Abstract
Abstract 1523
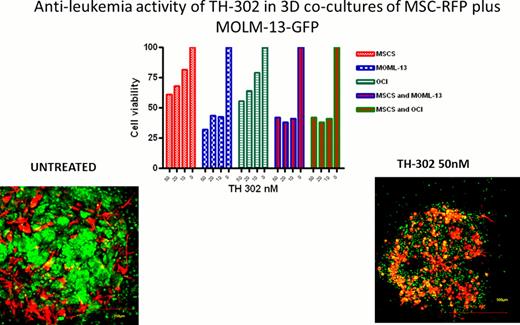
We have recently demonstrated that the leukemic bone marrow (BM) niche is highly hypoxic and that hypoxia promotes resistance of leukemic cells to chemotherapy (Benito et al., PLoS One 2011, e23108). Our preliminary data indicate that AML cells surviving chemotherapy in the human xenograft mouse models of leukemia reside within hypoxic areas of BM microenvironment as documented by staining with the hypoxia marker CAIX. These findings support utility of hypoxia-activated pro-drugs with the goal to eliminate leukemic blasts and leukemic stem cells residing in hypoxic BM microenvironment. TH-302 is a 2-nitroimidazole linked bromo-isophosphoramide mustard cytotoxin that upon hypoxia-dependent activation induces DNA cross-linking. TH-302 exhibited potent hypoxia-selective anti-leukemia activity in pre-B ALL (REH, NALM6), AML (OCI-AML3, MOLM-13, KG-1) and CML cell lines (KBM5), with IC50s at 1% O2 ranging from 0.04μM to 2.3μM and hypoxia cytotoxicity ratio (HCR) ranging from 116 for KBM5 to 11 for REH cells. TH-302 at 5–7.5μM also exhibited hypoxia-dependent anti-leukemia activity in primary ALL and AML samples (N=3; normoxia, 2–8%; hypoxia, 28–65% apoptotic cells).To better recapitulate the multidimensional BM niche we utilized co-cultures of GFP-labeled leukemic cells with bone marrow-derived RFP-labeled mesenchymal stromal cells (MSC) immobilized within Matrigel. MSC and leukemic cells generated three-dimensional (3D) structures- “spheroids”- and co-proliferated over time with colonies of leukemic cells firmly attached to MSC, as monitored by confocal microscopy (Fig. 1). Pimonidazole staining shows that vast hypoxia is present in the MSC/AML spheroids grown at normal oxygen tension, in contrast to what is observed in plastic-based (2-D) stromal co-cultures. Anti-leukemia activity of TH-302 was next determined in 2D vs 3D co-cultures of MSCs plus MOLM-13 or OCI-AML3 cells. In 2D co-cultures, MSC protected MOLM-13 and OCI-AML3 cells from TH-302-induced cytotoxicity, while extensive apoptosis was documented in hypoxic spheroid co-cultures (at 50nM TH-302, reduction in viability 10–15% vs >60%, Fig. 1). These findings suggest that culture conditions faithfully mimicking BM microenvironment promote pathologic hypoxia generated by rapidly proliferating AML cells, which in turn leads to their increased sensitivity to hypoxia-activated cytotoxins. To validate these findings in vivo, we next tested anti-leukemia efficacy of TH-302 in the in vivo model of primary AML established in NSG mice. TH-302 (50 mg/kg IP 3 times a week for three weeks) reduced the number of circulating AML cells (control, 13.2+/−5.7 ×106/ml; TH-302, 2.5+/−2.1 ×106/ml) and prolonged survival of NSG mice engrafted with primary AML cells compared to the vehicle treated mice (median survival time: TH-302=75 days; Control=56 days; P=0.003, n= 8 mice/group). To test the ability of hypoxia-activated prodrug to target leukemia-initiating cells, secondary transplant experiments were performed in which BM cells from control or TH-302 treated mice (collected after two weeks of therapy) were serially diluted and injected into secondary NSG recipient mice at 0.01, 0.005 or 0.0001×106̂ cells/mouse (N=5 mice/dilution). Although all mice transplanted with higher cell doses died from leukemia, we observed significantly prolonged survival of animals injected with 0.01×106 cells from TH-302-treated primary recipients compared with vehicle-treated controls (median survival control=68 days; TH-302=79 days; P=0.0031) or with 0.005×106 cells (control=79 days; TH-302=83 days; P=0.0462). In summary, our findings suggest that pathologic hypoxia is a prevalent condition of leukemic BM microenvironment that promotes survival of leukemic blasts and leukemia-initiating cells. The results support targeting hypoxia with hypoxia-activated cytotoxins such as TH-302 to enhance the efficacy of therapeutic regimens in AML. A Phase 1 single agent clinical trial of TH-302 in patients with relapsed/refractory hematologic malignancies is ongoing.
Spheroids of MSCs and MOLM-13 cells were incubated with or without TH-302 for 72hr. Effect on cell viability was determined by WST-1 assay.
Spheroids of MSCs and MOLM-13 cells were incubated with or without TH-302 for 72hr. Effect on cell viability was determined by WST-1 assay.
Handisides:Threshold Pharmaceuticals: Employment. Hart:Threshold Pharmaceuticals: Employment. Konopleva:Threshold Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal