Abstract
Abstract 105
Erythrocytes are the major cellular component of blood and they have been shown to contribute to primary hemostasis, predominantly due to their rheological properties. Direct platelet-erythrocyte interaction has been published but no information is available on the mechanism of interaction and the physiological function. Our aim was to characterize platelet-erythrocyte interactions under near physiological conditions in-vitro.
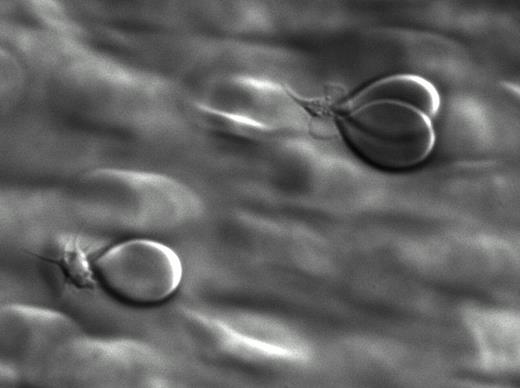
At first we studied whether erythrocytes are able to bind to platelets adhered to surfaces coated with different adhesive proteins at different flow-rates. For this purpose, an in vitro perfusion system connected to a light microscope and a digital camera was used. Erythrocytes bind to platelets both in buffer (washed platelets and erythrocytes) and in whole blood. Erythrocytes were attached to platelets with a sort of “focal adhesion point”, resulting in a tear-drop shape (Fig 1a, b, erythrocyte binding to platelets under flow). Erythrocyte-platelet adhesion was inversely correlated with flow rate and predominately occurred at shear rates lower than 300S−1. The addition of platelet agonists, i.e collagen related peptide (CRP), adenosine diphosphate (ADP), thrombin and arachidonic acid increased erythrocyte binding to platelets 3 to 6 folds indicating that platelet activation is involved in capturing erythrocytes from the circulation.
An Arg-Gly-Asp (RGD) containing peptide (d-RGDW), known to inhibit αIIbβ3 mediated platelet aggregation inhibited erythrocyte-platelet adhesion with 29% to 72%, depending on the agonist used (p<0.05, n=4). As erythrocyte ICAM-4 has been reported to be a ligand for platelet activated αIIbβ3(Hermand P. et al, J.Biol.Chem, 2003,), we tested whether ICAM-4 and platelet αIIbβ3 are the ligand/receptor pair responsible for the erythrocyte-platelet adhesion. Experiments with inhibitory antibodies revealed that the erythrocyte-platelet adhesion under conditions of flow was inhibited with both anti-ICAM-4 (40%, p<0.01, n=8) and anti- integrin β3 (CD61) (46%, p<0.001, n=8). In addition, an ICAM-4 peptide resembling the extracellular domain of human ICAM-4 demonstrated a significant inhibitory effect on erythrocyte-platelet adhesion.
To further characterize the binding between ICAM-4 and αIIbβ3, flow cytometry analysis was performed. We found a decreased fibrinogen binding to platelets (43% at ADP concentration of 125μM, p<0.05, n=5) and an increased P-selectin expression (60%, p<0.01, n=5) on platelets upon ADP stimulation in the presence of ICAM-4 peptide. This finding suggests that ICAM-4 peptide compete with fibrinogen for binding to activated αIIbβ3. The increase of P-selectin expression in the presence of ICAM-4 peptide suggests that binding of ICAM peptide to αIIbβ3 results in outside-in signalling and further platelet activation.
In conclusion, we found direct erythrocyte-platelet interaction under conditions of low shear stresses. This interaction is partly mediated via erythrocyte receptor ICAM-4 and platelet activated integrin αIIbβ3. In addition we found an indication that interaction with erythrocytes further enhances platelet activation. Direct erythrocyte-platelet adhesion seems to play a role in platelet depending thrombus formation.
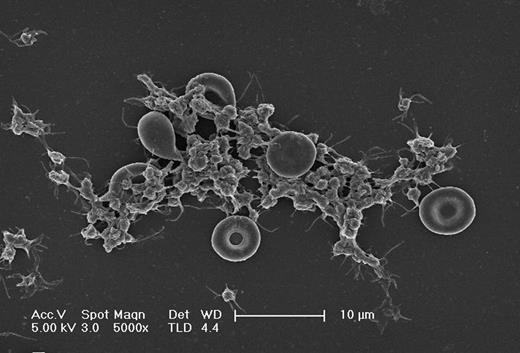
Scanning electron microscopy picture of erythrocyte-platelet interaction under flow
Scanning electron microscopy picture of erythrocyte-platelet interaction under flow
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal