose metabolism. As there are currently no CLL cell lines we used primary patient samples for these studies. CLL cells harvested from PBMCs were cultured in both glucose free and glucose containing media. Our results show that when CLL cells are cultured in these media, there is variation in sensitivity to glucose deprivation ranging from very sensitive to highly resistant (Figure 1). We have investigated possible resistance mechanisms and alternate sources of energy in CLL that could be responsible for maintaining viability even during glucose-withdrawal. We first investigated a role for glutamine. CLL cells sensitive to glucose withdrawal and cultured in the absence of glutamine, did not exhibit enhanced toxicity. These results suggest that cells resistant to glucose withdrawal were not maintaining viability via glutamine metabolism. The role of the mitochondrial metabolism was also investigated. We observed that when the CLL cells are cultured in the absence of glucose and are substituted with galactose, there is a rescue effect, with cell viability being restored. This rescue effect is also observed (although not to the same extent as with galactose) when the CLL cells in the absence of glucose are treated with 2-methylpyruvate (2MP). 2MP feeds directly into the mitochondria and can bypass the glycolytic pathway. CLL cells were also treated with metformin, which is complex-1 inhibitor, and we observed enhanced cell death. The results with galactose, 2MP and metformin all suggest that mitochondrial metabolism is an integral part of CLL metabolism, potentially playing a compensatory role upon glucose-withdrawal. The role of autophagy was also investigated and, using chloroquine we observed that autophagy was pro-survival in CLL patient samples.
In summary we have observed varying sensitivity of CLL patient samples to glucose deprivation, and identified resistance mechanisms and alternative sources of energy in CLL cells. The reduction of extracellular glucose has been shown to induce resistance of normal cells to DNA damaging therapeutics, and enhancement of sensitivity in cancer cells, suggesting that glycolysis inhibition may expand the therapeutic window of currently used therapeutics. Targeting these unique metabolic dependencies in CLL could provide strategies to chemo-sensitize and target CLL more effectively with less toxicity.
Variable sensitivity of CLL patient samples to 48-hour glucose free culture
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal