Abstract
Understanding the mechanisms that regulate angiogenesis and translating these into effective therapies are of enormous scientific and clinical interests. In this report, we demonstrate the central role of CDP-diacylglycerol synthetase (CDS) in the regulation of VEGFA signaling and angiogenesis. CDS activity maintains phosphoinositide 4,5 bisphosphate (PIP2) availability through resynthesis of phosphoinositides, whereas VEGFA, mainly through phospholipase Cγ1, consumes PIP2 for signal transduction. Loss of CDS2, 1 of 2 vertebrate CDS enzymes, results in vascular-specific defects in zebrafish in vivo and failure of VEGFA-induced angiogenesis in endothelial cells in vitro. Absence of CDS2 also results in reduced arterial differentiation and reduced angiogenic signaling. CDS2 deficit-caused phenotypes can be successfully rescued by artificial elevation of PIP2 levels, and excess PIP2 or increased CDS2 activity can promote excess angiogenesis. These results suggest that availability of CDS-controlled resynthesis of phosphoinositides is essential for angiogenesis.
Introduction
Angiogenesis is a process that involves the growth of new blood vessels from preexisting vessels and is important during embryonic development and in many physiologic and pathologic conditions.1,2 The complex process of angiogenesis, including endothelial cell proliferation, tip/stalk cell formation, migration, extracellular matrix remodeling, and lumen formation, is managed by angiogenic signaling networks.3-5 Numerous proangiogenic and antiangiogenic factors have been identified, among which VEGF is a key mediator that promotes angiogenesis. In solid tumors, new blood vessels grow and infiltrate into the tumor, providing it with essential nutrients and oxygen and a route for tumor metastasis. Many types of tumors can produce massive amounts of VEGFA to promote their progression. Thus, antiangiogenesis, especially neutralizing VEGFA and blocking its signaling, has been regarded as a highly promising avenue for anticancer therapy.6,7
VEGFA exerts its biologic effects by binding to and activating its receptors. VEGF receptor 2 (VEGFR2) is the main receptor that transduces VEGF-induced signaling in endothelial cells. Activation of VEGFR2 leads to phosphorylation of specific downstream signal transduction mediators, mainly phospholipase C γ (PLCG) and PI3K. Phosphoinositide 4,5 bisphosphate (PI4,5P2, PIP2) plays a key role in intracellular downstream transmission of VEGF-dependent signaling, serving as the proximal substrate for both major signaling arms. PI3K uses PIP2 to produce PI(3,4,5)P3, which activates Akt signaling, whereas PLCγ hydrolyzes PIP2 to generate inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), which further activate Ca2+ metabolism, protein kinase C, NFAT, and ERK. Disruption of either of these 2 signaling arms causes defects in angiogenesis (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).8,9
Breakdown and turnover products of PIP2 enter the phosphoinositide-recycling pathway for regeneration. One of the key steps in this recycling pathway is the synthesis of CDP-DAG from phosphatidic acid and cytidine triphosphate, catalyzed by the enzyme CDP-DAG synthase (CDS; supplemental Figure 1B).10,11 A variety of evidence suggests that CDS activity can regulate both the availability of PIP2 and the extent of PIP2-dependent signaling. The amplitude of the light response in Drosophila photoreceptor cells is modulated by CDS levels, showing that CDS-dependent PIP2 recycling limits phospholipase C–mediated phototransduction in the fly retina; flies carrying a cds1 mutation that displays light-induced irreversible loss of phototransduction and retinal degeneration.12 A more recent study that used cultured endothelial cells suggested that competition between PI3K and PLCG for limiting PIP2 substrate modulates vascular tube formation and vessel stability.9
The zebrafish has emerged as an important new model for uncovering novel genes and pathways that regulate vessel formation during development, notably through forward-genetic screens for mutants with vascular phenotypes.13,14 Many of the genes identified are also important players in vascular pathologies.15-20 Here, we report 2 new zebrafish mutants with specific defects in angiogenesis identified in a screen for N-ethyl-N-nitrosourea (ENU)–induced vascular mutants. These mutants both result from loss-of-function mutations in the CDS2 gene (cds2; vertebrates, including mice, humans, and zebrafish, have 2 CDS genes, cds1 and cds221-23 ), leading to defects in VEGF signaling activity and angiogenic capacity.
Methods
Zebrafish methods
Zebrafish (Danio rerio) embryos were obtained and raised, and fish were maintained as described.24,25 The Tg(fli1a:EGFP)y1 transgenic zebrafish line was described previously.26 An ENU F3 genetic screen was performed with this line to isolate the cds2y25 and cds2y54 mutants. Embryos imaged after 1.5 days post fertilization (dpf) were treated with 1-phenyl-2-thiourea to inhibit pigment formation.24 The zebrafish fish husbandry and research protocol were reviewed and approved by the National Institutes of Health Animal Care and Use Committee.
Molecular cloning of Cds2, the defective gene in y25 and y54 mutants
To identify the defective gene in y54 mutants, we performed meiotic mapping to localize y54 in the zebrafish genome. Initial bulked segregant mapping placed y54 on LG5, and fine genetic mapping with the use of 745 mutant embryos from a polymorphic mapping cross localized y25 and y54 to a 0.32-cM interval between markers z30487 and z6214 (Figure 2A). Chromosome walks initiated from both markers refined this to a 39-Kb, 0.06-cM critical interval defined by 1 recombinant on the z30487 end and 1 recombinant on the z6214 end. This interval included cds2, arhGAP24, bmp10, SH2D1A, and Oz protein (only the 5′ end). The complete nucleotide coding sequence of each of these genes was obtained from cDNA sequenced from y25 and y54 mutants and their wild-type siblings. Comparison with wild-type and mutant sequences for each of the genes showed that only cds2 has coding changes in both mutants, leading to premature stops at position 110 (TAC to TAA, Tyr to STOP) in the y54 mutant and at position 158 (CGA to TGA, Arg to STOP) in the y25 mutant. Sequences of CA repeat genetic markers are available at http://zebrafish.mgh.harvard.edu/zebrafish/ssrQuery.aspx. BAC clones are listed at http://vega.sanger.ac.uk/Danio_rerio/Info/Index. BAC DNA was prepared with the use of Nucleobond columns (Clontech).
Genotyping assay for cds2y54 mutants
To genotype for the cds2y54 mutation, PCR was performed on zebrafish embryo genomic DNA extracts with the use of REDTaq ReadyMix PCR Reaction Mix and the following primers: cds2genF, 5′-AACAGCTTGATGTAGCACAGCAGAGTA-3′, and cds2genR, 5′-ATGAGGGTGTGGATGATGATGATA-3′. PCR products were digested with MseI, cutting PCR products carrying mutant cds2 into 193-bp and 124-bp fragments, but not PCR products (317 bp) from wild-type cds2.
Cloning of full-length zebrafish cds2 and cds1
cds2.
Sequences were obtained with the use of National Center for Biotechnology Information reference sequence (accession no. BC165336). The full-length cDNA of cds2 was obtained by high-fidelity PCR on zebrafish embryo cDNA at 24 hours post fertilization (hpf) with the use of Pfu Ultra High-Fidelity DNA polymerase II (Stratagene) and the primers 5′-CCCACCATGACAGAATTACGACAGCGAGGAGCGACG-3′ and 5′-CAGAAGTTAGGCTGCTTCTTCCAGGGCAGGAAGCAG-3′. A single product was obtained, cloned into pCRII-TOPO (Invitrogen), and verified by sequencing.
cds1.
Sequences were obtained with the use of National Center for Biotechnology Information reference sequence (accession no. XM_001341863). The full-length cDNA of cds1 was obtained by high-fidelity PCR on 24-hpf cDNA with the use of Pfu Ultra High-Fidelity DNA polymerase II (Stratagene) and the primers 5′-TAAGACAAAATGACGGAGCTGAGACGGCGA-3′ and 5′-TCATTGGGCTTCCTCTACAGCAGGTGGCAG-3′. A single product was obtained, cloned into pCRII-TOPO (Invitrogen), and verified by sequencing.
Expression constructs, in vitro transcription/translation, and RNA injection
pCS2/cds2.
The cds2 pCRII-TOPO clones described in “cds2”were used as templates in a PCR reaction with Pfu Ultra High-Fidelity DNA polymerase II (Stratagene), and primers were extended with a BamHI site (5′-AGGGGATCCCCCACCATGACAGAATTACGACAGCGAGGAGCGACG-3′) and an XbaI site (5′-GCTCTAGACAGAAGTTAGGCTGCTTCTTCCAGGGCAGGAAGCAG-3′). The resulting PCR product was digested with BamHI and XbaI and cloned into Bam/XbaI-digested pCS2+. The construct was verified by sequencing.
pCS2/cds1.
The cds1 pCRII-TOPO clones described in “cds1” were used as templates in a PCR reaction with Pfu Ultra High-Fidelity DNA polymerase II (Stratagene), and primers were extended with a BamHI site (5′-AGGGGATCCTAAGACAAAATGACGGAGCTGAGACG-3′) and an XbaI site (5′-GCTCTAGATCATTGGGCTTCCTCTACAGCAGGT-3′). The resulting PCR product was digested with BamHI and XbaI and cloned into Bam/XbaI-digested pCS2+. The construct was verified by sequencing.
Capped mRNA for injection was synthesized from NotI-digested pCS2 expression plasmids with the use of the mMessage mMachine SP6 kit (Ambion), according to the provider's protocol. Capped mRNA was injected into 1- to 4-cell embryos either into a single blastomere or into the streaming yolk cytoplasm, just beneath the blastomeres, using a pneumatic picopump and micromanipulator (World Precision Instruments), as described previously.24
I-SceI [kdrl:mcherry-v2a-cds2].
To generate an endothelium-specific transgenic construct, a red fluorescent mCherry cassette was PCR-amplified with the use of primers that contained attB1 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTACATGGTGAGCAAGGGCGAGGAG-3′) and v2A (5′-AGGTCCAGGGTTCTCCTCCACGTCTCCAGCCTGCTTCAGCAGGCTGAAGTTAGTAGCTCCGCTTCCCTTGTACAGCTCGTCCATGCC-3′) peptide sequences. The cds2 coding sequence was PCR-amplified with primers that contained v2A peptide (5′-GGAAGCGGAGCTACTAACTTCAGCCTGCTGAAGCAGGCTGGAGACGTGGAGGAG AACCCTGGACCTATGACAGAATTACGACAGCGA-3′) and attB2 (5′-ggggaccactttgtacaagaaagctgggtacagaagttaggctgcttcttccagggc-3′) sequences. A bridging PCR generated an attB1-mCherry-2A-attB2 cassette, which was cloned into the pENTRY/D-TOPO vector (Invitrogen). The resultant vector was recombined with pDEST-I-SceI-flk (Aniket V. Gore, unpublished data, June 6, 2012) and p3E-polyA to final DNA construct in a LR clonase reaction (40 ng of DNA was used in the endothelial rescue experiment).27 In the endothelial rescue experiment, 40 ng/mL DNA was predigested with I-SceI endonuclease at 37° for 30 minutes. Digestion mixture (1 nl) was injected into the 1-cell stage blastomere of zebrafish embryos. At 48 hpf, injected mCherry-positive zebrafish embryos were sorted, imaged (representative image shown in Figure 1J), and then confirmed with genotyping analysis.
pCS2(+)-zvegf165 is a gift from Dr Ruowen Ge (National University of Singapore).28 pCS2(+)-zvegfc is commercially available from Open Biosystems. CMV promoter-containing zebrafish vegf165 or zebrafish vegfc DNA fragments for injection were generated as previously described.29
Zebrafish cds1 and cds2 were cloned into the pLenti6/V5 TOPO vector (Invitrogen) that contained a blasticidin resistance gene with the use of the following primers: cds1, forward BamH1 (5′-GGATCCGCCACCATGCGGAGCTGAGACGGCGAG-3′); reverse Xho1 (5′-CTCGAGTCATTGGGCTTCCTCTACAGCAGG-3′); cds2, forward BamH1 (5′-GGATCCGCCACCATGACAGAATTACGACAGCGAG-3′); and reverse Xho1 (5′-CTCGAGGCCACCTTAGGCTGCTTCTTCCAGGGC-3′). Enhanced green fluorescent protein (EGFP) cloned into the pLenti6/V5 TOPO vector was used as a control.
All constructs were verified through sequencing analysis.
Quantitative RT-PCR analysis
Total RNA from the trunk region of control and morpholino oligonucleotide (MO)–injected embryos (20-30 embryos pooled for each sample) at 24 hpf was extracted and then reversely transcribed. The PCR primers were characterized for the expected product length and sensitivity as follows: Fli1a forward, 5′-CGTCAAGCGAGAGTATGACC-3′, and reverse, 5′-AGTTCATCTGAGACGCTTCG-3′; Flt4 forward, 5′-TAACCAACCCCTCCATCAGA-3′, and reverse, 5′-CTGAATGCTGAGAGTCCGATT-3′; dll4 forward, 5′-ACTCTTCTGCTACGGTATGTTT-3′, and reverse, 5′-CAA TGCTGGTTGAAGGTTTT-3′; and β-actin forward, 5′-GCTGTTTTCCCCTCCA TTGTT-3′, and reverse, 5′-TCCCATGCCAACCATCACT-3′.30 The SYBR Green Supermix (Bio-Rad) was used in quantitative PCR on the Bio-Rad CFX96 Real-Time PCR system. All the experiments were repeated 3 times with quadruplicates. We analyzed the data with the ΔΔCt method. Data were represented as mean ± SD, and t test was performed for comparison between control and experimental groups.
Morpholino injections
Morpholino-modified antisense oligonucleotides (Genetools) used in this study included the following: cds2-ATG (5′-TCGCTGTCGTAATTCTGTCATGGTG-3′) that targeted −4 to 21 of the 5′ untranslated region and coding region of cds2 (1.8 ng was used as full dosage); cds1-spl2, 5′-GTACCTGAAAAACAAGGTGCATAAC-3′, that targeted intron 4/exon 5 boundary (11 ng was used as full dosage); vegfaa, 5′-CTCGTCTTATTTCCGTGACTGTTTT-3′31 (6 ng); and plcg1, 5′-ATTAGCATAGGGAACTTACTTTCG-3′17 (3 ng). Morpholino injections were performed on 1- to 4-cell embryos as described previously.26 The morpholino “full” doses were titrated to levels that yielded maximal vascular-specific phenotypes with little or no nonspecific toxic effects.
Whole-mount in situ hybridization
To derive cds2, cds1-like riboprobes, the corresponding pCRII-TOPO constructs were linearized with XhoI or NotI, respectively, and transcribed with SP6 RNA polymerase. Antisense mRNA probes dll4, flt4, and fli1a were prepared as described.32-37 Whole-mount in situ hybridization was performed essentially as described elsewhere.38
PIP2 quantitation and delivery into cultured HUVECs or zebrafish embryos
Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were obtained from Avanti Polar Lipids Inc. PI, PIP2, PIP3, and Histone H1 carrier were purchased from Echelon Biosciences. For in vivo rescue experiments, control vesicles (molar ratio PC:PE = 19:1; final concentration of PC, 2.38mM) or PIP2 vesicles (molar ratio PC:PIP2:PE = 17:2:1; final concentration of PIP2 is 0.25mM) were generated by sonication. 1-2nl control or PIP2 vesicles were delivered into the extracellular spaces of the trunk of zebrafish embryos 16-18 hpf by microinjection through the horizontal myoseptum. Embryos were imaged and scored for intersegmental vessel (ISV) phenotypes at 30 hpf.
For delivery of ectopic PIP2 into the whole vasculature of wild-type embryos, 1nl of 20mM PIP2:Histone H1 carrier mixture (1:1 molar ratio) was injected into the vasculature of 50-hpf embryos via microangiography. At 72 hpf, embryos were fixed for AP staining, followed by imaging and subintestinal vessel (SIV) phenotype scoring.
Endothelial cell culture, stable expression of cds, and 3-dimensional angiogenesis analysis
Recombinant human VEGFA-165, stromal cell-derived factor 1α (CXCL12), SCF (Kit ligand), and IL-3 are from R&D Systems. Basic fibroblast growth factor was purchased from Millipore. As previously described,39,40 for monolayer culture HUVECs (Lonza) were cultured in bovine hypothalamus extract and 20% FBS in M199 (Gibco) on 1 mg/mL gelatin-coated flasks and used from passages 3-6.
Lentivirus was prepared according instruction (ViraPower Lentiviral Gateway Expression Kit; Invitrogen). Low-passage HUVECs were allowed to grow to 80% confluence and were infected with lentivirus (3 mL of cell culture media, 6 mL of lentiviral supernatant, and 12 μg/mL polybrene/hexadimethrine bromide). Seventy-two hours after infection, cells were selected in the presence of blasticidin for 10-14 days, and the expression levels of the genes were examined by RT-PCR to verify increased mRNA transcript levels above control cells (EGFP-expressing).
For 3-dimensional (3D) in vitro angiogenesis, 2.5 mg/mL collagen type I gels were prepared, including SCF, stromal cell–derived factor 1α, IL-3, and VEGFA-165 in the gel at 200 ng/mL, and were seeded with endothelial cells on the surface at a density of 40 000 cells/well.41 Assays were fixed in 3% glutaraldehyde at predetermined time points and processed for future analysis.
siRNA transfection and validation
Dharmacon SmartPOOL validated small interfering RNA (siRNA), targeting luciferase (as control); cds1 or cds2 were purchased and resuspended in H2O at a concentration of 40μM. siPORT Amine (Ambion) was used as the transfection agent. A double transfection protocol was used with siPORT Amine (Ambion) and with a final concentration of 200nM siRNA added per transfection. In 3D culture, cells were established in 3D collagen type I assays and allowed to invade until predetermined time points. Collagen gels were removed, collagenase was treated, and cells were lysed with the Ambion ToTALLY RNA kit (no. 1910) to isolate total RNA. RNA was produced according to the manufacturer's recommendations. cDNA was produced with the Stratagene AccuScript High Fidelity First Strand cDNA Synthesis Kit, and RT-PCR and analysis of differentially expressed genes were performed. Primer sets were made (300-400 bp) in both a gene- and species-specific manner and follows. GAPDH (control) forward, 5′-GCCAAAAGGGTCATCATCTC-3′, and reverse, 5′-GTAGAGGCAGGGATGATGTTC-3′; CDS1 forward, 5′-TCATGCTGATGCTTCTTG-3′, and reverse, 5′-AACCATATCATGCCT TCAAAC-3′; and CDS2 forward, 5′CAACTTGTCTTCAAGATGG-3′, and reverse, 5′-CTGCA GTCGATAATGCTTC-3′. Either cds1 or cds2 siRNA provides > 90% knockdown efficacy at the mRNA level.
Immunoanalysis
Zebrafish trunk tissues were collected with a dissection microscope with no. 55 superfine forceps and directly lysed in 1× SDS sample buffer (5 μL/embryo, final volume). HUVECs in 3D culture were directly harvested into 1× SDS sample buffer. Rabbit anti-phospho ERK1/2 and total ERK1/2 antibodies were purchased from Cell Signaling Technologies. HRP-conjugated goat anti–rabbit IgG secondary antibody was purchased from Jackson ImmunoResearch Laboratories. Quantitation of relative intensity was performed by Quantity One image analysis software (Bio-Rad), with results shown from 3 independent assays.
Microscopic imaging methods
For imaging angiogenesis during zebrafish embryonic development, transmitted light and fluorescence images were obtained with a Leica MZ12 microscope equipped with a ProgRes mF digital camera (Jenoptik). Confocal images were obtained with an Olympus Fluoview 1000 confocal microscope equipped with a 20× 0.9NA water immersion lens. For imaging in vitro 3D angiogenesis, time-lapse videomicroscopy and imaging were performed with an inverted microscope (Eclipse TE2000-E; Nikon) and the analysis software MetaMorph (Molecular Devices). After fixation of the collagen I assays, the cells were stained with 1% toluidine blue in 30% methanol for increased visualization before imaging. Data analysis was performed by imaging the invasive front of the endothelial cells at 3 depths below the monolayer of cells (50μM apart in depth). Images were obtained at each depth, and the number of endothelial cells was counted. The number of cells at each depth was counted, and the sums of the 3 depths were added together to give a total number of invading cells per well. A minimum of 5 replicate wells were averaged to give each value within one experiment. At least 3 total replicates of each experiment were performed. All microscopic imaging was performed at 25°C.
Results
Identification of 2 angiogenesis-defective mutants in the zebrafish
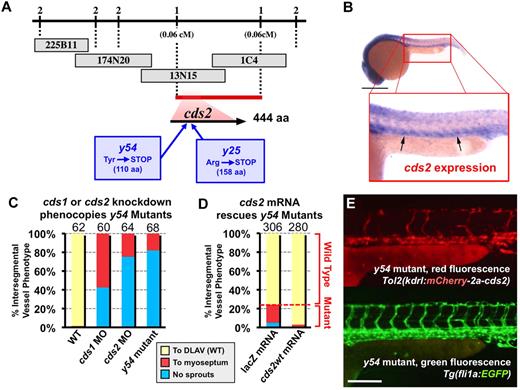
Blood vessel growth is regulated by a complex but still not fully understood network of extracellular cues and intracellular signaling pathways. Many of the same mechanisms that regulate vessel growth during development have also been shown to be important for postnatal vascular pathologies, including the excess angiogenesis required for tumor growth and progression.3-5,7,8,42,43 Developmental model organisms such as mice and zebrafish have been critical in uncovering these mechanisms and in identifying new regulatory molecules. Forward-genetic mutagenesis screens in the zebrafish provide a powerful tool for identifying novel genes that play key roles in vessel formation.44 We identified 2 ENU-induced recessive mutants, y25 and y54, with similar phenotypes in a genetic screen for vascular mutants in the zebrafish. The defects in both mutants are specific to angiogenesis. No gross structural defects are evident in mutant animals, and nonvascular organs and tissues develop normally (Figure 1A-B). Furthermore, endothelial specification and vasculogenic assembly of the trunk axial vessels (dorsal aorta and cardinal vein) and cranial primary vessels (lateral dorsal aorta and primordial hindbrain channel) also occur normally in y25 or y54 homozygotes (Figure 1C-I), and these primitive vessels carry robust blood flow (data not shown). However, subsequent growth of vessels by angiogenesis is defective, including the trunk ISVs (Figure 1D-G) and cranial central arteries (Figure 1H-I).
Zebrafish y54 mutants show angiogenic defects in embryogenesis. (A-B) Transmitted light (A) and fluorescence microscopy (B) images of 30-hours postfertilization (hpf) Tg(fli-EGFP)y1 y54 mutant and wild-type sibling zebrafish. (C-G) Trunk intersegmental vessel angiogenesis defects in y54 mutants. (C) Diagram of a zebrafish embryo with the red box highlighting the region shown in panels D through G and with the blue box highlighting the region shown in panels H and I. (D-G) Confocal images of trunk vessels in 30-hpf (D-E) or 54-hpf (F-G) Tg(fli-EGFP)y1 wild-type (WT) sibling (D,F) or y54 mutant (E,G) zebrafish, showing formation of the vasculogenic dorsal aorta (DA) and posterior cardinal vein (PCV) but failure to properly form the angiogenic intersegmental vessels (arrows) in y54 mutants (lateral view, anterior to the left). (H,I) Confocal images of cranial vessels in 40-hpf Tg(fli-EGFP)y1 wild-type sibling (H) or y54 mutant (I) zebrafish (lateral view, anterior to the left). The lateral dorsal aorta (LDA), primordial hindbrain channel (PHBC), and basilar artery (BA) are present, but the angiogenic central arteries (CtAs) fail to form in y54 mutants. Scale bars = 100 μm (D-G) and 50 μm (H-I).
Zebrafish y54 mutants show angiogenic defects in embryogenesis. (A-B) Transmitted light (A) and fluorescence microscopy (B) images of 30-hours postfertilization (hpf) Tg(fli-EGFP)y1 y54 mutant and wild-type sibling zebrafish. (C-G) Trunk intersegmental vessel angiogenesis defects in y54 mutants. (C) Diagram of a zebrafish embryo with the red box highlighting the region shown in panels D through G and with the blue box highlighting the region shown in panels H and I. (D-G) Confocal images of trunk vessels in 30-hpf (D-E) or 54-hpf (F-G) Tg(fli-EGFP)y1 wild-type (WT) sibling (D,F) or y54 mutant (E,G) zebrafish, showing formation of the vasculogenic dorsal aorta (DA) and posterior cardinal vein (PCV) but failure to properly form the angiogenic intersegmental vessels (arrows) in y54 mutants (lateral view, anterior to the left). (H,I) Confocal images of cranial vessels in 40-hpf Tg(fli-EGFP)y1 wild-type sibling (H) or y54 mutant (I) zebrafish (lateral view, anterior to the left). The lateral dorsal aorta (LDA), primordial hindbrain channel (PHBC), and basilar artery (BA) are present, but the angiogenic central arteries (CtAs) fail to form in y54 mutants. Scale bars = 100 μm (D-G) and 50 μm (H-I).
Loss of function mutations in the cds2 gene are responsible for angiogenic failure in the y25 and y54 mutants
Genetic mapping and positional cloning were performed to identify the defective loci in the y25 and y54 mutants. Both mutants map to cds2 (Figure 2A), a gene encoding an enzyme required for resynthesis of phosphoinositides (see “Introduction”). Sequence analysis of genomic DNA from y25 and y54 shows mutations that create early nonsense codons predicted to generate truncated cds2 polypeptides that lack the catalytic domain (Figure 2A). The cds2 gene shows preferential expression in developing trunk blood vessels (Figure 2B; supplemental Figure 2), as does the closely related cds1 gene (supplemental Figure 2), consistent with a functional role for both genes in the vasculature. Injection of MOs that target the translation start site of cds2 closely phenocopies cds2 mutants (Figure 2C; supplemental Figure 3A-B), whereas injection of wild-type cds2 mRNA rescues the vascular defect in cds2y54 mutants (Figure 2D), confirming that cds2 is indeed the defective gene in these mutants. Transient transgenic endothelial-specific expression of wild-type zebrafish cds2 in cds2y54 mutants results in efficient rescue of ISV growth, showing that endothelial-autonomous restoration of cds2 function is sufficient to rescue the mutant vascular phenotype in vivo (Figure 2E).
y25/y54 are mutations in cds2. (A) Genetic map of the cds2 interval on linkage group LG5, showing the number of recombinants (of a total of 745 mutant embryos) for tested markers in the interval (numbers above line), BAC clones (gray boxes), the critical interval defined by 1 recombinant on each side (red bar), and the position of the cds2 gene. Missense mutations leading to premature stop codons in the 2 separate cds2 mutations are also noted. (B) In situ hybridization of 24-hours postfertilization (hpf) wild-type zebrafish embryo probed for cds2, showing vascular-enriched expression (arrows). (C) Quantitation of the intersegmental vessel phenotypes of wild-type–, CDS1-, or CDS2-morpholino–injected wild-type and y54 mutant 30-hpf Tg(fli-EGFP)y1 zebrafish. (D) Quantitation of the intersegmental vessel phenotypes of 30-hpf Tg(fli-EGFP)y1 y54 mutant zebrafish injected with either lacZ mRNA (left column) or wild-type cds2 mRNA (cds2wt; right column). The bars in panels C and D show the percentages of intersegmental vessels (ISVs) that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (DLAV; yellow). The number of embryos counted for each injection condition in panels C and D is shown at the top of each column, with 10 trunk segments counted per embryo. (E) Fluorescence microscopy images of the trunk of a 48-hpf Tg(fli-EGFP)y1 y54 mutant injected at the 1 cell-stage with a Tol2(flk1:mCherry-2a-cds2) transgene driving simultaneous expression of mCherry (top panel, red) and wild-type CDS2 in endothelial cells (marked by EGFP expression in bottom panel). Growth of ISVs is rescued. Scale bars = 800 μm (B) and 200 μm (E). CDS indicates CDP-diacylglycerol synthetase; EGFP, enhanced green fluorescent protein; MO, morpholino oligonucleotide; and WT, wild-type.
y25/y54 are mutations in cds2. (A) Genetic map of the cds2 interval on linkage group LG5, showing the number of recombinants (of a total of 745 mutant embryos) for tested markers in the interval (numbers above line), BAC clones (gray boxes), the critical interval defined by 1 recombinant on each side (red bar), and the position of the cds2 gene. Missense mutations leading to premature stop codons in the 2 separate cds2 mutations are also noted. (B) In situ hybridization of 24-hours postfertilization (hpf) wild-type zebrafish embryo probed for cds2, showing vascular-enriched expression (arrows). (C) Quantitation of the intersegmental vessel phenotypes of wild-type–, CDS1-, or CDS2-morpholino–injected wild-type and y54 mutant 30-hpf Tg(fli-EGFP)y1 zebrafish. (D) Quantitation of the intersegmental vessel phenotypes of 30-hpf Tg(fli-EGFP)y1 y54 mutant zebrafish injected with either lacZ mRNA (left column) or wild-type cds2 mRNA (cds2wt; right column). The bars in panels C and D show the percentages of intersegmental vessels (ISVs) that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (DLAV; yellow). The number of embryos counted for each injection condition in panels C and D is shown at the top of each column, with 10 trunk segments counted per embryo. (E) Fluorescence microscopy images of the trunk of a 48-hpf Tg(fli-EGFP)y1 y54 mutant injected at the 1 cell-stage with a Tol2(flk1:mCherry-2a-cds2) transgene driving simultaneous expression of mCherry (top panel, red) and wild-type CDS2 in endothelial cells (marked by EGFP expression in bottom panel). Growth of ISVs is rescued. Scale bars = 800 μm (B) and 200 μm (E). CDS indicates CDP-diacylglycerol synthetase; EGFP, enhanced green fluorescent protein; MO, morpholino oligonucleotide; and WT, wild-type.
Injection of morpholinos that target cds1 causes a vascular phenotype similar to but somewhat milder than that observed in cds2y54 mutants or after cds2 knockdown (Figure 2C; supplemental Figure 3C). Coinjection of cds1 and cds2 morpholinos at full doses results in early embryonic lethality, consistent with a pleiotropic requirement for phosphoinositides. However, coinjection at lower doses causes additive vascular-specific phenotypes, with more severe defects noted on coinjection of half doses of both MOs together than when either morpholino alone is injected at its maximal dose (supplemental Figure 3C).
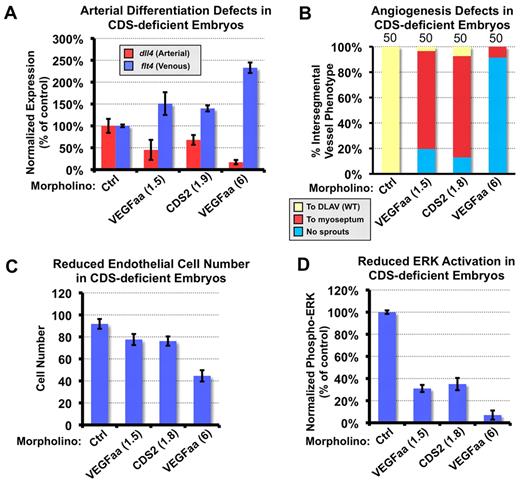
Reduced arterial differentiation, endothelial ERK activation, and cell proliferation in CDS2-deficient zebrafish
To begin to understand how cds2 deficit causes failure in vascular morphogenesis, we examined endothelial specification and differentiation in CDS2 morphants. In situ hybridization with the use of probes for endothelial cell specification markers, including ets1a, ets1b, fli1a, cdh5, plxnd1 and plcg1, found no significant alteration in initial expression of these genes in CDS2 morphants (supplemental Figure 4). However, quantitative RT-PCR found a reduction in arterial marker dll4 and an increase in venous marker flt4 in the trunks of cds2 morphants at 24 hpf (Figure 3A). Arterial differentiation and angiogenesis both require endogenous VEGFA signaling and plcg1-dependent dll4 induction and ERK phosphorylation.17,45-49 cds2y54 Mutants are phenotypically similar to kdrly17 mutants, which carry loss-of-function mutation in 1 of 2 kdr (VEGFR2) genes in zebrafish. This suggests that angiogenic defects in CDS2-deficient animals might be because of a reduced VEGFA signaling capacity. Indeed, partial knockdown of VEGFA phenocopies the effects of CDS2 knockdown on ISV growth and arterial-venous marker expression (Figure 3A-B), although more complete knockdown results in more severe vascular defects (Figure 3B). Because VEGFA signaling is also required for endothelial cell proliferation, we examined the effect of CDS2 silencing on endothelial cell number in the Tg(fli1a:nEGFP)y7 transgenic line, which highlights endothelial cell nuclei. Cds2 morpholino injection results in modest reduction in endothelial cell numbers at 24 hpf, an effect that can also be mimicked by partial knockdown of VEGFA morpholino (Figure 3C).
CDS2 deficiency blocks VEGFA signaling transduction. (A) cds2 knockdown results in decreased arterial and increased venous marker expression in zebrafish embryos. Wild-type zebrafish embryos were injected at the 1- to 2-cell stage with either control morpholino (ctrl; 6 pg), low-dose (1.5 pg) vegfaa morpholino, full-dose (1.8 pg) cds2 morpholino, or full-dose (6 pg) vegfaa morpholino. Trunk tissues were collected at 24 hours after fertilization (hpf) and used for quantitative RT-PCR with primers amplifying either arterial marker dll4, venous marker flt4 (vegfr3), or β-actin (internal control). Red columns show dll4 expression, and blue columns show flt4 expression; both were normalized to levels in control morpholino-injected animals. (B) Low-dose vegfa morphants phenocopy cds2 morphants. Quantitation of the intersegmental vessel (ISV) phenotypes of 48-hpf Tg(fli-EGFP)y1 wild-type zebrafish injected as in panel A. The bars show percentages of ISVs that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (DLAV; yellow). The number of embryos (n = 50) counted for each injection is shown at the top of each column, with 10 trunk segments counted per embryo (for a total of 500 segments). (C) CDS2 deficit causes reduced endothelial cell proliferation. Tg(fli-nEGFP)y7 transgenic zebrafish embryos were injected with morpholinos as in panel A. The average number of endothelial cells present in the 3 posterior-most trunk segments was measured in each of 10 embryos at 26 hpf. (D) Reduced VEGFA signaling in cds2 and vegfa morphants. Wild-type zebrafish embryos injected as in panel A were collected at 24 hpf for trunk tissue harvest and immunoanalysis with the use of anti-total ERK1/2 and anti–phospho-ERK1/2 antibodies. The level of activated phospho-ERK is normalized to total ERK level. ERK activation level in control embryos was set to 100%. Ctrl indicates control; CDS, CDP-diacylglycerol synthetase; and WT, wild-type.
CDS2 deficiency blocks VEGFA signaling transduction. (A) cds2 knockdown results in decreased arterial and increased venous marker expression in zebrafish embryos. Wild-type zebrafish embryos were injected at the 1- to 2-cell stage with either control morpholino (ctrl; 6 pg), low-dose (1.5 pg) vegfaa morpholino, full-dose (1.8 pg) cds2 morpholino, or full-dose (6 pg) vegfaa morpholino. Trunk tissues were collected at 24 hours after fertilization (hpf) and used for quantitative RT-PCR with primers amplifying either arterial marker dll4, venous marker flt4 (vegfr3), or β-actin (internal control). Red columns show dll4 expression, and blue columns show flt4 expression; both were normalized to levels in control morpholino-injected animals. (B) Low-dose vegfa morphants phenocopy cds2 morphants. Quantitation of the intersegmental vessel (ISV) phenotypes of 48-hpf Tg(fli-EGFP)y1 wild-type zebrafish injected as in panel A. The bars show percentages of ISVs that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (DLAV; yellow). The number of embryos (n = 50) counted for each injection is shown at the top of each column, with 10 trunk segments counted per embryo (for a total of 500 segments). (C) CDS2 deficit causes reduced endothelial cell proliferation. Tg(fli-nEGFP)y7 transgenic zebrafish embryos were injected with morpholinos as in panel A. The average number of endothelial cells present in the 3 posterior-most trunk segments was measured in each of 10 embryos at 26 hpf. (D) Reduced VEGFA signaling in cds2 and vegfa morphants. Wild-type zebrafish embryos injected as in panel A were collected at 24 hpf for trunk tissue harvest and immunoanalysis with the use of anti-total ERK1/2 and anti–phospho-ERK1/2 antibodies. The level of activated phospho-ERK is normalized to total ERK level. ERK activation level in control embryos was set to 100%. Ctrl indicates control; CDS, CDP-diacylglycerol synthetase; and WT, wild-type.
VEGF-dependent arterial differentiation and angiogenesis both require vegfr2- and plcγ1-dependent ERK phosphorylation,8,17,31,47 so we examined phospho-ERK levels in the 24-hpf zebrafish trunk, whereby ERK activation is enriched specifically in the developing vasculature.29 Injection of full-dose VEGFA morpholino completely blocked ERK activation in trunk tissues collected from zebrafish embryos (supplemental Figure 5), consistent with previous reports that ERK activation is observed exclusively in the axial vasculature in the 24-hpf zebrafish trunk.45 Partial knockdown of VEGFA or CDS deficiency both resulted in a comparable partial reduction in ERK activation (Figure 3D). Taken together, our results suggest that angiogenic defects in CDS2 mutants or morphants are caused by partial reduction of VEGFA signaling activity in the endothelium.
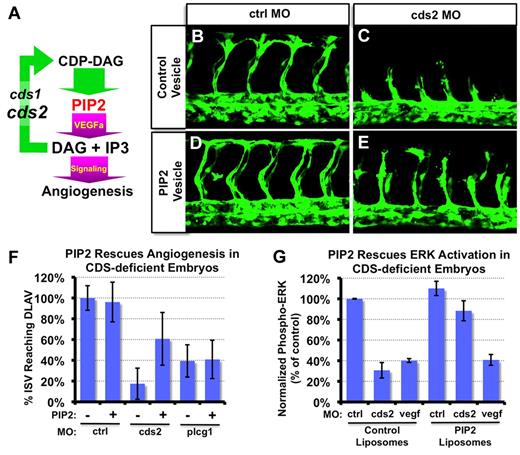
Exogenous PIP2 rescues the angiogenic defects in cds2 morphants
PIP2 is a critical substrate consumed during both PLCG1- and PI3K-dependent VEGFA signaling,8,9,47 whereas CDS controls its resynthesis. We hypothesize that reduced VEGF signaling and angiogenesis defects in CDS2 mutants and morphants could be because of impaired recycling of this critical substrate (Figure 4A). To test this idea we used liposome-mediated delivery to examine whether exogenously supplied PIP2 can rescue the defects in CDS-deficient endothelial cells in vivo. Delivery of liposome-conjugated PIP2 via a single injection into the trunk interstitial spaces significantly improves ISV growth in cds2 morpholino-injected animals (Figure 4B-F), although the same injection shows no effect on ISV growth in animals injected with low doses of a morpholino that targets PLCγ1 (Figure 4F). PIP2 delivery also improves ISV growth in cds2y25 mutants (supplemental Figure 6). To determine whether the rescue effects of PIP2 liposomes are because of recovery of downstream angiogenic signaling, we examined ERK activation in 30-hpf zebrafish trunk tissues (Figure 4G). A single injection of PIP2-containing liposomes restored trunk ERK activation in CDS2 morphants, but it did not restore trunk ERK activation in animals injected with low-dose VEGFA morpholino, as might be expected because VEGFA MO-injected animals are deficient in VEGF activation but still possess intact downstream signaling. Thus, delivery of PIP2-containing liposomes restores angiogenesis by rescuing deficient VEGFA-induced ERK activation in CDS2 morphants. Interestingly, we found that a single intravascular injection of exogenous PIP2 can also promote ectopic branching of new lumenized vascular sprouts from the subintestinal vein that normally delimits the ventral border of the subintestinal vascular plexus (Figure 5A-F), in a manner similar to zebrafish embryos injected with cmv:vegfa-containing plasmid (Figure 5C). Exogenous PIP2 dramatically increases both the number (Figure 5D) and length (Figure 5E) of SIV ectopic sprouts but does not alter the width of the normal endogenous SIV plexus (Figure 5F). Together, these data suggest that the endothelium is uniquely sensitive to defects in CDS-controlled phosphoinositide resynthesis and that the availability of endogenous PIP2 can limit the extent of angiogenesis in vivo.
Exogenous PIP2 restores angiogenesis in CDS2 morphants. (A) Simplified schematic diagram of phosphoinositide recycling and its relation to VEGF signal transduction and angiogenesis. VEGF/phospholipase C γ (PLCG) signaling converts phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3). CDP-DAG synthetase (CDS) activity is required for resynthesis of CDP-DAG and PIP2. (B-F) Exogenous PIP2 rescues intersegmental vessel (ISV) growth in cds2-deficient zebrafish in vivo. Tg(fli-EGFP)y1 zebrafish, injected with control (1.8 pg) cds2 (1.8 pg), low-dose plcg1 (3 pg), or low-dose VEGFaa (1.5 pg) morpholino at 1- to 2-cell stage, received an additional intermyotomal injection of liposome without (−) or with (+) PIP2 at 18 hours after fertilization (hpf), as noted. (B-E) Shown are representative confocal images of zebrafish trunk vasculature, which were obtained at 30 hpf. (F) Quantitation of the percentage of ISVs reaching dorsally to form the dorsal longitudinal anastomotic vessel (DLAV). Twenty embryos were counted for each group, with 11 trunk segments counted per embryo. (G) Exogenous PIP2 rescues ERK activation in cds2-deficient zebrafish in vivo. Trunk tissues were collected at 30 hpf and then assayed by immunoblotting with anti-ERK1/2 and anti–phospho-ERK1/2 antibodies. Normalization was performed as described in Figure 3D. Scale bars = 100 μm (B-E). Ctrl indicates control; MO, morpholino oligonucleotide; plcg1, phospholipase C γ1.
Exogenous PIP2 restores angiogenesis in CDS2 morphants. (A) Simplified schematic diagram of phosphoinositide recycling and its relation to VEGF signal transduction and angiogenesis. VEGF/phospholipase C γ (PLCG) signaling converts phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3). CDP-DAG synthetase (CDS) activity is required for resynthesis of CDP-DAG and PIP2. (B-F) Exogenous PIP2 rescues intersegmental vessel (ISV) growth in cds2-deficient zebrafish in vivo. Tg(fli-EGFP)y1 zebrafish, injected with control (1.8 pg) cds2 (1.8 pg), low-dose plcg1 (3 pg), or low-dose VEGFaa (1.5 pg) morpholino at 1- to 2-cell stage, received an additional intermyotomal injection of liposome without (−) or with (+) PIP2 at 18 hours after fertilization (hpf), as noted. (B-E) Shown are representative confocal images of zebrafish trunk vasculature, which were obtained at 30 hpf. (F) Quantitation of the percentage of ISVs reaching dorsally to form the dorsal longitudinal anastomotic vessel (DLAV). Twenty embryos were counted for each group, with 11 trunk segments counted per embryo. (G) Exogenous PIP2 rescues ERK activation in cds2-deficient zebrafish in vivo. Trunk tissues were collected at 30 hpf and then assayed by immunoblotting with anti-ERK1/2 and anti–phospho-ERK1/2 antibodies. Normalization was performed as described in Figure 3D. Scale bars = 100 μm (B-E). Ctrl indicates control; MO, morpholino oligonucleotide; plcg1, phospholipase C γ1.
Exogenous PIP2 promotes ectopic angiogenesis in wild-type zebrafish in vivo. (A-C) Confocal images of subintestinal vessels (SIVs) extending over the yolk cell of 3 days postfertilization (dpf) Tg(fli-EGFP)y1 zebrafish that were untreated (A), received a single intravascular injection of carrier-loaded phosphatidylinositol-4,5-bisphosphate [(PI(4,5)P2] at 2 dpf (B), or received an injection of CMV:vegfaa at the 1-cell stage (C). Lateral views, anterior to the left. (D-F) Quantitation of the average number of ectopic SIV ventral sprouts (D), average total length of ectopic SIV ventral sprouts (E), and average width of the normal SIV plexus (F) in 3-dpf embryos. Scale bars = 200 μm (A-C). EGFP indicates enhanced green fluorescent protein.
Exogenous PIP2 promotes ectopic angiogenesis in wild-type zebrafish in vivo. (A-C) Confocal images of subintestinal vessels (SIVs) extending over the yolk cell of 3 days postfertilization (dpf) Tg(fli-EGFP)y1 zebrafish that were untreated (A), received a single intravascular injection of carrier-loaded phosphatidylinositol-4,5-bisphosphate [(PI(4,5)P2] at 2 dpf (B), or received an injection of CMV:vegfaa at the 1-cell stage (C). Lateral views, anterior to the left. (D-F) Quantitation of the average number of ectopic SIV ventral sprouts (D), average total length of ectopic SIV ventral sprouts (E), and average width of the normal SIV plexus (F) in 3-dpf embryos. Scale bars = 200 μm (A-C). EGFP indicates enhanced green fluorescent protein.
CDS2 regulates angiogenesis of human endothelial cells in vitro
To further explore the role of CDS2 in the endothelium, we performed in vitro 3D collagen gel endothelial cell invasion assays after siRNA-mediated knockdown of the orthologous human CDS1 or CDS2 genes in HUVECs (Figure 6A).39,50 Transfection of CDS1 or CDS2 siRNAs into HUVECs results in > 95% reduction in their mRNA levels (supplemental Figure 7A) and leads to significant decreases in endothelial cell invasion, with > 50% or 90% inhibition after CDS1 or CDS2 silencing, respectively (Figure 6B-C). As in zebrafish embryos, CDS deficiency in HUVECs results in strong reduction in ERK activation (Figure 6D). It also results in reduced activation of known downstream modulators of VEGF signaling Akt and NFAT, but does not result in altered expression of VEGFR2 (supplemental Figure 7).51 In addition, in vivo increased CDS-controlled resynthesis of phosphoinositides promotes excess angiogenesis in vitro (Figure 5). We examined this by increasing the CDS expression level or supplying PIP2 to normal endothelial cells. We introduced lentiviral vectors for expression of GFP (as control), CDS1, or CDS2 into normal HUVECs in vitro and measured their activity in the EC invasion assay (Figure 6E). HUVECs that overexpress either CDS1 or CDS2 show strongly increased activity in the EC invasion assay compared with GFP-transduced controls (Figure 6E). A single transient treatment of HUVECs with PIP2 liposome just before the assay also significantly increases their activity in the in vitro EC invasion assay (Figure 6F). Together, these data suggest that PIP2 substrate availability can limit angiogenesis under conditions of VEGF stimulation.
CDS2 regulates angiogenesis in vitro. (A) Schematic diagram that shows the HUVEC invasion assay used to model angiogenesis in vitro. HUVECs are plated on the surface of a collagen gel that contains VEGFA and other factors (see “PIP2 quantitation and delivery into cultured HUVECs or zebrafish embryos”), which they invade and grow into. Invasion is imaged and quantified at specific depths. (B-D) CDS2 or CDS1 knockdown inhibits ERK activation and in vitro angiogenesis of HUVECs. (B) Sample DIC microscopy images of HUVECs transfected with control (ctrl), CDS1, or CDS2 small interfering RNA (siRNA) for 48 hours and then transferred into a 3-dimensional (3D) invasion assay for an additional 24 hours. Images show HUVECs in the monolayer at the surface of the collagen gel (monolayer) and at 2 separate depths (depth 1, depth 2) to which some of the cells have migrated within the collagen gel. Images such as these are collected and used for quantitation of HUVEC angiogenic invasion. (C) Quantitation of the percentage of invading control, CDS1, or CDS2 siRNA-treated HUVECs with the use of the assay shown in panel A. The percentage of invading cells was normalized to control siRNA-treated HUVECs. (D) Protein extracts from panels B and C were assayed by immunoblotting with the use of anti-ERK1/2 and anti–phospho-ERK1/2 antibodies. (E) CDS overexpression promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs transduced with either green fluorescent protein (GFP; control), zCDS1, or zCDS2 producing lentiviral vectors, using the assay shown in panel A. The percentage of invading cells was normalized to control HUVECs. (F) Exogenous phosphatidylinositol-4,5-bisphosphate (PIP2) promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs that were either untreated (control) or treated with carrier alone (carrier only) or carrier-loaded phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2; carrier + PIP2] before plating on the collagen gel with the use of the assay shown in panel A. Scale bars = 25 μm (B). CDS indicates CDP-diacylglycerol synthetase.
CDS2 regulates angiogenesis in vitro. (A) Schematic diagram that shows the HUVEC invasion assay used to model angiogenesis in vitro. HUVECs are plated on the surface of a collagen gel that contains VEGFA and other factors (see “PIP2 quantitation and delivery into cultured HUVECs or zebrafish embryos”), which they invade and grow into. Invasion is imaged and quantified at specific depths. (B-D) CDS2 or CDS1 knockdown inhibits ERK activation and in vitro angiogenesis of HUVECs. (B) Sample DIC microscopy images of HUVECs transfected with control (ctrl), CDS1, or CDS2 small interfering RNA (siRNA) for 48 hours and then transferred into a 3-dimensional (3D) invasion assay for an additional 24 hours. Images show HUVECs in the monolayer at the surface of the collagen gel (monolayer) and at 2 separate depths (depth 1, depth 2) to which some of the cells have migrated within the collagen gel. Images such as these are collected and used for quantitation of HUVEC angiogenic invasion. (C) Quantitation of the percentage of invading control, CDS1, or CDS2 siRNA-treated HUVECs with the use of the assay shown in panel A. The percentage of invading cells was normalized to control siRNA-treated HUVECs. (D) Protein extracts from panels B and C were assayed by immunoblotting with the use of anti-ERK1/2 and anti–phospho-ERK1/2 antibodies. (E) CDS overexpression promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs transduced with either green fluorescent protein (GFP; control), zCDS1, or zCDS2 producing lentiviral vectors, using the assay shown in panel A. The percentage of invading cells was normalized to control HUVECs. (F) Exogenous phosphatidylinositol-4,5-bisphosphate (PIP2) promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs that were either untreated (control) or treated with carrier alone (carrier only) or carrier-loaded phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2; carrier + PIP2] before plating on the collagen gel with the use of the assay shown in panel A. Scale bars = 25 μm (B). CDS indicates CDP-diacylglycerol synthetase.
Lithium treatment mimics angiogenic failure by CDS2 deficit
To further verify that the angiogenic defect in CDS-deficient endothelium is because of a failure in phosphoinositide recycling, we used LiCl treatment to inhibit inositol monophosphatase (IMPase), resulting in blockage of myo-inositol production and phosphoinositide synthesis (Figure 7A).52 LiCl treatment inhibited angiogenesis either in vitro (Figure 7B) or in vivo (Figure 7C-G). In either case, the angiogenesis defect was ameliorated by providing an exogenous source of myo-inositol, suggesting that the effects are specific to inhibition of phosphoinositide recycling and not other potential targets of lithium (Figure 7B-G). Together, these results suggest that the angiogenesis defect in CDS-deficient animals is caused by a failure to fully restore phosphoinositide consumed during angiogenesis, mainly mediated by VEGFA.
Myo-inositol rescues LiCl suppression of angiogenesis in vivo and in vitro. (A) Simplified schematic diagram of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) recycling to phosphatidylinositol-4,5-bisphosphate [PIP2; PI(4,5)P2], showing the step of myoinositol generation catalyzed by inositol monophosphatase (IMP) that is inhibited by lithium ions. CDS indicates CDP-DAG synthetase; PA, phosphatidic acid; PI, phosphatidylinositol; and PI4P, phosphatidylinositol-4-phosphate. (B) Myo-inositol rescues LiCL suppression of HUVEC invasion in vitro. HUVECs in in vitro angiogenesis assay are incubated in media with or without 10mM NaCl or LiCl and with or without exogenous myoinositol (same quantitation methods as shown in Figure 6). (C-G) Myo-inositol rescues LiCL suppression of HUVEC invasion in vivo. (C-F) Confocal images of trunk vessels in 30-hours postfertilization (hpf) Tg(fli-EGFP)y1 zebrafish that were untreated (C), incubated with 200mM LiCl from 12 hpf zebrafish (D), received a yolk cell injection of myoinositol at the 1-cell stage (E), or received a yolk cell injection of myo-inositol and then were incubated with LiCl (F). Lateral views, anterior to the left. (G) Quantitation of the intersegmental vessel (ISV) phenotypes of zebrafish embryos in panels C-F. The bars show percentages of ISVs that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (yellow). The number (n = 50) of embryos counted is shown at the top of each column, with 10 trunk segments counted per embryo (same quantitation methods as shown in Figure 2). Scale bars = 100 μm (C-F).
Myo-inositol rescues LiCl suppression of angiogenesis in vivo and in vitro. (A) Simplified schematic diagram of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) recycling to phosphatidylinositol-4,5-bisphosphate [PIP2; PI(4,5)P2], showing the step of myoinositol generation catalyzed by inositol monophosphatase (IMP) that is inhibited by lithium ions. CDS indicates CDP-DAG synthetase; PA, phosphatidic acid; PI, phosphatidylinositol; and PI4P, phosphatidylinositol-4-phosphate. (B) Myo-inositol rescues LiCL suppression of HUVEC invasion in vitro. HUVECs in in vitro angiogenesis assay are incubated in media with or without 10mM NaCl or LiCl and with or without exogenous myoinositol (same quantitation methods as shown in Figure 6). (C-G) Myo-inositol rescues LiCL suppression of HUVEC invasion in vivo. (C-F) Confocal images of trunk vessels in 30-hours postfertilization (hpf) Tg(fli-EGFP)y1 zebrafish that were untreated (C), incubated with 200mM LiCl from 12 hpf zebrafish (D), received a yolk cell injection of myoinositol at the 1-cell stage (E), or received a yolk cell injection of myo-inositol and then were incubated with LiCl (F). Lateral views, anterior to the left. (G) Quantitation of the intersegmental vessel (ISV) phenotypes of zebrafish embryos in panels C-F. The bars show percentages of ISVs that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (yellow). The number (n = 50) of embryos counted is shown at the top of each column, with 10 trunk segments counted per embryo (same quantitation methods as shown in Figure 2). Scale bars = 100 μm (C-F).
Discussion
CDS is essential for VEGFA-induced angiogenesis
In this study, we report the essential role of CDS2 in VEGFA signaling–induced angiogenesis. Two CDS2 mutants identified from a large-scale forward genetic screen in zebrafish show severe angiogenic defects and phenocopy VEGFA signaling–defective animals such as VEGFR2 mutants, low-dose VEGFA morphants, or low-dose PLCγ1 morphants. Cds2 mutants and morphants both showed significant reduction in ERK activation, arterial differentiation, and cell proliferation in the developing endothelium. Given that (1) VEGFA-VEGFR2-PLCγ1 signaling is required for arterial differentiation and ERK activation,8,9,17,47,48 (2) the cds2 mutant phenotype can be successfully rescued by endothelial-specific expression of wild-type cds2 and phenocopied by cds2 knockdown (Figures 2C,E), and (3) siRNA-mediated silencing of human CDS2 also strongly blocks angiogenesis in vitro (Figure 6), we conclude that cds2 is required for the transduction of VEGFA signaling and VEGFA-mediated angiogenesis. Among the many isoforms of phosphoinositides, PIP2 is the biochemical target for VEGFA signaling to generate further signaling messengers, including PIP3, IP3, and DAG. Exogenously supplied PIP2 can rescue angiogenic defects in cds2 morphants (Figure 4B-G) and can even promote ectopic angiogenesis of SIVs in wild-type animals (Figure 5). Independently interfering with phosphoinositide recycling by lithium inhibition of inositol monophosphatase also inhibits angiogenesis in vitro (Figure 7A-B) and in vivo (Figure 7C-G). Taken together, we conclude that PIP2 availability can limit VEGFA signaling and VEGFA-induced angiogenesis both in vivo and in vitro.
VEGFA-stimulated endothelium is uniquely sensitive to CDS-controlled PIP2 availability
Although PIP2 is required for the transduction of many PLC- or PI3K-dependent signaling pathways in a wide variety of different cell types, zebrafish cds2 mutants show a surprisingly vascular-specific phenotype, suggesting that the endothelium is uniquely sensitive to deficiencies in phosphoinositide recycling. Although the reasons for this are still not clear, previous reports have shown that activated ERK is highly enriched in the developing endothelium of the trunk,45 and manipulation of VEGFA expression alone can result in strong changes in the levels of activated ERK in samples collected from whole zebrafish embryos.53 Increased turnover or production of phosphoinositides, especially PIP2, has been observed in several different types of cells under physiologic or cellular signaling stimulation.54-58 We would hypothesize that endothelial cells have elevated downstream PIP2-dependent ERK activation and greater PIP2 consumption than other cell types, at least during development. Greater PIP2 consumption would necessitate more active resynthesis of this substrate, making the endothelium more sensitive to deficits in PIP2 resynthesis. In other words, in the presence of impaired CDS-controlled PIP2 resynthesis, VEGFA-stimulated endothelial cells will run out of PIP2 “fuel” faster than other cells, resulting in a vascular-specific phenotype. Further studies will be needed to determine whether endothelium is preferentially susceptible to disruption of phosphoinositide recycling in adult or pathologic angiogenesis and in nonpiscine vertebrates.
Targeting phosphoinositide recycling as a novel antiangiogenic therapy
If the endothelium is indeed preferentially sensitive to partial reduction in phosphoinositide recycling during pathologic angiogenesis in mammals, then this could serve as a valuable new paradigm for antiangiogenic therapies. Inhibition of phosphoinositide recycling has a potent effect on VEGF-dependent signaling and angiogenesis. These effects are exacerbated, not alleviated, by increased stimulation by VEGF and, potentially, other angiogenic signals transduced via phosphoinositide second messengers. Hypothetically, if a tumor were to respond to vascular insufficiency caused by therapeutic inhibition of PIP2 recycling by increasing its own VEGF production, then it might actually increase the effectiveness of the therapy rather than overcome it, because increased VEGF stimulation would lead to more rapid and complete depletion of limiting PIP2 substrate in the responding endothelial cells and more rapid and profound collapse of PIP2-dependent signaling. Further work will be needed to determine whether this potential new paradigm for angiogenic therapy will prove useful.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Igor Dawid and members of the Weinstein laboratory for critical comments on this manuscript. They thank Young Cha, Andrew Davis, Misato Fujita, Aniket Gore, Miho Matsuda, Gregory Palardy, and Matthew Swift for technical support and reagents.
This work was supported by the intramural program of the National Institute of Child Health and Human Development, National Institutes of Health (NIH; B.M.W.), the intramural program of the National Institute of Dental and Craniofacial Research, NIH (J.S.G.), the Leducq Foundation (B.M.W.), and by the NIH (grant HL59393, G.E.D.).
National Institutes of Health
Authorship
Contribution: M.K., K.R.K., B.D.L., K.M.S., A.N.S., and J.T.-V. performed ENU treatment and genetic screen; W.P., V.N.P., A.N.S., D.C., and C.M.M. performed experiments; W.P., V.N.P., A.N.S., J.S.G., G.E.D., and B.M.W. analyzed results and made the figures; AND W.P., V.N.P., A.N.S., G.E.D., and B.M.W. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brant M. Weinstein, Program in Genomics of Differentiation, National Institute of Child Health and Human Development, National Institutes of Health, 6 Center Dr, Bethesda, MD 20892; e-mail: flyingfish2@nih.gov.
References
Author notes
W.P. and V.N.P. contributed equally to this study.
M.K., K.R.K., B.D.L., K.M.S. and J.T.-.V. contributed equally to this study.


![Figure 5. Exogenous PIP2 promotes ectopic angiogenesis in wild-type zebrafish in vivo. (A-C) Confocal images of subintestinal vessels (SIVs) extending over the yolk cell of 3 days postfertilization (dpf) Tg(fli-EGFP)y1 zebrafish that were untreated (A), received a single intravascular injection of carrier-loaded phosphatidylinositol-4,5-bisphosphate [(PI(4,5)P2] at 2 dpf (B), or received an injection of CMV:vegfaa at the 1-cell stage (C). Lateral views, anterior to the left. (D-F) Quantitation of the average number of ectopic SIV ventral sprouts (D), average total length of ectopic SIV ventral sprouts (E), and average width of the normal SIV plexus (F) in 3-dpf embryos. Scale bars = 200 μm (A-C). EGFP indicates enhanced green fluorescent protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2012-02-408328/4/m_zh89991293850005.jpeg?Expires=1770947071&Signature=Q76f25h-PBi59E1vNuO1G7R8w4MeeOE3kej~Q85WKA-tSq3jvfXONU5p6IER2lTqhFygUqrxZ83GjM-5mAGFbxbjREvC-heerzpGafy6v4hmfEduS339pUmQG~ZhZM45IiIcdR8JN-pu~kBAyFWNJgpkBBTO6IcL40sSSndc97KgW4XRWzVP8o1CAw9M9g4ACkqkCdHEUI2aDavpnq9dbu1nheADtCRmPzM8F01p57JdteeA8VxpDUzYIW5vHOBa92bP3b17NVL2wczfS1LUrwMNN4ucfJhOwUzaTPvMEqH9VHLyiViSus1R-uW-QmfC0pJe6SlH7hp53varS8Ap1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. CDS2 regulates angiogenesis in vitro. (A) Schematic diagram that shows the HUVEC invasion assay used to model angiogenesis in vitro. HUVECs are plated on the surface of a collagen gel that contains VEGFA and other factors (see “PIP2 quantitation and delivery into cultured HUVECs or zebrafish embryos”), which they invade and grow into. Invasion is imaged and quantified at specific depths. (B-D) CDS2 or CDS1 knockdown inhibits ERK activation and in vitro angiogenesis of HUVECs. (B) Sample DIC microscopy images of HUVECs transfected with control (ctrl), CDS1, or CDS2 small interfering RNA (siRNA) for 48 hours and then transferred into a 3-dimensional (3D) invasion assay for an additional 24 hours. Images show HUVECs in the monolayer at the surface of the collagen gel (monolayer) and at 2 separate depths (depth 1, depth 2) to which some of the cells have migrated within the collagen gel. Images such as these are collected and used for quantitation of HUVEC angiogenic invasion. (C) Quantitation of the percentage of invading control, CDS1, or CDS2 siRNA-treated HUVECs with the use of the assay shown in panel A. The percentage of invading cells was normalized to control siRNA-treated HUVECs. (D) Protein extracts from panels B and C were assayed by immunoblotting with the use of anti-ERK1/2 and anti–phospho-ERK1/2 antibodies. (E) CDS overexpression promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs transduced with either green fluorescent protein (GFP; control), zCDS1, or zCDS2 producing lentiviral vectors, using the assay shown in panel A. The percentage of invading cells was normalized to control HUVECs. (F) Exogenous phosphatidylinositol-4,5-bisphosphate (PIP2) promotes increased HUVEC invasion of 3D collagen matrices in vitro. Quantitation of the percentage of invading HUVECs that were either untreated (control) or treated with carrier alone (carrier only) or carrier-loaded phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2; carrier + PIP2] before plating on the collagen gel with the use of the assay shown in panel A. Scale bars = 25 μm (B). CDS indicates CDP-diacylglycerol synthetase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2012-02-408328/4/m_zh89991293850006.jpeg?Expires=1770947071&Signature=dCFTwWWKxfXufx1rn8KS7KGLTXqEJueY5MQAiF1RYzBkAuIBOOyorsUFLsqPf-rkAguoDBakGFoOHNJTjZVJsR18Z0xsLqxXUSNr1iIY87EugplrzCvb96KKnHNptDOFjEjgrbk5DVWA9PchMHcNODoW0fNGK1WlMf7geF2Jbmo2R18wJdJafww7lSPQq8XmwsmfUaDYsky3UwvZmMypBCtb1ObS7g1YTS3iGGVixT4Kbcs5LqX~K3q1~aXu-Yl7vuluN-4QWuk5YUUAUI02L7LQ909qvGBFbzGcoIadZLDq2AfwlHW3SlONo1kSfkkyolUoIyDjmn9KIiCBmfzyXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Myo-inositol rescues LiCl suppression of angiogenesis in vivo and in vitro. (A) Simplified schematic diagram of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) recycling to phosphatidylinositol-4,5-bisphosphate [PIP2; PI(4,5)P2], showing the step of myoinositol generation catalyzed by inositol monophosphatase (IMP) that is inhibited by lithium ions. CDS indicates CDP-DAG synthetase; PA, phosphatidic acid; PI, phosphatidylinositol; and PI4P, phosphatidylinositol-4-phosphate. (B) Myo-inositol rescues LiCL suppression of HUVEC invasion in vitro. HUVECs in in vitro angiogenesis assay are incubated in media with or without 10mM NaCl or LiCl and with or without exogenous myoinositol (same quantitation methods as shown in Figure 6). (C-G) Myo-inositol rescues LiCL suppression of HUVEC invasion in vivo. (C-F) Confocal images of trunk vessels in 30-hours postfertilization (hpf) Tg(fli-EGFP)y1 zebrafish that were untreated (C), incubated with 200mM LiCl from 12 hpf zebrafish (D), received a yolk cell injection of myoinositol at the 1-cell stage (E), or received a yolk cell injection of myo-inositol and then were incubated with LiCl (F). Lateral views, anterior to the left. (G) Quantitation of the intersegmental vessel (ISV) phenotypes of zebrafish embryos in panels C-F. The bars show percentages of ISVs that have failed to sprout (blue), ISVs that have grown only up to the horizontal myoseptum half way up the trunk (red), and ISVs that have grown all the way to the dorsal trunk to form the dorsal longitudinal anastomotic vessel (yellow). The number (n = 50) of embryos counted is shown at the top of each column, with 10 trunk segments counted per embryo (same quantitation methods as shown in Figure 2). Scale bars = 100 μm (C-F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2012-02-408328/4/m_zh89991293850007.jpeg?Expires=1770947071&Signature=P9FMk1Aw5SacxmcRj3bgdYzySxp7Ttb7exVLcQ~MMYseRxUsMJpkEQ9ElxQHRtvN4uFNlkAMNqj~cI41NaCd39Tq-0iiJngDrpsuev0Mrojei~p5-8mN1on1pw2ZW0mhu94mNgv8hDKjo-D8F~qse337riWF9EQhU9-T6vHOgwWI04a0ii9xlnzVrxy2mlGxu6JHLozI5~4FD5MNxuXvjrJbf67kYOGtNUmeInLWXLr9bZUQDsuGdONOy7XE-dpu-M08gLFnonblh3JNT2SpXbK-Roqf8yxSNJjF08cW3vEQYiEdfi~BXfXQqxCkz42IYnbuf2qtNgMMUKwe-8aUuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal