Abstract
Multiple signaling pathways control the specification of endothelial cells (ECs) to become arteries or veins during vertebrate embryogenesis. Current models propose that a cascade of Hedgehog (Hh), vascular endothelial growth factor (VEGF), and Notch signaling acts instructively on ECs to control the choice between arterial or venous fate. Differences in the phenotypes induced by Hh, VEGF, or Notch inhibition suggest that not all of the effects of Hh on arteriovenous specification are mediated by VEGF. We establish that full derepression of the Hh pathway in ptc1;ptc2 mutants converts the posterior cardinal vein into a second arterial vessel that manifests intact arterial gene expression, intersegmental vessel sprouting, and HSC gene expression. Importantly, although VEGF was thought to be absolutely essential for arterial fates, we find that normal and ectopic arterial differentiation can occur without VEGF signaling in ptc1;ptc2 mutants. Furthermore, Hh is able to bypass VEGF to induce arterial differentiation in ECs via the calcitonin receptor-like receptor, thus revealing a surprising complexity in the interplay between Hh and VEGF signaling during arteriovenous specification. Finally, our experiments establish a dual function of Hh during induction of runx1+ HSCs.
Introduction
Among the first fully differentiated cells to arise in the vertebrate embryo are the mesodermally derived hematopoietic and endothelial lineages, which compose the circulatory system, itself the first functional organ system to develop. Unraveling the signaling networks that govern the formation of the vasculature has taken on added significance with the recent demonstration of the emergence of HSCs from ventral endothelium of the dorsal aorta (DA).1-3 In zebrafish, hematopoietic and endothelial lineages arise from a common bipotential progenitor, the hemangioblast.4 Hemangioblasts are specified within ventral mesoderm, alongside unipotential hematopoietic progenitors and angioblasts. These 3 cell types are transported via the cellular rearrangements and morphologic movements of gastrulation to reside as 2 bilateral cell populations within the lateral plate mesoderm. The first major vessels of vertebrate embryos form via vasculogenesis, whereby migratory angioblasts coalesce to generate lumenized vessels. In zebrafish, angioblasts within the posterior lateral mesoderm (PLM) migrate medially beneath the somites and coalesce ventral to the notochord and hypochord at the midline. These cells form the major trunk vessels, the DA and posterior cardinal vein (PCV).5
The exact regulation and timing of endothelial specification are an important developmental question, but published data are currently difficult to reconcile. A genetic basis for arterial and venous differentiation was established with the identification of Ephrin-B2 and EphB4 receptors as markers of arteries and veins, respectively.6,7 Using lineage tracking, clusters of endothelial cells (ECs) were identified within the zebrafish PLM, which either contributed to the DA or PCV, but never both, leading to the suggestion that determination of arterial and venous fate within ECs occurs before angioblast migration.8 The molecular basis of this proposed fate restriction is unclear because differential expression of known arterial or venous markers (ephrinb2a, kdrl, and flt4) is observed after the migrating angioblasts arrive at the midline9-11 ; however, the first angioblasts to reach the midline and contribute to the DA express activated ERK during their migration.12 Importantly, recent studies show that some flk1/kdrl+ (arterial) angioblasts migrate ventrally and contribute to the PCV after their arrival at the midline.13 Taken together, these studies suggest that endothelial progenitors are specified as arterial or venous before circulation, but it is unclear whether they are fully determined before migration to the midline.
The Notch signaling pathway is a key determinant in the establishment of arterial identity.9 In mice, Notch receptors and ligands exhibit arterially restricted expression and targeted inactivation of Notch pathway components results in defective arterial specification.14 Zebrafish mindbomb (mib) mutants, which are defective for Notch signaling, exhibit arteriovenous shunts, defective PCV formation, and reduced arterial gene expression, whereas gridlock (grl) mutants, which contain a lesion in hey2, exhibit reduced arterial gene expression and DA maturation defects.9,15,16
Although Notch signaling guides arterial fate, elegant studies in zebrafish and more recently in mouse revealed Hedgehog (Hh) and VEGF signaling to act upstream of Notch in arterial specification.17,18 Zebrafish sonic-you (syu) mutants, which lack midline Shha activity, exhibit a drastic reduction in arterial gene expression. This failure to specify the DA correctly was proposed to result from failure to express vegfa in the adjacent somites.17 How this transcriptional activation occurs has not been addressed. Recently, a study positioned Crlra downstream of Hh signaling and upstream of VEGF within this hierarchy, but the precise molecular details remain unclear.19 Whatever the exact mechanism of VEGF induction, vegfa morphants exhibit reduced arterial gene expression, whereas vegfa overexpression induces ectopic arterial differentiation and can rescue the loss of arterial differentiation in syu mutant embryos, indicating its indispensability.17 VEGF binds to Kdrl or Kdr, a receptor tyrosine kinase, thereby activating downstream phospholipase C gamma1 (Plcg1).20 Zebrafish kdrl and plcg1 mutants are defective in arterial but not in venous specification.21,22 Less is known about venous determination; in mice, active repression of Notch signaling may occur in venous ECs.23 In zebrafish, PI3K signaling downstream of VEGF may maintain venous identity via its ability to block MAPK signaling, which itself favors arterial differentiation via activation of ERK.12
Hh signaling inhibition in zebrafish embryos results in a failure to form 2 distinct trunk axial vessels, a phenotype more severe than in embryos lacking VEGF signaling, implying that additional components act downstream of Hh to regulate arterial differentiation.9,17,20 Consistent with this, ectopic lumenized vessels can be induced by exogenous Shh but not by VEGF.24 Differences in the response of ECs to Hh and VEGF may account for this25 ; however, recent studies in mammals have revealed VEGF-independent roles for Hh signaling in vascular lumenization, yet the underlying mechanism remains unclear.18 In zebrafish, activation of Hh signaling can induce arterial differentiation at the expense of venous identity26 ; here we extend these findings through analysis of animals doubly mutant for the Hh receptors Ptc1 and Ptc2, which exhibit constitutive activation of Hh signaling.27,28 We show that Hh induces arterial differentiation at the expense of venous fate, effectively converting the PCV into a second DA, which displays intact arterial expression in addition to arterial characteristics, such as precocious vessel sprouting and HSC gene expression. In addition, we show that, in ptc1;ptc2 mutants, arterial differentiation can occur independently of VEGF signaling, via a parallel pathway whereby Hh signaling acts via Crlra to induce arterial differentiation. Given the high conservation of these genetic hierarchies between zebrafish and mammals, the identification of Crlr signaling in parallel with VEGF may account for the VEGF-independent effects of Hh signaling during mammalian arterial development.18
Methods
Zebrafish husbandry
Zebrafish (Danio rerio) embryos from wild-type, ptch1hu1602/+;ptch2tj222, smohi1640Tg/+, plcg1t26480/+, Tg(fli1a:EGFP)y1/+, and Tg(gata1:dsred)sd2/+ strains were raised at 28.5°C as described.29 All animal studies were conducted with approval from the United Kingdom Home Office.
Drug treatments
Purmorphamine (Chemistry Research Laboratory; stock solution in DMSO: 2.5 mg/mL) was used as previously described.30 SU5416 and Cyclopamine (Merck; stock solution 10mM in DMSO and 95% ethanol, respectively) were used in E3 medium at a concentration of 2μM from tailbud and 50μM from high stage, respectively.
Whole-mount RNA in situ hybridization
Experiments were performed as previously described.11 Myc expression was detected using anti–c-Myc primary antibody (9E10; Abcam; 1:500) alongside anti-DIG antibody. Embryos were incubated with AlexaFluor-488 secondary antibody (Invitrogen; 1:200) and imaged, followed by developing of in situ staining.
Crlra overexpression constructs
Full-length crlra was amplified by RT-PCR with the SuperScript One-Step RT-PCR System (Invitrogen) using the primers 5′-ggatcccgctccggtactctgacatc-3′ and 5′-ctcgagcacattgccatgttgagtgg-3′. The resulting BamHI/XhoI fragment was cloned into pCS2+. Capped mRNA was generated using mMessage Machine SP6 Kit (Ambion).
In situ probes
wnt16 probe was amplified using the primers 5′-cctttgtgctctcagggaag-3′ and 5′-gcgttgctctttatccttgc-3′ and cloned into pGem-T-Easy (Promega). crlra probe was created via PCR generated from IMAGE:7141310 (Source Bioscience) using primers 5′-tggataaccgtattaccgcc-3′ and 5′-cgcgcaattaac cctcactaaagcactagtcataccaggatc-3′.
Fli1a:Myc-dnPKA:IRES-EGFP construct
Morpholino injections
Embryos were injected with 1nl crlra morpholino (Gene Tools; 0.67mM dissolved in distilled water) 5′-agctcgctgtcatcttctttggcat-3′19 ; 0.5 nl (0.5mM) Su(H) morpholino 5′-caaacttccctgtcacaacaggcgc-3′34 ; 0.5 nl (10 μg/μL) kdr morpholino 5′-gttttcttgatctcacctgaaccct-3′22 ; and 0.5 nl (10 μg/μL) kdrl morpholino 5′-ccgaatgatactccgtatgtcacctt-3′.35
Confocal imaging
Confocal images were collected using a Leica DM IRE2 microscope at 20× magnification. Time-lapse confocal images were collected using a PerkinElmer Ultraview Vox microscope at 10× and 20× magnification. Images were analyzed using Volocity Version 6.0.1 (PerkinElmer) to reconstruct a 3-dimensional representation.
Results
Zebrafish ptc1;ptc2 mutants show defective angioblast migration and fail to establish circulation
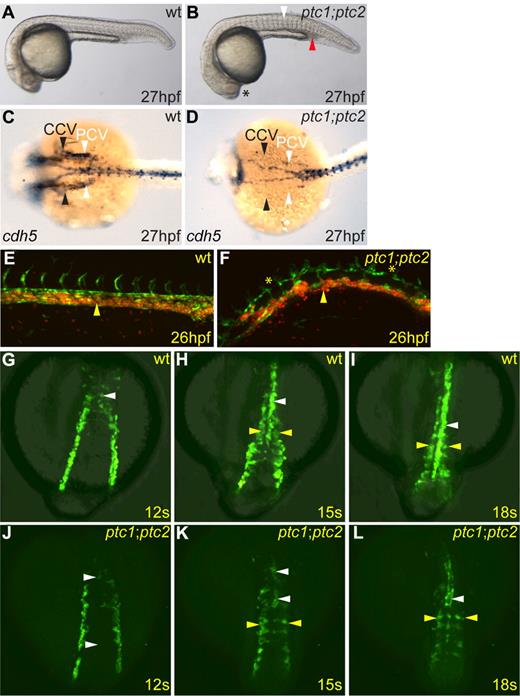
Although both ptc1 heterozygous and homozygous embryos exhibit increased levels of Hh signaling, vascular development is not obviously affected in either, and the circulatory loop is established normally. Similarly, ptc2 homozygotes have no detectable defects in the circulatory system.28 However, double-mutant ptc1;ptc2 embryos, which exhibit constitutive Hh signaling, by contrast, failed to establish circulation, resulting in pooling of primitive erythrocytes in the intermediate cell mass and posterior blood island (Figure 1A-B red arrowhead). This was further confirmed using the Tg(fli1a:EGFP)y1 and Tg(gata1:dsred)sd2 transgenes36,37 to visualize both developing vasculature and primitive erythrocytes at onset of circulation. Using time-lapse confocal microscopy, we observed that, in wild-type embryos, primitive erythrocytes entered circulation via the PCV, as previously described13 (Figure 1E yellow arrowhead; supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), whereas, in ptc1;ptc2 embryos, they failed to enter the circulation, mainly residing within the PCV (Figure 1F yellow arrowhead; supplemental Video 2). Double-mutant embryos also exhibit disorganized vasculature. In wild-type embryos at 26 hpf, the DA and PCV were juxtaposed after the entry of primitive erythrocytes into circulation (Figure 1E), whereas in ptc1;ptc2 mutants, the 2 axial vessels were clearly separated (Figure 1F). In more anterior regions, at 27 hpf, ptc1;ptc2 embryos exhibited a drastic reduction of endothelial cdh5 staining in the common cardinal vein (Figure 1C-D black arrowheads), and the adjoining PCV (Figure 1C-D white arrowheads), indicating these vessels were absent, which would explain the failure to establish a circulatory loop in ptc1;ptc2 double mutants.
Zebrafish ptc1;ptc2 double mutants fail to establish circulation and exhibit multiple endothelial defects and defective angioblast migration. (A-B) ptc1;ptc2 mutant (B) embryos at 27 hpf showed somitic flattening (white arrowhead) and loss of lens (asterisk). ptc1;ptc2 embryos showed noncirculating primitive erythrocytes in the posterior intermediate cell mass (red arrowhead). (C-D) cdh5 expression in ptc1;ptc2 mutants (D) revealed defective formation of the common cardinal vein (CCV; black arrowheads) and PCV (white arrowheads) compared with wild-type (C). (E-F) Confocal image of trunk vasculature in Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+ (E) and Tg(gata1:dsred)sd2/+;ptc1;ptc2 (F) embryos; anterior to the left, posterior right. The primary vasculature formed normally in Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+ embryos, and circulation commenced normally (yellow arrowhead; supplemental Video 1), whereas Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+;ptc1;ptc2 embryos had disorganized vasculature, axial vessels were noncontinuous along their anteroposterior axis (asterisks), and embryos lacked circulation (yellow arrowhead; supplemental Video 2). (G-L) Confocal images using a Tg(fli1a:EGFP)y1/+ background to visualize migrating angioblasts in indicated genetic backgrounds. Dorsal views are shown. (G-I) Normal angioblast migration in Tg(fli1a:EGFP)y1/+ embryos. (J-L) Fewer angioblasts migrated to the midline in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos and formed a discontinuous endothelial cord by 18 somites (supplemental Video 3; white arrowheads). Angioblasts were present in more lateral positions (supplemental Video 4; L yellow arrowheads) than in controls at the corresponding stage (I yellow arrowheads).
Zebrafish ptc1;ptc2 double mutants fail to establish circulation and exhibit multiple endothelial defects and defective angioblast migration. (A-B) ptc1;ptc2 mutant (B) embryos at 27 hpf showed somitic flattening (white arrowhead) and loss of lens (asterisk). ptc1;ptc2 embryos showed noncirculating primitive erythrocytes in the posterior intermediate cell mass (red arrowhead). (C-D) cdh5 expression in ptc1;ptc2 mutants (D) revealed defective formation of the common cardinal vein (CCV; black arrowheads) and PCV (white arrowheads) compared with wild-type (C). (E-F) Confocal image of trunk vasculature in Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+ (E) and Tg(gata1:dsred)sd2/+;ptc1;ptc2 (F) embryos; anterior to the left, posterior right. The primary vasculature formed normally in Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+ embryos, and circulation commenced normally (yellow arrowhead; supplemental Video 1), whereas Tg(fli1a:EGFP)y1;Tg(gata1:dsred)sd2/+;ptc1;ptc2 embryos had disorganized vasculature, axial vessels were noncontinuous along their anteroposterior axis (asterisks), and embryos lacked circulation (yellow arrowhead; supplemental Video 2). (G-L) Confocal images using a Tg(fli1a:EGFP)y1/+ background to visualize migrating angioblasts in indicated genetic backgrounds. Dorsal views are shown. (G-I) Normal angioblast migration in Tg(fli1a:EGFP)y1/+ embryos. (J-L) Fewer angioblasts migrated to the midline in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos and formed a discontinuous endothelial cord by 18 somites (supplemental Video 3; white arrowheads). Angioblasts were present in more lateral positions (supplemental Video 4; L yellow arrowheads) than in controls at the corresponding stage (I yellow arrowheads).
The medial migration of angioblasts is regulated by Hh signaling11,26 ; thus, to investigate whether a migration defect could contribute to the failure to form proper vessels, we used the Tg(fli1a:EGFP)y1 transgene to follow medial migration of blood and endothelial progenitors in live ptc1;ptc2 embryos (Figure 1G-L). In control Tg(fli1a:EGFP)y1/+ embryos, GFP+ angioblasts migrated, such that by 12 somites, the first GFP+ DA angioblasts had reached the midline (supplemental Video 3; Figure 1G white arrowhead). By 15 somites, these cells had begun to coalesce into a vascular cord at the midline (Figure 1H white arrowhead), closely followed by angioblasts, which contribute to the PCV (Figure 1H yellow arrowheads). By 18 somites, the vascular cord extended posteriorly (Figure 1I white arrowhead), whereas some angioblasts remained more lateral and had not yet reached the midline (Figure 1I yellow arrowheads). In Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos, by contrast, quantification using ImageJ Version 1.44 software revealed a 38% decrease in GFP+ cells throughout the embryo at 12 somites and 55% fewer angioblasts migrated toward the midline at this stage (supplemental Video 4; Figure 1J white arrowheads), such that by 15 somites, 43% fewer angioblasts had reached the midline, forming a discontinuous vascular cord (Figure 1K white arrowheads) with many angioblasts present in more lateral positions (Figure 1K yellow arrowheads). By 18 somites, the vascular cord extended anteroposteriorly in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos yet remained discontinuous and contained fewer GFP+ cells along the anteroposterior axis (Figure 1I,K white arrowheads). Many GFP+ cells also remained in more lateral positions at 18 somites in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos (Figure 1L yellow arrowheads). Thus, aberrant migration could contribute to the observed circulation defect. We also examined the effect of Hh signaling deficiency on angioblast migration using Tg(fli1a:EGFP)y1/+;smohi1640Tg embryos.38 In contrast to recent studies,26 we observed that angioblasts in Tg(fli1a:EGFP);smohi1640Tg embryos migrated in 2 waves yet failed to coalesce into an endothelial cord at the midline (supplemental Video 6; supplemental Figure 1D-F). Taken together, these results suggest that either elevated or absent Hh signaling results in impaired angioblast migration, implying that the levels of Hh signaling perceived by angioblasts are important in their successful migration to the midline.
More detailed analysis of angioblast migration for a period of 17 hours from 16 somite stage revealed that, whereas the vasculature formed normally in Tg(fli1a:EGFP)y1/+ embryos (supplemental Video 7), Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos exhibited delayed formation of the DA, resulting from aberrant medial migration of angioblasts; however, the DA ultimately formed a continuous vessel in these embryos (supplemental Video 8). Importantly, no angioblasts were observed to migrate ventrally and contribute to the PCV, as described previously.13 Furthermore, in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos, angioblasts in the position of the PCV in the posterior trunk were observed to migrate dorsally and contribute to the nascent DA (supplemental Video 8). VEGF has been shown to limit the ventral migration of angioblasts13 ; thus, in ptc1;ptc2 mutants, where VEGF signaling is elevated (see Figure 3L green arrowhead), angioblasts migrated dorsally to contribute to the dorsal aorta, thereby underlining the importance of VEGF signaling as a directional cue in this process.
ptc1;ptc2 mutants exhibit ectopic arterial differentiation at the expense of venous differentiation, resulting in conversion of the PCV into an arterial vessel
During normal development, intersegmental vessels (ISVs) sprout from the DA from 24 hpf onwards, whereas venous ISVs sprout later between 1.5 dpf and 2 dpf.39,40 In ptc1;ptc2 embryos, by contrast, precocious ISV formation was clearly observed in the PCV at 27 hpf (Figure 2B-D white arrowheads), suggesting that the PCV had acquired arterial characteristics. Optical sectioning and 3-dimensional reconstruction confirmed that the sprouting ISVs originated from the PCV and not the DA (Figure 2F white arrowhead). In addition to the precocious PCV sprouts, ptc1;ptc2 mutants displayed atypical endothelial connections between the DA and PCV (Figure 2B gray arrowheads), which were not present in control embryos (Figure 2A,C).
ptc1;ptc2 mutants exhibit precocious vessel sprouting from the posterior cardinal vein. Confocal images of developing trunk vasculature in Tg(fli1a:EGFP)y1/+ (A,C,E) and Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos at 27 hpf (B,D,F); anterior is left, posterior right. (A) Normal trunk vasculature. Highlighted area is shown in panel C. (B) Endothelial connections between the DA and PCV were present in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos (gray arrowheads), and ectopic vessel sprouts arising from PCVs were present (B,D white arrowheads). Highlighted area is shown in panel D. 3-Dimensional reconstruction: transverse plane of embryo shown in panels A, C, and E and embryo shown in panels B, D, and F) show the sprouting capacity of ECs in the PCV region in ptc1;ptc2 mutants (white arrowhead).
ptc1;ptc2 mutants exhibit precocious vessel sprouting from the posterior cardinal vein. Confocal images of developing trunk vasculature in Tg(fli1a:EGFP)y1/+ (A,C,E) and Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos at 27 hpf (B,D,F); anterior is left, posterior right. (A) Normal trunk vasculature. Highlighted area is shown in panel C. (B) Endothelial connections between the DA and PCV were present in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos (gray arrowheads), and ectopic vessel sprouts arising from PCVs were present (B,D white arrowheads). Highlighted area is shown in panel D. 3-Dimensional reconstruction: transverse plane of embryo shown in panels A, C, and E and embryo shown in panels B, D, and F) show the sprouting capacity of ECs in the PCV region in ptc1;ptc2 mutants (white arrowhead).
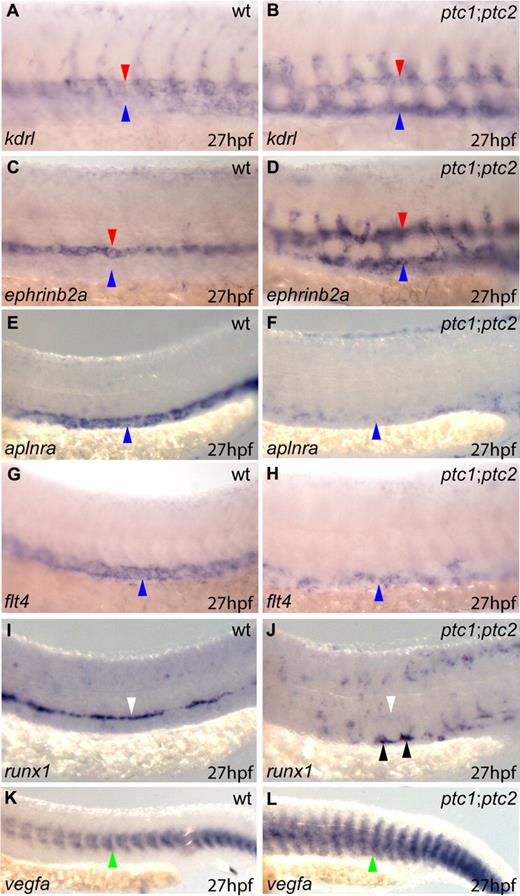
To evaluate the differentiation status of the vessels in ptc1;ptc2 embryos, we assayed arterial and venous marker gene expression by in situ hybridization. Expression of the VEGF receptor kdrl normally exhibits an arterial bias, originally being present throughout the nascent vasculature, but becoming down-regulated in the PCV and restricted to the DA by 26 to 27 hpf (Figure 3A red arrowhead). In ptc1;ptc2 mutants, by contrast, kdrl expression is maintained in the PCV during vascular development (Figure 3A-B blue arrowheads). Whereas the arterial marker ephrinb2a was ectopically expressed in the PCV (Figure 3C-D blue arrowhead), the expression of aplnra in venous endothelium41 was absent or substantially down-regulated in the PCV of ptc1;ptc2 mutants (Figure 3E-F blue arrowheads). Furthermore, expression of the VEGF receptor flt4, which is restricted to the PCV at 27 hpf, was substantially down-regulated in ptc1;ptc2 mutants (Figure 3G-H blue arrowheads). Ectopic arterial gene expression in the PCV of ptc1;ptc2 mutants, combined with a loss of venous gene expression, indicates that the PCV had undergone a fate change and was converted into an artery. We also assessed the onset of arterial gene expression within the ptc1;ptc2 mutants and did not observe any induction of arterial markers within premigratory angioblasts (data not shown); however, ectopic expression of the arterial marker dll4 was observed as early as 18 somites (supplemental Figure 2A-B red arrowheads) and was detectable in angioblasts, which had not yet reached the midline in ptc1;ptc2 mutants (supplemental Figure 2D asterisks). Similarly, precocious midline expression of ephrinb2a was observed at 18 somites (supplemental Figure 2E-H red arrowheads) and 19 somites (supplemental Figure 2I-L red arrowheads) in ptc1;ptc2 embryos. Thus, activation of Hh can change both spatial and temporal profile of arterial gene expression.
ptc1;ptc2 mutants exhibit ectopic arterial differentiation at the expense of venous differentiation, resulting in conversion of the PCV into a second artery. Lateral views of the trunk region are shown, oriented anterior to left, posterior right. (A) kdrl exhibited arterial preference in wild-type embryos (red arrowheads) but was present ectopically in the PCV in ptc1;ptc2 embryos (B blue arrowheads). (C) ephrinb2a was restricted to the DA in wild-type embryos (red arrowhead) but present ectopically in the PCV in ptc1;ptc2 embryos (D blue arrowheads). (E) aplnra and (G) flt4 expression was restricted to the PCV in wild-type embryos (blue arrowheads) and down-regulated in ptc1;ptc2 embryos (F,H blue arrowheads). (I) runx1 was restricted to the DA in wild-type embryos (white arrowhead), down-regulated in the DA of ptc1;ptc2 embryos (J white arrowhead), and ectopically expressed in the PCV of ptc1;ptc2 embryos (J black arrowheads). (K) Increased trunk vegfa expression was present in ptc1;ptc2 embryos (L green arrowhead) compared with wild-type (K green arrowhead).
ptc1;ptc2 mutants exhibit ectopic arterial differentiation at the expense of venous differentiation, resulting in conversion of the PCV into a second artery. Lateral views of the trunk region are shown, oriented anterior to left, posterior right. (A) kdrl exhibited arterial preference in wild-type embryos (red arrowheads) but was present ectopically in the PCV in ptc1;ptc2 embryos (B blue arrowheads). (C) ephrinb2a was restricted to the DA in wild-type embryos (red arrowhead) but present ectopically in the PCV in ptc1;ptc2 embryos (D blue arrowheads). (E) aplnra and (G) flt4 expression was restricted to the PCV in wild-type embryos (blue arrowheads) and down-regulated in ptc1;ptc2 embryos (F,H blue arrowheads). (I) runx1 was restricted to the DA in wild-type embryos (white arrowhead), down-regulated in the DA of ptc1;ptc2 embryos (J white arrowhead), and ectopically expressed in the PCV of ptc1;ptc2 embryos (J black arrowheads). (K) Increased trunk vegfa expression was present in ptc1;ptc2 embryos (L green arrowhead) compared with wild-type (K green arrowhead).
Analysis of runx1+ HSCs in ptc1;ptc2 embryos confirms conversion of the PCV to an arterial vessel and indicates a dual function of Hh in their induction
Expression of the transcription factor runx1 is normally restricted to cells in the ventral DA and also marks emerging definitive hematopoietic progenitors and HSCs.11,42,43 In ptc1:ptc2 mutants, runx1 was expressed ectopically in the PCV, supporting the hypothesis that the PCV had acquired arterial character (Figure 3J black arrowheads). Quantification of the number of runx1+ cells in ptc1;ptc2 mutants revealed an 89% reduction (supplemental Figure 3), and these cells were absent from the DA (Figure 3I,J white arrowheads). Recent studies have revealed a novel signaling pathway in the zebrafish somite,44 whereby somitic wnt16 signaling upstream of deltaC/deltaD was necessary for initiation of runx1 expression within the DA. Because ptc1;ptc2 embryos exhibit somite patterning defects, we analyzed wnt16 expression and found that it was persistently reduced in the somites of ptc1;ptc2 mutant embryos from 10 somites onwards (Figure 4A-D black arrowheads). In line with this, expression of deltaD was lost within the somitic mesoderm from 10 somites (Figure 4G-H black arrowheads), as was deltaC (Figure 4E-F).27 That runx1 is expressed at all is surprising given the early down-regulation of wnt16 and deltaC/deltaD; however, we have previously demonstrated that bmp4 expression is required for initiation of runx1 expression in the DA.30 Furthermore, ptc1;ptc2 mutants exhibit strong up-regulation of bmp4 within the pronephric ducts (data not shown). It is therefore possible that Bmp signaling is able to rescue the loss of somitic wnt16-DeltaC/D by inducing runx1 expression within arterial ECs close to the source of bmp4. Taken together, these data indicate that ectopic Hh signaling affects runx1+ HSCs in 2 opposing ways: first by promoting arterial fate in the ventral vessel allowing ectopic HSCs to form, but at the same time antagonizing somitic wnt16 and deltaC/D, leading to a reduction in overall HSC numbers.
Somitic wnt16-deltaC/deltaD signaling required for HSC specification is abrogated in ptc1;ptc2 mutants. Zebrafish flat mounts are shown with anterior to the left and posterior to the right. (A-B) Somitic wnt16 expression is strongly down-regulated in ptc1;ptc2 embryos at 10 somites (B black arrowheads), and this down-regulation persisted at the 15-second stage (C-D black arrowheads). (E-F) Expression of deltaC was absent from the somitic mesoderm (SM) of ptc1;ptc2 embryos (F black arrowheads) but was present within the presomitic mesoderm (PSM; F gray arrowheads). (G-H) deltaD expression was strongly down-regulated within the SM (H black arrowheads) and PSM (H gray arrowheads) of ptc1;ptc2 embryos.
Somitic wnt16-deltaC/deltaD signaling required for HSC specification is abrogated in ptc1;ptc2 mutants. Zebrafish flat mounts are shown with anterior to the left and posterior to the right. (A-B) Somitic wnt16 expression is strongly down-regulated in ptc1;ptc2 embryos at 10 somites (B black arrowheads), and this down-regulation persisted at the 15-second stage (C-D black arrowheads). (E-F) Expression of deltaC was absent from the somitic mesoderm (SM) of ptc1;ptc2 embryos (F black arrowheads) but was present within the presomitic mesoderm (PSM; F gray arrowheads). (G-H) deltaD expression was strongly down-regulated within the SM (H black arrowheads) and PSM (H gray arrowheads) of ptc1;ptc2 embryos.
The level of Hh and VEGF signaling determines the balance between venous and arterial fates
Because Hh sits atop a signaling hierarchy that activates VEGF,17,19 we analyzed vegfa expression in ptc1;ptc2 mutants and found that it was significantly up-regulated throughout the trunk of mutant embryos (Figure 3K-L green arrowheads), consistent with previous studies.7 We also treated wild-type embryos with the Smoothened agonist Purmorphamine (PMA) from tailbud stage onwards45 because PMA is capable of activating Hh signaling to a greater level than that observed in ptc1 mutants but less than that of ptc1;ptc2 mutants.30 PMA-treated embryos demonstrated increased kdrl and ephrinb2a expression within the DA (supplemental Figure 4A-D red arrowheads). PMA-treated embryos also retained higher kdrl expression within the PCV (supplemental Figure 4A-B blue arrowheads), whereas ephrinb2a was not ectopically induced. In contrast to ptc1;ptc2 mutants, PMA-treated embryos exhibited a moderate increase in vegfa expression (supplemental Figure 4G-H), and expression of the venous marker aplnra was considerably reduced or absent in the PCV of PMA-treated embryos (supplemental Figure 4E-F blue arrowheads). Taken together, these results indicate that the levels of Hh signaling and subsequent vegfa expression are critical for the balance between arterial versus venous differentiation.
Arterial specification occurs independently of VEGF signaling in ptc1;ptc2 mutants
Up-regulated vegfa expression in ptc1;ptc2 mutants (Figure 3K-L green arrowheads) implies that the aberrant PCV specification was the result of an increase in VEGF signaling. To address this, we crossed the plcg1t26480 mutant,40 which blocks VEGF signaling downstream of its receptor20 into the Tg(fli1a:EGFP)y1/+;ptc1;ptc2 background. Whereas the vasculature of Tg(fli1a:EGFP)y1/+ siblings exhibited normal ISV sprouting (Figure 5A), ectopic ISVs were observed in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos (Figure 5B). Furthermore, whereas the axial vessels were juxtaposed in Tg(fli1a:EGFP)y1/+ embryos (Figure 5A), the 2 vessels could be observed discretely in Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos (Figure 5B). In contrast, no ISVs were present in Tg(fli1a:EGFP)y1/+;plcg1 embryos, indicating defective angiogenesis arising from inhibition of VEGF signaling (Figure 5C).20,46,47 Whereas ISVs were absent in Tg(fli1a:EGFP)y1/+;ptc1;ptc2;plcg1 embryos (Figure 5D-E), the developing vasculature consisted of a noncontinuous dorsal vessel, which was not closely juxtaposed to the ventral vessel. Expression of kdrl at 27 hpf was reduced in the PCV (Figure 5F blue arrowhead) of Tg(fli1a:EGFP)y1/+ siblings and retained in the DA (Figure 5F red arrowhead) and ISVs (Figure 5F yellow arrowhead). In contrast, ectopic kdrl expression was retained in both vessels in Tg(fli1a:EGFP)y1/+;ptc1;ptc2;plcg1 mutant embryos (Figure 5G red arrowheads). Consistent with this, arterial ephrinb2a was retained in all Tg(fli1a:EGFP)y1/+;ptc1;ptc2;plcg1 embryos and demonstrated punctate expression throughout the trunk in the position of the dorsal and ventral vessels (Figure 5I red arrowheads). Both kdrl and ephrinb2a are strongly down-regulated in plcg1 mutants.36
Arterial specification occurs independently of VEGF signaling in ptc1;ptc2;plcg1 triple mutants. (A-E) Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos formed 2 separate vessels, both with sprouting capacity (B), whereas Tg(fli1a:EGFP)y1/+;plcg1t26480 embryos formed a single vessel without ISV sprouts and lacking arterial gene expression (data not shown; C). Two separate vessels were present in triple mutant embryos (D, zoomed view of panel E), but no ISVs formed. kdrl (F-G) and ephrinb2a (H-I) expression indicated that the DA remained duplicated in the triple mutants, albeit with fewer cells.
Arterial specification occurs independently of VEGF signaling in ptc1;ptc2;plcg1 triple mutants. (A-E) Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos formed 2 separate vessels, both with sprouting capacity (B), whereas Tg(fli1a:EGFP)y1/+;plcg1t26480 embryos formed a single vessel without ISV sprouts and lacking arterial gene expression (data not shown; C). Two separate vessels were present in triple mutant embryos (D, zoomed view of panel E), but no ISVs formed. kdrl (F-G) and ephrinb2a (H-I) expression indicated that the DA remained duplicated in the triple mutants, albeit with fewer cells.
Although the ptc1;ptc2;plcg1 triple mutant is expected to have no VEGF signaling, bifurcations in the downstream signaling cascade imply that the use of alternative effectors cannot be excluded. To control for this possibility, we used an inhibitor of VEGF signaling, SU5416, which acts at the level of the VEGF receptor.48,49 We treated the progeny of Tg(fli1a:EGFP)y1/+;ptc1/+;ptc2/+ x ptc1/+;ptc2/+ crosses, with high concentrations of SU5416 (2μM) from tailbud stage to ensure maximal inhibition of VEGF signaling (supplemental Figure 5). In treated ptc1;ptc2 mutants, expression of arterial markers, such as dll4 and ephrinb2a, were retained, albeit with reductions in the number of arterial ECs (supplemental Figure 5H,L red arrowheads) relative to DMSO-treated ptc1;ptc2 mutants (supplemental Figure 5F,J red arrowheads). However, in SU5416-treated siblings, all arterial differentiation was absent (supplemental Figure 5E,G,I,K red arrowheads). Moreover, ectopic arterial differentiation was retained in SU5416-treated ptc1;ptc2 mutants (supplemental Figure 5H,L red arrowhead). These results indicate that activation of Hh signaling can induce arterial differentiation independently of VEGF signaling.
This result was surprising given previously demonstrated requirements for VEGF in arterial differentiation. However, it remains formally possible that the levels of SU5416 used, which were determined by its nonspecific toxicity at higher doses, were insufficient to inhibit elevated VEGF signaling in ptc1;ptc2 embryos. To address this issue, we asked whether the same dose of SU5416 was sufficient to inhibit the effects of exogenous vegf121 mRNA. Embryos were injected with 50 pg vegfa mRNA, more than 2 times higher concentration than that sufficient to induce substantial ectopic arterial differentiation.17 As expected, embryos injected with vegf121 mRNA and treated with DMSO exhibited ectopic ephrinb2a expression throughout the trunk (supplemental Figure 6B red arrowhead). By contrast, 63% of injected embryos treated with SU5416 exhibited total loss of ephrinb2a in the trunk (supplemental Figure 6D red arrowhead), whereas the remaining 37% exhibited weak expression and no ectopic ephrinb2a expression was observed (data not shown). These data clearly indicate that the concentration of SU5416 used is sufficient to inhibit very high levels of VEGF signaling.
As a further control, we coinjected ptc1;ptc2 mutant embryos with morpholinos targeting the VEGF receptors kdr and kdrl,22,35 which together are required to transduce the VEGF signal. Combinatorial knockdown abolished arterial ephrinb2a expression in non–double mutant siblings (supplemental Figure 7A,C red arrowhead). By contrast, ptc1;ptc2 mutant embryos retained ectopic ephrinb2a expression (supplemental Figure 7D red arrowhead), although no vessel sprouting was observed and fewer ephrinb2a+ cells were present (supplemental Figure 7B red arrowhead).
Finally, to demonstrate endothelial cell autonomy of Hh signaling, we mosaically activated Hh signaling within ECs by coinjecting a Fli1a:Myc-dnPKA:IRES-EGFP DNA construct and Tol2 mRNA into embryos, which were treated with cyclopamine, to inhibit Hh signaling and downstream VEGF (supplemental Figure 8). Murine dnPKA is a well-established activator of Hh signaling.33 Uninjected embryos treated with cyclopamine displayed a total absence of the early and late arterial markers dll4 (supplemental Figure 8I-J black arrowheads) and ephrinb2a within the vasculature (supplemental Figure 8D,F black arrowheads).11 By contrast, injected embryos treated with cyclopamine exhibited ECs coexpressing Myc-dnPKA and dll4 (supplemental Figure 8G-H arrowheads) and ephrinb2a (supplemental Figure 8K-L arrowheads). This rescue of arterial differentiation indicates that Hh signaling is able to act cell autonomously in ECs. Taken together, our triple-mutant analysis, SU5416 exposure, VEGF receptor knockdown, and demonstration of cell autonomy all show that high levels of Hh signaling are able to induce arterial differentiation in a VEGF-independent manner.
Arterial specification in ptc1;ptc2 mutants is dependent on Notch signaling
To determine whether loss of Notch, which acts downstream of VEGF, could prevent ectopic arterial differentiation in ptc1;ptc2 embryos, we injected a morpholino targeting Su(H),34 a protein essential for Notch signal transduction.50 Knockdown of Su(H) in non–double mutant sibling embryos resulted in blood vessels (Figure 6A-B yellow arrowheads), which failed to express arterial ephrinb2a (Figure 6C-D red arrowheads). Interestingly, knockdown of Su(H) in ptc1;ptc2 double-mutant embryos also resulted in a total loss of vascular ephrinb2a expression (Figure 6E-F red arrowheads). These data indicate that the VEGF-independent signaling in ptc1;ptc2 embryos occurs upstream of Notch.
Arterial specification in ptc1;ptc2 mutants is dependent on Notch signaling. (A) Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos exhibit blood vessel formation (B, yellow arrowhead; n = 15 of 230), even after Su(H) morpholino injection (B yellow arrowhead; n = 5 of 95). (C) Normal DA ephrinb2a expression in uninjected non–double mutant embryos (C red arrowhead; n = 157 of 167). (D) Loss of ephrinb2a in DA of non–double mutant Su(H) morphants (red arrowhead; n = 159 of 180). (E) Increased (red arrowhead), ectopic (green arrowhead) ephrinb2a expression in uninjected ptc1;ptc2 embryos (n = 10 of 167). (F) Loss of ephrinb2a in DA of ptc1;ptc2 Su(H) morphants (n = 16 of 180).
Arterial specification in ptc1;ptc2 mutants is dependent on Notch signaling. (A) Tg(fli1a:EGFP)y1/+;ptc1;ptc2 embryos exhibit blood vessel formation (B, yellow arrowhead; n = 15 of 230), even after Su(H) morpholino injection (B yellow arrowhead; n = 5 of 95). (C) Normal DA ephrinb2a expression in uninjected non–double mutant embryos (C red arrowhead; n = 157 of 167). (D) Loss of ephrinb2a in DA of non–double mutant Su(H) morphants (red arrowhead; n = 159 of 180). (E) Increased (red arrowhead), ectopic (green arrowhead) ephrinb2a expression in uninjected ptc1;ptc2 embryos (n = 10 of 167). (F) Loss of ephrinb2a in DA of ptc1;ptc2 Su(H) morphants (n = 16 of 180).
Knockdown of crlra in combination with VEGF inhibition prevents arterial differentiation in ptc1;ptc2 mutants
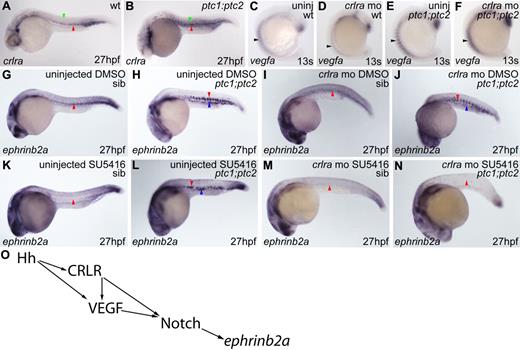
Our finding that arterial differentiation can occur independently of VEGF signaling but is dependent on Notch raises the important question of how Hh is able to bypass VEGF to activate downstream target genes. We evaluated crlra, a downstream target of Hh with a role in arterial induction.19 In wild-type embryos, crlra was diffusely and weakly expressed throughout the somites (Figure 7A green arrowhead) but also weakly expressed in the DA (Figure 7A red arrowhead). Conversely, in ptc1;ptc2 embryos, crlra was considerably up-regulated both in the somites (Figure 7B green arrowhead) and in the DA (Figure 7B red arrowhead). Thus, increased Crlra might account for the retention of arterial differentiation in VEGF inhibited ptc1;ptc2 mutants.
Knockdown of crlra in combination with VEGF inhibition prevents arterial differentiation in ptc1;ptc2 mutants. (A) Wild-type somitic (green arrowhead) and DA crlra expression (red arrowhead). (B) Increased somitic (green arrowhead) and DA (red arrowhead) crlra expression in ptc1;ptc2 embryos. (C) Normal somitic crlra expression in uninjected wild-type non–double mutant embryos (arrowhead; n = 58 of 66). (D) Decreased somitic crlra expression in non–double mutant crlra morphants (n = 58 of 71). (E-F) No difference in crlra expression (arrowheads) was detected between uninjected (n = 5 of 66) and crlra morphant (n = 4 of 71) ptc1;ptc2 embryos. Uninjected wild-type siblings treated with SU5416 from tailbud until collection exhibited total absence of vascular ephrinb2a expression (K red arrowhead; n = 40 of 50) compared with controls (G red arrowhead; n = 46 of 49). SU5416-treated ptc1;ptc2 mutants showed increased ephrinb2a (L red arrowhead) and ectopic expression in PCV region (L blue arrowhead; n = 4 of 50), as did uninjected ptc1;ptc2 mutants (H red and blue arrowheads; n = 3 of 49). Uninjected SU5416-treated ptc1;ptc2 embryos exhibited no vessel sprouting compared with DMSO-treated ptc1;ptc2 embryos. ephrinb2a expression was down-regulated in the DA of non–double mutant crlra morphants treated with DMSO (I red arrowhead; n = 78 of 96), whereas non–double mutant crlra morphants treated with SU5416 exhibited a loss of vascular ephrinb2a expression (M red arrowhead; n = 165 of 183). ptc1;ptc2 crlra morphants treated with DMSO showed strong ephrinb2a expression (J red arrowhead), which was present ectopically in the region of the PCV (J blue arrowhead; n = 8 of 96), whereas vascular ephrinb2a was absent in ptc1;ptc2 crlra morphants treated with SU5416 (N red arrowhead; n = 10 of 186). (O) Proposed model for arterial differentiation.
Knockdown of crlra in combination with VEGF inhibition prevents arterial differentiation in ptc1;ptc2 mutants. (A) Wild-type somitic (green arrowhead) and DA crlra expression (red arrowhead). (B) Increased somitic (green arrowhead) and DA (red arrowhead) crlra expression in ptc1;ptc2 embryos. (C) Normal somitic crlra expression in uninjected wild-type non–double mutant embryos (arrowhead; n = 58 of 66). (D) Decreased somitic crlra expression in non–double mutant crlra morphants (n = 58 of 71). (E-F) No difference in crlra expression (arrowheads) was detected between uninjected (n = 5 of 66) and crlra morphant (n = 4 of 71) ptc1;ptc2 embryos. Uninjected wild-type siblings treated with SU5416 from tailbud until collection exhibited total absence of vascular ephrinb2a expression (K red arrowhead; n = 40 of 50) compared with controls (G red arrowhead; n = 46 of 49). SU5416-treated ptc1;ptc2 mutants showed increased ephrinb2a (L red arrowhead) and ectopic expression in PCV region (L blue arrowhead; n = 4 of 50), as did uninjected ptc1;ptc2 mutants (H red and blue arrowheads; n = 3 of 49). Uninjected SU5416-treated ptc1;ptc2 embryos exhibited no vessel sprouting compared with DMSO-treated ptc1;ptc2 embryos. ephrinb2a expression was down-regulated in the DA of non–double mutant crlra morphants treated with DMSO (I red arrowhead; n = 78 of 96), whereas non–double mutant crlra morphants treated with SU5416 exhibited a loss of vascular ephrinb2a expression (M red arrowhead; n = 165 of 183). ptc1;ptc2 crlra morphants treated with DMSO showed strong ephrinb2a expression (J red arrowhead), which was present ectopically in the region of the PCV (J blue arrowhead; n = 8 of 96), whereas vascular ephrinb2a was absent in ptc1;ptc2 crlra morphants treated with SU5416 (N red arrowhead; n = 10 of 186). (O) Proposed model for arterial differentiation.
Embryos were injected with crlra morpholino19 and treated with SU5416. As previously described, SU5416 treated wild-type embryos revealed a total absence of arterial differentiation within the trunk vasculature (Figure 7G,K red arrowhead). In ptc1;ptc2 mutants, by contrast, treatment with SU5416 was unable to block arterial ephrinb2a expression (Figure 7H,L blue arrowhead). Wild-type embryos injected with crlra morpholino exhibited considerable down-regulation of ephrinb2a (Figure 7G,I red arrowhead), whereas those injected with crlra morpholino and treated with SU5416 displayed a total absence of ephrinb2a throughout the trunk vasculature (Figure 7M red arrowhead). However, knockdown of crlra in ptc1;ptc2 embryos failed to prevent induction of ephrinb2a, which was expressed ectopically (Figure 7J blue arrowhead). In ptc1;ptc2 embryos, only combined knockdown of crlra and VEGF inhibition by SU5416 resulted in a total loss of ephrinb2a within the trunk vasculature (Figure 7N red arrowhead).
A confounding aspect of the latter experiment is that crlra normally activates somitic VEGF expression,19 so morpholino knockdown of crlra would be expected to lead to down-regulation both of Crlra and VEGF. To address whether activation of Hh can bypass this function of Crlra, we assayed vegfa expression in ptc1;ptc2 mutant embryos and found that, in non–double mutant siblings, knockdown of crlra resulted in a decrease in somitic vegfa expression (Figure 7C-D arrowhead) as previously reported.19 However, in ptc1;ptc2 mutants, crlra knockdown produced no observable difference in vegfa expression (Figure 7F arrowhead) relative to uninjected ptc1;ptc2 mutants (Figure 7E arrowhead). We conclude that Hh is indeed able to signal in parallel to Crlr to induce expression of vegfa. Taken together with our observations that Notch signaling is required for arterial differentiation in ptc1;ptc2 mutant embryos, these data indicate that Hh signaling is capable of inducing arterial differentiation via Crlr independently of VEGF signaling, and, conversely, via VEGF independently of Crlr, but that these signals converge again at the level of Notch (Figure 7O).
Discussion
Hh signaling plays a crucial role in inducing arterial fate in the zebrafish embryo. Elegant experiments by Lawson et al revealed a hierarchy of signaling, initiated by Hh and leading, via the induction of VEGF, to activation of hey2/gridlock and Notch signaling to provide the final transcriptional output.17 More recently, an additional layer in this control has been suggested involving the Crlr receptor.19 We have exploited the constitutive activation of the Hh pathway in double-mutant ptc1;ptc2 zebrafish embryos to explore further the relationships between Hh and VEGF in early vessel formation. In keeping with previous findings,17 we showed that activation of Hh leads to ectopic arterial gene expression, ultimately resulting in the absence of a functional circulatory loop. However, this endogenous hyperactivation of Hh signaling results in such strong repression of venous development that a correctly specified PCV fails to develop: rather, a second arterial vessel forms with an intact arterial marker signature, vessel sprouting, and initiation of HSC gene expression.
An earlier role has been postulated for Hh signaling in guiding endothelial migration.11,26 We have demonstrated that the levels of Hh signaling received by angioblasts are critical for their successful migration and that activation of the Hh pathway results in more severe defects in initial angioblast migration toward the midline than loss of Hh signaling. Previous studies have demonstrated that smo embryos exhibit a loss of arterial ECs and concomitant gain in venous ECs, and it was proposed that Hh is required cell autonomously already within the PLM to regulate arteriovenous specificity.26 Importantly, we find that, in smohi1640 mutants, the first wave of angioblast migration, which largely contributes to DA formation, is not lost as has previously been reported.26 The discrepancy between our findings and those of Williams et al26 may be explained by the higher resolution of the confocal imaging of angioblast migration used in our study. However, smo embryos lack arterial ECs, arguing that these 2 migratory waves are not differentially specified before migration. In line with this, the Hh receptors ptc1 and ptc2 are not expressed in the PLM,26,51 implying that cells of the PLM are unlikely to receive the Hh signal directly. From our high-resolution time-lapse movies, we suggest that Hh signaling is most important in the final stages of medial migration because smo−/− angioblasts begin their medial migration normally yet fail to form a continuous endothelial cord as they reach the midline.
Interestingly, the arterial transformation of the ventral vessel leads to the generation of ISVs from this location, in addition to formation of atypical connections between the 2 axial vessels. Such connections have been observed in mutants with defects in arteriovenous specification, and it is probable that separation of the 2 vessels requires them to have different molecular identities.14 Considering the body of data implicating VEGF in arterial induction, we were initially surprised to find that mutation of plcg1, chemical ablation of VEGF signaling, or knockdown of the VEGF receptors failed to block normal and ectopic induction of arterial fates in double mutants. However, results from mice and also differences in phenotypic strength of VEGF and Hh pathway mutants18,25 indicated that VEGF-independent functions of Hh signaling in these processes were likely. It is important to stress, however, that under normal circumstances VEGF is essential for arterial induction.
Although Nicoli et al suggested that Crlr was induced by Hh in the somites and acted via VEGF, they could not exclude that direct effects of Crlr on ECs might occur.19 Indeed, our experiments indicate that such direct effects are probable because down-regulation of VEGF signaling is not sufficient to block arterial differentiation, arguing against a simple Hh-Crlr-VEGF pathway. If somitic VEGF was the sole effector acting on ECs to induce arterial fate, then inhibition of VEGF signaling in ptc1;ptc2 mutants should have resulted in the absence of arterial differentiation. Furthermore, it follows that vegfa overexpression would also be predicted to phenocopy the vascular defects of ptc1;ptc2 mutants, a situation that did not occur (supplemental Figure 6B).17
The expression of crlra both in somites and ECs prompted us to analyze its potential role in bypassing a normal requirement for VEGF in double mutants. Indeed, crlra expression was also induced in double mutants. Importantly, knockdown of crlra did not affect VEGF up-regulation in the somites; thus, it is likely that Crlr acts in the ECs in VEGF-inhibited double mutants. This finding raises several questions: most importantly, how Crlr signaling is regulated. A recent report indicated that in certain cells CRLR might act to “transactivate” the VEGF receptor independently of the VEGF ligand.52 However, these authors also show that the VEGF inhibitor we used (SU5416) should block the action of both CRLR and VEGF receptor activation. Our data show that CRLR functions in the presence of SU5416, and thus this mechanism does not appear to be acting in zebrafish. A second simple assumption would be that signals via VEGF and Crlr could both independently induce arterial fate in ECs. This is not supported by experiments by us (supplemental Figure 9) and others.19 crlr overexpression is not sufficient to induce ectopic arterial fates in wild-types or rescue arterial fates in Hh signaling-deficient embryos. In contrast, VEGF has been shown to be an instructive factor with respect to arterial induction, and injection in wild-type or Hh signaling-deficient embryos can induce ectopic arteries (supplemental Figure 6B).17 One interesting assumption concerning the role of Crlr may be that it acts to enhance or modify Hh signaling in ECs to induce arteries rather than act directly in the induction steps. We have looked at mRNA expression of the classic Hh target ptc1 in crlr MO-injected embryos but did not see changes (R.N.W., unpublished observations, February 2010). Another possibility is that crlr overexpression by itself is not sufficient to increase signaling via this pathway and other crucial components of this pathway also need to be up-regulated, such as RAMPs or adrenomedullin ligands.53 Then we have to assume that Hh activation in ptc1;ptc2 mutants is not just up-regulating crlr but also those other components required for its activation. We are currently evaluating this possibility.
The key outcome of Hh and VEGF signaling appears to be activation of Notch signaling. To further position the Crlr signaling pathway within this hierarchy, we inhibited Notch using a Su(H) morpholino capable of inhibiting the Notch pathway sufficiently to block artery formation.34 We find that knockdown of Su(H) in ptc1;ptc2 mutants can block artery formation, arguing that Crlr acts upstream of Notch. Thus, we suggest the model shown in Figure 7O for the signaling hierarchies involved in artery formation. Our results show that, although under normal circumstances VEGF is an essential signal for correct vessel patterning in the zebrafish embryo, in situations of elevated Hh signaling, Crlr can bypass this requirement. It may be interesting to evaluate whether similar mechanisms are at work in other settings, for example, in tumors where Hh signaling is activated. It has been reported that crlr down-regulation inhibits tumor angiogenesis in a zebrafish xenograft model.19 If this is a more widespread mechanism, inhibition of Crlr in addition to VEGF inhibition could be important to evaluate as a therapeutic strategy, not only for anticancer therapy, but also in the treatment of diseases, such as diabetic retinopathy or macular degeneration. Furthermore, the remarkable conservation of the genetic hierarchies involved in arterial specification between zebrafish and mammals indicates that signaling via Crlr represents a strong candidate pathway for recently identified, but as yet uncharacterized, mechanisms of vessel patterning shown to be Hh-dependent and VEGF-independent.18
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Pouget for reagents, assistance, and critical reading of the manuscript, and W. Clements for sharing unpublished data.
This work was supported by The Wellcome Trust (program grant 082962/Z/07/Z; P.W.I. and F.J.M.v.E.) and the Medical Research Council (R.K.P.). The CDBG is supported by the MRC Center (grant G0700091; P.W.I.).
Wellcome Trust
Authorship
Contribution: R.N.W. designed and performed research, analyzed data, and wrote the paper; M.J.K. designed and performed research and analyzed data; F.J.M.v.E. designed research, analyzed data, and wrote the paper; and P.W.I., R.K.P., and S.S.-M. discussed the data and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fredericus J. M. van Eeden, MRC Centre for Developmental and Biomedical Genetics/Department of Biomedical Sciences, Western Bank, Sheffield, S10 2TN, United Kingdom; e-mail: f.j.vaneeden@sheffield.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal