Abstract
Platelet dense granules are members of a family of tissue-specific, lysosome-related organelles that also includes melanosomes in melanocytes. Contents released from dense granules after platelet activation promote coagulation and hemostasis, and dense granule defects such as those seen in Hermansky-Pudlak syndrome (HPS) cause excessive bleeding, but little is known about how dense granules form in megakaryocytes (MKs). In the present study, we used SLC35D3, mutation of which causes a dense granule defect in mice, to show that early endosomes play a direct role in dense granule biogenesis. We show that SLC35D3 expression is up-regulated during mouse MK differentiation and is enriched in platelets. Using immunofluorescence and immunoelectron microscopy and subcellular fractionation in megakaryocytoid cells, we show that epitope-tagged and endogenous SLC35D3 localize predominantly to early endosomes but not to dense granule precursors. Nevertheless, SLC35D3 is depleted in mouse platelets from 2 of 3 HPS models and, when expressed ectopically in melanocytes, SLC35D3 localizes to melanosomes in a manner requiring a HPS-associated protein complex that functions from early endosomal transport intermediates. We conclude that SLC35D3 is either delivered to nascent dense granules from contiguous early endosomes as MKs mature or functions in dense granule biogenesis directly from early endosomes, suggesting that dense granules originate from early endosomes in MKs.
Introduction
Platelet functions are largely mediated by soluble factors released from membrane-bound storage organelles, including dense granules (DGs), α-granules, and lysosomes.1 DGs store calcium, ATP, ADP, phosphates, and serotonin.2 The high calcium concentration makes them electron dense, and 4-8 DGs per platelet can be identified by whole-mount electron microscopy.3 Contents released from DGs after platelet activation amplify coagulation at sites of vascular injury.2 Defective DG biogenesis causes δ–storage pool deficiency (δ-SPD), characterized by reduced or undetectable dense core structures by whole-mount electron microscopy, depleted DG components, and reduced DG content release after stimulation. This cellular defect causes bleeding diathesis with potential severe pathology or lethality.2,4 Understanding the cellular mechanisms that underlie DG formation in megakaryocytes (MKs) and platelets is crucial to improving δ-SPD diagnostic tools and therapies.
DGs harbor membrane transporters to import their contents from the MK or platelet cytosol, but few such transporters have been characterized.5 The paucity of known integral membrane proteins that localize specifically to DGs has hampered efforts to define DG intermediates as they form from electron lucent precursors during MK differentiation.6,7 Our understanding of DG biogenesis derives largely from analyses of platelets in syndromic forms of δ-SPD, such as Hermansky-Pudlak syndrome (HPS)8,9 and Chediak-Higashi syndrome,10 in which DGs and other tissue-specific lysosome-related organelles (LROs) are dysfunctional. HPS is characterized minimally by δ-SPD and oculocutaneous albinism due to malformation of platelet DGs and pigment cell melanosomes.8,9 Different HPS subtypes result from mutations in any of 9 genes in humans, and mutations in at least 15 genes (including orthologs of those in HPS) cause a similar disorder in mice.8,9 Most of these genes encode subunits of cytoplasmic multimeric protein complexes that are thought to regulate membrane trafficking of resident proteins from itinerant compartments to newly forming LROs.8,11 These include adaptor protein-3 (AP-3), a coat protein that sorts cargoes from early endosomes toward lysosomes or LROs in other cell types,12 and 3 less understood complexes called biogenesis of lysosome-related organelle complex-1 (BLOC-1), BLOC-2, and BLOC-3.13 Like AP-3, BLOC-1 and BLOC-2 regulate cargo transport from early endosomes14-16 ; in melanocytes, BLOC-1 and BLOC-2 function from distinct endosomal domains from AP-3,14,15,17 but BLOC-1 and AP-3 function together in neurons.16 BLOC-3 is not known to function in cargo transport and its molecular function remains unknown.
The pleiotropic defects in HPS patients and mouse models suggest that affected LROs share a common biogenetic origin irrespective of their tissue-specific functions. However, how AP-3 or BLOCs function in DG biogenesis is not known. Whereas cargoes destined for melanosomes derive from early endosomes in melanocytes, DG cargoes were proposed to derive from multivesicular late endosomes in MKs based on the behavior of CD63.18 However, because CD63 is not restricted to DGs in platelets,19-21 the compartments from which DG-specific cargoes are delivered to DGs remain unclear. Defining such compartments requires identifying DG-specific cargoes or other integral membrane proteins that regulate DG biogenesis.
Whereas proteomics approaches to defining DG-specific cargoes have had limited success,5 candidates can be deduced from genetic analyses of nonsyndromic δ-SPD. In the present study, we focused on Slc35d3, a gene that is mutated in the Roswell (Slc35d3ros/ros) mouse δ-SPD model.22,23 SLC35D3 is an orphan member of a family of transporters, most of which reside in the Golgi and import sugar nucleotides from the cytosol to assemble complex oligosaccharides on nascent glycoproteins and glycolipids.24 SLC35D3 is unusual among SLC35 family members in having a long C-terminal cytoplasmic extension with 2 potential YxxØ consensus endocytic sorting signals. We therefore hypothesized that SLC35D3 functions on DGs or intermediates in DG biogenesis. We tested this hypothesis in the present study by localizing SLC35D3 in megakaryocytoid cells. Our results provide evidence that DG-limiting membrane components derive from early endosomes during MK maturation and accumulate in platelets in a manner requiring the HPS-associated complexes BLOC-1 and AP-3.
Methods
Mice
Platelets and MKs were isolated from 12- to 13-week-old male C57BL/6J (wt), B6-Cg-Pldnpa/J (pallid), B6-Cg-AP3b1pe/J (pearl), and B6.C3-Pde6brd1Hps4le/J (light ear) mice (The Jackson Laboratory) bred in the animal facilities at the University of Pennsylvania under guidelines of the University Laboratory Animal Resources. The light ear mice also carry a mutation in phosphodiesterase 6B, which is not expressed in hematopoietic cells. Slc35d3ros/ros and control C3H/HeSnJ mice, obtained from Richard T. Swank's laboratory (Roswell Park Institute, Buffalo, NY), were bred at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (Beijing, China). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania or the Institute of Genetics and Developmental Biology. C3H/HeSnJ and Slc35d3ros/ros mice were genotyped by PCR (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Reagents, cell culture, plasmids, and transgene expression
Chemicals were from Sigma-Aldrich and tissue-culture reagents were from Invitrogen unless otherwise specified. Culture of Plate-E retroviral packaging cells25 and maintenance and differentiation of the Gata-1−/− embryonic stem cell–derived G1ME cells26 were as described previously.25,26 To induce megakaryocytic differentiation, G1ME cells were transduced with a recombinant retroviral vector encoding GATA-1.26 Retroviral vectors encoding native mouse SLC35D3 or N- or C-terminal HA11-epitope–tagged forms of human SLC35D3 were generated in the retroviral vector pBMN-IRES(X/N)-hygro15 (a gift from Andrew Peden, Cambridge Institute for Medical Research, Cambridge, United Kingdom) as described in supplemental Methods. Retroviruses were generated in Plat-E cells by transient transfection using Lipofectamine 2000 (Invitrogen) and harvested from cell supernatants 2 days later. G1ME cells were infected by double spinoculation with GATA-1– and SLC35D3-encoding retroviruses, and then cultured with 1% thrombopoietin (Tpo; R&D Systems)–conditioned medium for 4 days before analysis. At this time, 10%-20% of the cells were large, multinucleated cells that expressed both the SLC35D3 transgene and the megakaryocytoid markers VWF and P-selectin. The immortalized mouse melanocyte cell lines melan-Ink4a27 and melan-mu15 were cultured as described previously,15 transiently transfected using FuGENE-6 (Roche Diagnostics), or transduced with recombinant retroviruses as described above and analyzed 2 days later.
Antibodies
Rabbit polyclonal Ab to mouse SLC35D3 was generated by Genemed Synthesis to a synthetic peptide (CMKKDYLMENEALPSP, the C-terminal 15 residues of SLC35D3 preceded by cysteine) conjugated to keyhole limpet hemocyanin. Where indicated, the Ab was affinity purified using peptide conjugated to sulfo-link beads (Pierce/Thermo Scientific). Rabbit Ab to VWF was from Dako, rabbit anti–syntaxin 13 was a gift from Dr Peden, and goat anti-MRP4 was from Abcam. mAbs used were: TA99 (Mel-5) anti-Tyrp1 from the ATCC; 1D4B anti–LAMP-1 from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); 16B12 anti-HA.11 from Convance Inc; rat anti-HA.11 from Roche Diagnostics; rat anti–mouse transferrin receptor from BD Biosciences; and mouse anti–β-actin from Sigma-Aldrich. Dylight 488– or Dylight 594–conjugated donkey Abs to rat and goat Ig were from Jackson ImmunoResearch Laboratories, and alkaline phosphatase-conjugated goat Ab to rabbit or mouse and bovine Ab to goat Ig were from Invitrogen.
Platelet and primary MK isolation and immunoblotting
Platelets were isolated from blood drawn from anesthetized mice by differential centrifugation as described previously28 (see supplemental Methods for details). Mouse fetal liver cells were isolated from embryonic day 13.5-14.5 embryos and cultured as described previously.28 After 8 days, mature MKs were enriched on a BSA step gradient and collected by low-speed centrifugation.28 Cells were lysed in lysis buffer (2% Triton X-100, 150mM NaCl, 50mM Tris-HCl, 2mM EDTA, pH 8.0) containing a protease inhibitor cocktail (Roche Diagnostics). Lysates were denatured in 2% SDS at 37°C, fractionated by SDS-PAGE on a 10% polyacrylamide gel, and subjected to immunoblotting using enhanced chemifluorescence and a PhosphorImager (GE Healthcare) as described previously.29
Quantitative RT-PCR analysis
RT reactions were performed using Superscript II (Invitrogen) and 3 μg of total cellular RNA that was extracted using TRIzol reagent (Invitrogen). Results were quantified using real-time PCR with TaqMan probes/primers on an Applied Biosystems Prism 7900 system (Life Technologies). Primers (supplemental Table 1) were designed using Primer Express Version 3 software (Applied Biosystems). A standard curve for each primer pair was established by serial dilution of plasmid DNA or PCR products at known concentrations. Relative gene-expression levels were normalized to the GAPDH signal.
Mepacrine uptake and SLO permeabilization
Streptolysin O (SLO; 6 μg/μL) was preactivated for 30 minutes at 37°C in potassium acetate (KOAc) buffer (115mM KOAc, 25mM HEPES, 2.5mM MgCl2, and 10mM dithiothreitol, pH 7.4), and then CaCl2 was added to 1mM. G1ME cells or fetal liver cell (FLC)–derived MKs were incubated with 50μM mepacrine in culture medium for 30 minutes at 37°C in the dark, washed with PBS as described previously,30 resuspended in the activated SLO for 15 minutes at 4°, and washed in KOAc buffer containing 5mM EGTA for 30 minutes at 37°C. Cells were fixed and analyzed by fluorescence microscopy as described in the next section.
Immunofluorescence microscopy
Immunofluorescence microscopy (IFM) was performed essentially as described previously.31 Briefly, G1ME and FLC-derived MKs were prepared with cytospin and fixed with 2% formaldehyde in PBS, labeled with primary and fluorochrome-conjugated secondary Abs in buffer containing 0.2% saponin (for SLO-permeabilized cells, saponin was omitted), and then with Hoechst 33342 (1 μg/mL) to label nuclei. Cells were analyzed on a DM IRBE microscope (Leica Microsystems) equipped with a Retiga-SRV digital camera (QImaging) and OpenLab Version 5.5.2 image acquisition software (Perkin-Elmer). For G1ME cells, only large multinucleated cells with low transgene expression (requiring exposure times > 250 ms at > 85% digital gain) were included in the analysis. Melanocytes were grown on coverslips and then fixed, permeabilized, and analyzed as described above. Images were acquired from sequential z-stacks in 0.2-μm steps, analyzed, processed by volume deconvolution, and visualized with the extended focus mode using Volocity Version 5.3.1 software (Perkin-Elmer), and further processed using Adobe Photoshop CS4 Version 11.02. For additional details, see supplemental Methods.
Electron microscopy
Four days after infection with GATA-1 and HA-SLC35D3 viruses, differentiated G1ME cells were enriched using a BSA step gradient as described previously,28 then fixed with 2% (wt/vol) paraformaldehyde/ 0.2% (wt/vol) glutaraldehyde in PHEM buffer (60mM Pipes, 25mM HEPES, 10mM EGTA, and 2mM MgCl2, pH 6.9) and processed for ultracryomicrotomy as described previously.21,32 Ultrathin cryosections were prepared with an ultracryomicrotome Ultracut FCS (Leica Microsystems), and immunogold labeled with mouse or rat anti-HA.11 Ab (Roche Applied Science), followed by protein A conjugated to 10-nm gold particles (Cell Microscopy Center, University Medical Center Utrecht, Utrecht, Netherlands). Sections were analyzed by electron microscopy under a Philips CM120 (FEI) or a JEOL 1200CX microscope, and digital acquisitions were made with a numeric camera Keen View (Soft Imaging System).
Subcellular fractionation
Subcellular fractionation was performed as described previously33 with minor modifications. Briefly, 4 days after infection with GATA-1 retrovirus, differentiated G1ME cells were washed twice and resuspended in 2 mL of cold RCD buffer (180mM NaCl, 38mM KCl, 1.7mM NaHCO3, 21.2mM sodium citrate, 27.8mM D-glucose, 1.1mM MgCl2, and 1mM theophylline, pH 7.4) containing a protease inhibitor cocktail (Roche Diagnostics). Cells were disrupted by Dounce homogenization and postnuclear homogenates were fractionated over a 3-layer discontinuous Percoll gradient (Life Technologies) by centrifugation at 37 000g for 30 minutes at 4°C. Fractions were collected from the bottom, Percoll was removed by centrifugation at 100 000g for 90 minutes at 4°C, and aliquots of each fraction were analyzed by immunoblotting.
Results
Slc35d3 expression is induced during MK maturation
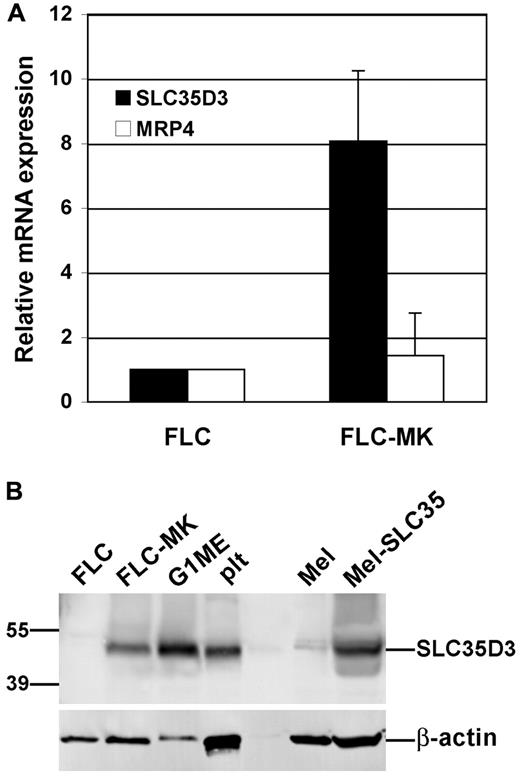
Slc35d3 mRNA is expressed most highly in brain tissue and less in other tissues.23 Because DG precursors form in MKs,34 we investigated whether, like genes for some α-granule cargoes,35 Slc35d3 expression is induced during MK differentiation from hematopoietic progenitors. FLCs isolated from C57BL/6J (wt) mice at prenatal day 13.5-14.5 were either harvested immediately or cultured for 8 days with Tpo to induce MK differentiation and then subjected to BSA gradient centrifugation. As previously shown,28 the resulting cells are enriched in granular, multinucleated mature MKs that express CD41, CD42 and platelet factor-4. By real-time qRT-PCR analysis, whereas MRP4—which localizes at least in part to DGs in platelets36 —was expressed at similar levels in FLCs and mature MKs, Slc35d3 mRNA was increased 8-fold in the mature MKs (Figure 1A). To test for protein expression, we probed immunoblots of FLCs, MKs, and platelet lysates with a polyclonal Ab raised against the C-terminal peptide of mouse SLC35D3 (Figure 1B). A specific band of Mr 48 000 (close to the predicted molecular weight of 45 000) was observed in MKs and platelets, but not in undifferentiated FLCs. The band was also observed in mouse melanocytes expressing exogenous mouse SLC35D3 from a recombinant retrovirus, but not in untransduced melanocytes, consistent with the absence of Slc35d3 mRNA in melanocytes23 and confirming the identity of the band as SLC35D3. Other bands observed in both untransduced and transduced melanocytes represented cross-reactive species and not modified forms of SLC35D3. These data indicate that Slc35d3 expression is induced during MK differentiation.
Slc35d3 expression is up-regulated during megakaryocytoid differentiation. (A) Quantitative real-time RT-PCR analysis of mRNA from undifferentiated FLCs (day 0) and FLC-derived MKs (FLC-MK) isolated 8 days after stimulation in culture with Tpo. mRNA levels for Slc35d3 and Mrp4 are normalized to 1 for FLC and internally controlled by Gapdh levels. (B) Immunoblot analysis of indicated cells or cell lines with affinity-purified anti-SLC35D3 Ab (top) or anti-actin Ab (bottom) as a control. Mel and Mel-SLC35 represent melan-Ink4a melanocytic cells that were untreated or transduced with retrovirus to express mouse SLC35D3 as negative and positive controls, respectively. Plt indicates platelets isolated from C57BL/6J mice. Only the relevant region of each gel is shown. Positions of molecular weight markers are shown on the left, and the specific SLC35D3 and actin bands are indicated by lines on the right.
Slc35d3 expression is up-regulated during megakaryocytoid differentiation. (A) Quantitative real-time RT-PCR analysis of mRNA from undifferentiated FLCs (day 0) and FLC-derived MKs (FLC-MK) isolated 8 days after stimulation in culture with Tpo. mRNA levels for Slc35d3 and Mrp4 are normalized to 1 for FLC and internally controlled by Gapdh levels. (B) Immunoblot analysis of indicated cells or cell lines with affinity-purified anti-SLC35D3 Ab (top) or anti-actin Ab (bottom) as a control. Mel and Mel-SLC35 represent melan-Ink4a melanocytic cells that were untreated or transduced with retrovirus to express mouse SLC35D3 as negative and positive controls, respectively. Plt indicates platelets isolated from C57BL/6J mice. Only the relevant region of each gel is shown. Positions of molecular weight markers are shown on the left, and the specific SLC35D3 and actin bands are indicated by lines on the right.
Epitope-tagged SLC35D3 does not accumulate in mepacrine-positive structures, α-granules, or lysosomes of megakaryocytoid cells
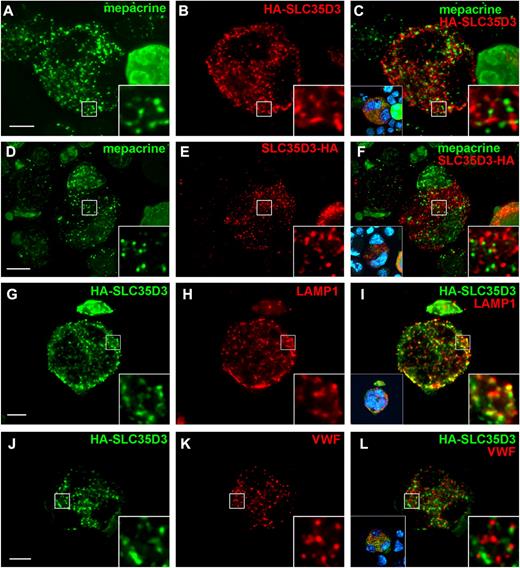
We used the G1ME cell line26 to determine whether SLC35D3 localizes to DGs. G1ME is derived from hematopoietic precursors that lack GATA-1, a transcription factor required for erythroid and megakaryocytic maturation.37 Restoration of Gata-1 expression in G1ME cells by recombinant retroviral transduction and culture with Tpo induces megakaryocytoid differentiation, characterized by enlarged cell volume, multilobular nuclei, expression of MK-specific markers, and the formation of α-granules and a demarcation membrane system.26 Differentiated G1ME cells express SLC35D3 (Figure 1B), but Ab cross-reactivity precluded immunolocalization analyses by microscopy. Therefore, to test for SLC35D3 localization, recombinant epitope-tagged forms of human SLC35D3 were coexpressed with GATA-1 in G1ME cells by concomitant retroviral transduction. Tagged SLC35D3 was then localized relative to endogenous markers by IFM in cells expressing low transgene levels. DG precursors were identified by labeling cells with the fluorescent compound mepacrine.38
Four days after viral infection, mepacrine-positive puncta were observed in enlarged, multinucleated G1ME cells (Figure 2A-F), as would be expected for DG precursors generated during MK differentiation.39 Labeling for SLC35D3 tagged at either the N- or C-terminus with the HA.11 epitope was also punctate and distributed throughout the cells (Figure 2 and Figure 3), unlike the perinuclear labeling observed for Golgi proteins (supplemental Figure 1 and Table 1). Therefore, SLC35D3 is not restricted to the Golgi like other SLC35 family members.24 However, HA-tagged SLC35D3 also did not overlap appreciably with mepacrine-enriched structures (Figure 2A-F, supplemental Figure 3M-P, supplemental Video 1, and Table 1). Moreover, SLC35D3 overlapped to only a small degree with LAMP1 or RAB7, which mark predominantly late endosomes and lysosomes, and not appreciably with VWF or P-selectin, which mark α-granules (Figure 2G-L, supplemental Figure 2D-F, supplemental Figure 3I-L, Table 1, and data not shown). Among multinucleated cells, results were independent of cell size. Therefore, most SLC35D3 in megakaryocytoid cells is not appreciably stored in granules that are destined for secretion in platelets, including mepacrine-positive DG or DG precursors, α-granules, or lysosomes.
SLC35D3 is largely absent from α-granules, dense granule precursors, and lysosomes in G1ME cells. G1ME cells were doubly infected with recombinant retroviruses expressing GATA-1 and SLC35D3 labeled at either the N-terminus (HA-SLC35D3) or C-terminus (SLC35D3-HA) with HA11 epitope and analyzed 4 days later by IFM and volume deconvolution using Volocity. (A-F) Differentiated G1ME cells were incubated with mepacrine (green) for 30 minutes, then the cells were permeabilized with SLO, fixed, and stained with Ab to the HA tag (red). (I-L) Fixed and permeabilized G1ME cells expressing HA-SLC35D3 were labeled with Abs to HA (green) and either LAMP1 (G-I) or VWF (J-L; red). Right insets show 5-fold magnification of boxed regions; left insets in panels C, F, I, and L, nondeconvolved images with nuclear labeling (blue). Scale bar indicates 10 μm.
SLC35D3 is largely absent from α-granules, dense granule precursors, and lysosomes in G1ME cells. G1ME cells were doubly infected with recombinant retroviruses expressing GATA-1 and SLC35D3 labeled at either the N-terminus (HA-SLC35D3) or C-terminus (SLC35D3-HA) with HA11 epitope and analyzed 4 days later by IFM and volume deconvolution using Volocity. (A-F) Differentiated G1ME cells were incubated with mepacrine (green) for 30 minutes, then the cells were permeabilized with SLO, fixed, and stained with Ab to the HA tag (red). (I-L) Fixed and permeabilized G1ME cells expressing HA-SLC35D3 were labeled with Abs to HA (green) and either LAMP1 (G-I) or VWF (J-L; red). Right insets show 5-fold magnification of boxed regions; left insets in panels C, F, I, and L, nondeconvolved images with nuclear labeling (blue). Scale bar indicates 10 μm.
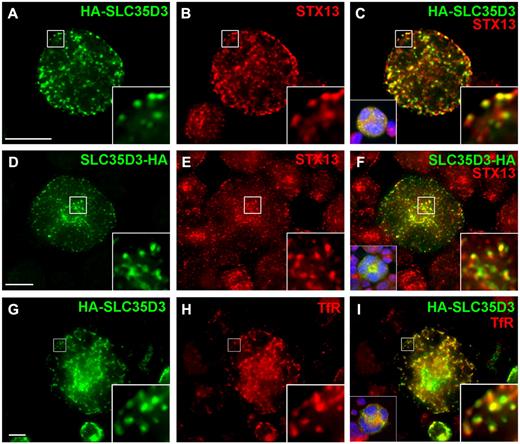
SLC35D3 is enriched in early endosomes in G1ME cells. G1ME cells were doubly infected with recombinant retroviruses expressing GATA-1 and HA-SLC35D3 or SLC35D3-HA and analyzed 4 days later by deconvolution IFM as in Figure 2. Epitope-tagged SLC35D3 (green) was labeled with anti-HA and early endosomes (red) were labeled with Abs to STX13 (A-F) or transferrin receptor (TfR; G-I). Right insets are 5-fold magnification of boxed regions; left insets in panels C, F, and I, nondeconvolved images with nuclear labeling (blue). Scale bar indicates 10 μm.
SLC35D3 is enriched in early endosomes in G1ME cells. G1ME cells were doubly infected with recombinant retroviruses expressing GATA-1 and HA-SLC35D3 or SLC35D3-HA and analyzed 4 days later by deconvolution IFM as in Figure 2. Epitope-tagged SLC35D3 (green) was labeled with anti-HA and early endosomes (red) were labeled with Abs to STX13 (A-F) or transferrin receptor (TfR; G-I). Right insets are 5-fold magnification of boxed regions; left insets in panels C, F, and I, nondeconvolved images with nuclear labeling (blue). Scale bar indicates 10 μm.
Pearson correlation coefficients for colocalization
| Comparison . | Compartment(s) . | Pearson correlation coefficient . | P . | |
|---|---|---|---|---|
| TfR . | LAMP1 . | |||
| HA-SLC35D3 vs STX13 | Early endosomes | 0.740 ± 0.115 | * | *** |
| HA-SLC35D3 vs TfR | Early endosomes | 0.614 ± 0.016 | ||
| HA-SLC35D3 vs RAB11 | Early (recycling) endosomes | 0.530 ± 0.094 | NS | *** |
| HA-SLC35D3 vs RAB5 | Early (sorting) endosomes | 0.496 ± 0.054 | NS | ** |
| HA-SLC35D3 vs LAMP1 | Late endosomes/ lysosomes | 0.341 ± 0.050 | *** | |
| HA-SLC35D3 vs RAB7 | Late endosomes | 0.281 ± 0.057 | *** | NS |
| HA-SLC35D3 vs TG-p230 | trans-Golgi network | 0.120 ± 0.045 | *** | *** |
| HA-SLC35D3 vs VWF | α granules | 0.022 ± 0.025 | *** | *** |
| HA-SLC35D3 vs giantin | Golgi | -0.049 ± 0.051 | *** | *** |
| HA-SLC35D3 vs mepacrine | Dense granule precursors | -0.072 ± 0.052 | *** | *** |
| Comparison . | Compartment(s) . | Pearson correlation coefficient . | P . | |
|---|---|---|---|---|
| TfR . | LAMP1 . | |||
| HA-SLC35D3 vs STX13 | Early endosomes | 0.740 ± 0.115 | * | *** |
| HA-SLC35D3 vs TfR | Early endosomes | 0.614 ± 0.016 | ||
| HA-SLC35D3 vs RAB11 | Early (recycling) endosomes | 0.530 ± 0.094 | NS | *** |
| HA-SLC35D3 vs RAB5 | Early (sorting) endosomes | 0.496 ± 0.054 | NS | ** |
| HA-SLC35D3 vs LAMP1 | Late endosomes/ lysosomes | 0.341 ± 0.050 | *** | |
| HA-SLC35D3 vs RAB7 | Late endosomes | 0.281 ± 0.057 | *** | NS |
| HA-SLC35D3 vs TG-p230 | trans-Golgi network | 0.120 ± 0.045 | *** | *** |
| HA-SLC35D3 vs VWF | α granules | 0.022 ± 0.025 | *** | *** |
| HA-SLC35D3 vs giantin | Golgi | -0.049 ± 0.051 | *** | *** |
| HA-SLC35D3 vs mepacrine | Dense granule precursors | -0.072 ± 0.052 | *** | *** |
Values represent the mean and SD from measurements of at least 5 cells each, except for giantin and TG-p230, which were from 4 cells each. Compartment(s) refers to the primary localization of the marker. Pairwise P values were calculated using a 1-way ANOVA and the Tukey multiple comparison test for all data and are represented relative to HA-SLC35D3 versus TfR or to HA-SLC35D3 versus LAMP1.
P < .05;
P < .01;
P < .005.
NS indicates not significant.
SLC35D3 localizes at steady state to early endosomes in megakaryocytoid cells and primary MKs
Whereas HA-tagged SLC35D3 did not colocalize with granule markers in differentiated G1ME cells, IFM showed that it overlapped substantially with syntaxin 13 (STX13; Figure 3A-F, supplemental Figure 3A-H, supplemental Video 2, and Table 1), an early endosomal tSNARE.15,40 Consistently, epitope-tagged SLC35D3 also overlapped substantially with the transferrin receptor (TfR; Figure 3G-I and Table 1), an integral membrane protein that cycles between early endosomes and the plasma membrane,41 and partially with both RAB5 and RAB11 (supplemental Figure 2A-C,G-I and Table 1), which mark sorting and recycling domains of early endosomes, respectively. Similarly, HA-SLC35D3 in retrovirally transduced, FL-derived MKs overlapped substantially with STX13 but poorly with mepacrine (supplemental Figure 4). The partial overlap between HA-SLC35D3 and STX13 reflects their presence in distinct domains of contiguous structures, as shown by XYZ plane analysis after 3-dimensional reconstruction of deconvolved IFM images (supplemental Figure 3A-H and supplemental Video 2). These data indicate that, despite its requirement for DG biogenesis and function, SLC35D3 localizes in megakaryocytoid cells and primary MKs predominantly to early endosomes and not to mepacrine-labeled DGs or DG precursors.
To further characterize SLC35D3-containing compartments, differentiated G1ME cells expressing HA-SLC35D3 were analyzed by immunoelectron microscopy (IEM). Ultrathin cryosections of fixed cells were immunogold labeled with anti-HA Ab and gold-conjugated protein A. Labeling was detected predominantly in tubulovesicular structures with morphologic characteristics of early endosomes (Figure 4 and supplemental Figure 5A). These structures were frequently situated in close proximity to the plasma membrane (Figure 4A) and occasionally near the Golgi (Figure 4C), but were most often closely opposed to late endosomal multivesicular bodies (MVBs) and/or vacuolar structures with few intraluminal membranes (Figure 4B,D and supplemental Figure 5A). This pattern is indicative of early endosomal domains. Occasional labeling was observed within the MVBs themselves—consistent with the modest overlap with LAMP1 and RAB7 by IFM (Table 1)—and also within small, round vacuolar structures approximately 150-200 nm in diameter (supplemental Figure 5A inset). These latter structures represent either tangential sections of early endosomal vacuolar domains40 or DG precursors, which appear as empty vacuoles in cryosections because of extraction of their contents.3,21 Labeling was not observed in the majority of MVBs, in lysosomes, or in the bean-shaped structures characteristic of α-granules (supplemental Figure 5B). These data confirm that SLC35D3 is most enriched in early endosomes and suggest that a small cohort might be present in DG precursors and late endosomes, but not in α-granules or lysosomes.
IEM analysis defines the SLC35D3-containing structures in G1ME cells as tubulovesicular endosomes. Large, multinucleated G1ME cells that had been doubly infected with GATA-1– and HA-SLC35D3–encoding retroviruses were enriched by fractionation on a BSA density gradient, then fixed and processed for IEM. Ultrathin cryosections were immunogold labeled with anti-HA Abs and 10-nm protein A gold. (A) Label is shown in vacuolar (E) and tubulovesicular structures near the plasma membrane (PM). (B-D) Immunogold-labeled tubulovesicular structures were frequently observed in close apposition to characteristic early endosomal vacuoles (E) with few luminal vesicles (B), near the Golgi (G) complex (C), and near MVBs (D). MVBs themselves were only occasionally labeled. Scale bars indicate 200 nm.
IEM analysis defines the SLC35D3-containing structures in G1ME cells as tubulovesicular endosomes. Large, multinucleated G1ME cells that had been doubly infected with GATA-1– and HA-SLC35D3–encoding retroviruses were enriched by fractionation on a BSA density gradient, then fixed and processed for IEM. Ultrathin cryosections were immunogold labeled with anti-HA Abs and 10-nm protein A gold. (A) Label is shown in vacuolar (E) and tubulovesicular structures near the plasma membrane (PM). (B-D) Immunogold-labeled tubulovesicular structures were frequently observed in close apposition to characteristic early endosomal vacuoles (E) with few luminal vesicles (B), near the Golgi (G) complex (C), and near MVBs (D). MVBs themselves were only occasionally labeled. Scale bars indicate 200 nm.
Endogenous SLC35D3 cofractionates with STX13 by subcellular fractionation of megakaryocytoid cells
To confirm SLC35D3 localization using a different approach, we assessed the distribution of endogenous SLC35D3 after subcellular fractionation of differentiated G1ME cells. Postnuclear homogenates from Gata-1–transduced, differentiated G1ME cells were fractionated by centrifugation over a Percoll density gradient designed to segregate DGs from other organelles.33 Fractions were analyzed by SDS-PAGE and immunoblotting for SLC35D3, STX13, VWF, and the DG marker MRP436 (Figure 5). Endogenous SLC35D3 distributed over a wide range of fractions with a peak at fractions 13-14. Most of these fractions were also enriched in STX13, which also peaked in fractions 13-14. Although α-granules were not well resolved from early endosomes in this gradient, the peak activity of VWF was shifted relative to STX13 and SLC35D3. Interestingly, a small percentage of SLC35D3 was detected in dense fractions 7-9, from which STX13 was absent. These fractions overlapped with the peak detection of MRP4. These data support the conclusion that SLC35D3 localizes predominantly to early endosomes, and suggest that a small fraction might be present in DGs or DG precursors.
Endogenous SLC35D3 and STX13 cofractionate in subcellular fractions of G1ME cells.Gata-1–transduced G1ME cells were lysed by Dounce homogenization, and membranes were fractionated by Percoll gradient centrifugation. Individual fractions were analyzed by Western blotting with Abs to endogenous SLC35D3 or to MRP4 (a marker of DGs), STX13 (a marker of early endosomes [EE]) or vWF (a marker of α granules [αGs]. Note that the peak of reactivity for SLC35D3 overlaps best with STX13, supporting early endosome localization, and a small trail cofractionates with MRP-4.
Endogenous SLC35D3 and STX13 cofractionate in subcellular fractions of G1ME cells.Gata-1–transduced G1ME cells were lysed by Dounce homogenization, and membranes were fractionated by Percoll gradient centrifugation. Individual fractions were analyzed by Western blotting with Abs to endogenous SLC35D3 or to MRP4 (a marker of DGs), STX13 (a marker of early endosomes [EE]) or vWF (a marker of α granules [αGs]. Note that the peak of reactivity for SLC35D3 overlaps best with STX13, supporting early endosome localization, and a small trail cofractionates with MRP-4.
SLC35D3 accumulation in platelets is ablated by the mutation in Roswell mice and requires the HPS-associated complexes BLOC-1 and AP-3 but not BLOC-3
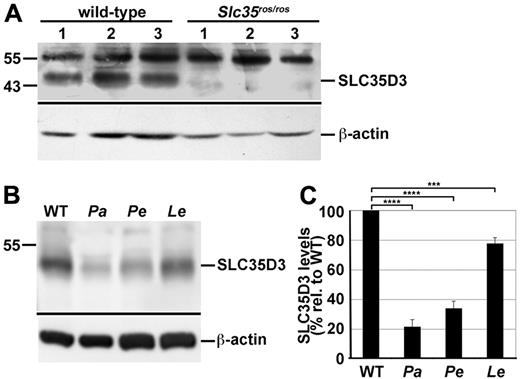
The enrichment of SLC35D3 in MK early endosomes and not in mepacrine-labeled DGs was surprising, given the bleeding diathesis and δ-SPD in Slc35d3ros/ros mice.22,23 In these mice, an IAP insertion in the Slc35d3 gene causes unusually high expression of an aberrant mRNA and is predicted to generate an in-frame substitution of 7 amino acids for the 10 N-terminal residues of wild-type SLC35D3.23 The consequences of this substitution and the increased mRNA expression on SLC35D3 stability and expression are not known. IFM analyses of transfected G1ME cells revealed no major effect of the amino acid substitution on the expression or localization of epitope-tagged SLC35D3 (data not shown). To assay for endogenous mutant protein expression in platelets, we probed immunoblots of platelet lysates from wild-type and Slc35d3ros/ros mice (Figure 6A). Whereas the Mr 48 000 SLC35D3-specific band was detected in each of 3 platelet preparations from wild-type mice, it was absent from corresponding Slc35d3ros/ros platelets. This indicates that the ros/ros mutation either destabilizes SLC35D3 in platelets or alters its intracellular itinerary during MK differentiation into proplatelets, and supports the earlier conclusion23 that a loss of SLC35D3 function underlies the malformation of DGs in Roswell platelets.22
SLC35D3 is depleted in platelets from ash-Roswell mice and mouse models of HPS2 and HPS9, but not of HPS4. Lysates of platelets isolated from wild-type and disease model platelets were fractionated by SDS-PAGE and analyzed by immunoblotting with Abs to SLC35D3 or to actin as a control. Relevant regions of the immunoblot are shown with positions of molecular weight markers (left) and specific SLC35D3 and actin bands indicated by lines (right). (A) SLC35D3 (detected with unfractionated antiserum) is absent from Slc35d3ros/ros platelets. Lysates from platelets isolated from 3 control C3H/HeSnJ (wild-type) and 3 Slc35d3ros/ros mice were analyzed. Note that background bands (Mr approximately 55 000) were present in all lysates. (B) SLC35D3 (detected with affinity-purified Ab) is reduced in platelets of HPS2 and HPS9 mouse models. Lysates of platelets from wild-type C57BL/6J and congenic pallid (Pa; BLOC-1–deficient HPS9 model), pearl (Pe; AP-3–deficient HPS2 model), and light ear (Le; BLOC-3–deficient HPS4 model) mice were analyzed. Note the marked depletion of SLC35D3 from both pallid and pearl, but not light ear platelets. (C) SLC35D3 band intensities were measured from at least 3 replicates of blots as in panel B, normalized to actin levels for each lysate, and then expressed as the percentage (mean ± SD) of the mean normalized value for wild-type lysates. The normalized values for HPS models were evaluated relative to normalized wild-type by unpaired 2-tailed Student t test. ****P < .0005; ***P < .001.
SLC35D3 is depleted in platelets from ash-Roswell mice and mouse models of HPS2 and HPS9, but not of HPS4. Lysates of platelets isolated from wild-type and disease model platelets were fractionated by SDS-PAGE and analyzed by immunoblotting with Abs to SLC35D3 or to actin as a control. Relevant regions of the immunoblot are shown with positions of molecular weight markers (left) and specific SLC35D3 and actin bands indicated by lines (right). (A) SLC35D3 (detected with unfractionated antiserum) is absent from Slc35d3ros/ros platelets. Lysates from platelets isolated from 3 control C3H/HeSnJ (wild-type) and 3 Slc35d3ros/ros mice were analyzed. Note that background bands (Mr approximately 55 000) were present in all lysates. (B) SLC35D3 (detected with affinity-purified Ab) is reduced in platelets of HPS2 and HPS9 mouse models. Lysates of platelets from wild-type C57BL/6J and congenic pallid (Pa; BLOC-1–deficient HPS9 model), pearl (Pe; AP-3–deficient HPS2 model), and light ear (Le; BLOC-3–deficient HPS4 model) mice were analyzed. Note the marked depletion of SLC35D3 from both pallid and pearl, but not light ear platelets. (C) SLC35D3 band intensities were measured from at least 3 replicates of blots as in panel B, normalized to actin levels for each lysate, and then expressed as the percentage (mean ± SD) of the mean normalized value for wild-type lysates. The normalized values for HPS models were evaluated relative to normalized wild-type by unpaired 2-tailed Student t test. ****P < .0005; ***P < .001.
The δ-SPD in Slc35d3ros/ros mice resembles that in mouse models of HPS.22,23 Both BLOC-1 and AP-3, subunits of which are deficient in several human HPS subtypes and mouse HPS models, function on early endosomes,11,12,42 where SLC35D3 accumulates in MKs. In melanocytes, AP-3 and BLOC-1 regulate cargo transport from endosomes to melanosomes.14,15,17 If BLOC-1 and/or AP-3 function similarly at late stages of MK differentiation to target SLC35D3 from early endosomes to DGs, then BLOC-1- and/or AP-3–deficient platelets might be depleted of SLC35D3. Consistently, whole-cell lysates of platelets from BLOC-1–deficient pallid and AP-3–deficient pearl mice were depleted of SLC35D3 relative to wild-type platelets, as shown by immunoblotting relative to an actin control (Figure 6B-C). In contrast to BLOC-1 and AP-3, BLOC-3 (subunits of which are defective in HPS types 1 and 4) does not function in the same cargo-transport pathways. Consistently, lysates of BLOC-3–deficient light ear mice were only modestly depleted of SLC35D3 (Figure 6B-C). These data indicate that SLC35D3 partitioning in platelets requires both BLOC-1 and AP-3, which are likely to regulate SLC35D3 trafficking from early endosomes, whereas BLOC-3 plays at most an ancillary role.
Exogenously expressed SLC35D3 localizes to a LRO in melanocytes
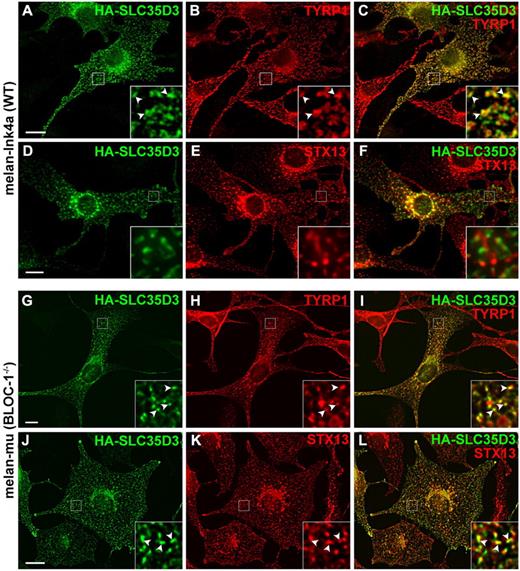
If SLC35D3 functions within DGs in platelets, then it might be expected to accumulate in LROs that derive from similar origins in other cell types, such as pigment cell melanosomes. To test this possibility, HA-SLC35D3 was transiently expressed in the pigmented mouse melanocyte cell line, melan-Ink4a,27 and its localization was analyzed by IFM. In cells expressing low levels, HA-SLC35D3 was largely detected in a reticular network characteristic of the endoplasmic reticulum (Figure 7J-L bottom left). However, when expressed at higher levels, HA-SLC35D3 was detected predominantly in pigmented structures that also labeled for the melanosomal marker TYRP1 (Figure 7A-C), indicating that HA-SLC35D3 was recognized as a melanosomal cargo. HA-SLC35D3 was not detected in STX13-containing early endosomes in melan-Ink4a cells (Figure 7D-F). To determine whether SLC35D3 melanosomal localization requires HPS-associated complexes, HA-SLC35D3 was expressed in melan-mu melanocytes derived from BLOC-1–deficient muted mice, in which TYRP1 and other melanosome cargoes are mislocalized to early endosomes.15 HA-SLC35D3 in these cells overlapped substantially with both the mislocalized TYRP1 and STX13 (Figure 7G-L), indicating that HA-SLC35D3 was also mislocalized to early endosomes. These data indicate that SLC35D3 has intrinsic LRO-sorting signals that are recognized in a different cell type, and that LRO localization requires BLOC-1.
SLC35D3 accumulates in melanosomes upon ectopic expression in wild-type but not muted (BLOC-1–deficient) melanocytes. Immortalized wild-type melan-Ink4a melanocytes (A-F) or BLOC-1–deficient melan-mu melanocytes (derived from muted mice; G-L) were infected with HA-SLC35D3 retrovirus, and then analyzed by IFM with Abs to the HA epitope (green; panels A, D, G, and J) and either the melanosome-resident protein TYRP1 (red; panels B and H) or the early endosomal SNARE protein STX13 (red; panels E and K) and appropriate secondary Abs. Images were deconvolved using OpenLab (A-C) or Volocity (D-L). Overlays of the 2 labels are shown in panels C, F, I, and L, and the boxed regions are magnified 5-fold (A-F) or 4-fold (G-L) in the insets. Note the extensive overlap of HA-SLC35D3 with TYRP1 on pigment granules (A-C) but not with STX13 (D-F) in wild-type melanocytes. In BLOC-1–deficient melan-mu cells, SLC35D3 also overlaps extensively with the mislocalized TYRP1 (G-I) on early endosomes marked by STX13 (J-L). Arrowheads in insets show examples of overlap. Scale bar indicates 10 μm.
SLC35D3 accumulates in melanosomes upon ectopic expression in wild-type but not muted (BLOC-1–deficient) melanocytes. Immortalized wild-type melan-Ink4a melanocytes (A-F) or BLOC-1–deficient melan-mu melanocytes (derived from muted mice; G-L) were infected with HA-SLC35D3 retrovirus, and then analyzed by IFM with Abs to the HA epitope (green; panels A, D, G, and J) and either the melanosome-resident protein TYRP1 (red; panels B and H) or the early endosomal SNARE protein STX13 (red; panels E and K) and appropriate secondary Abs. Images were deconvolved using OpenLab (A-C) or Volocity (D-L). Overlays of the 2 labels are shown in panels C, F, I, and L, and the boxed regions are magnified 5-fold (A-F) or 4-fold (G-L) in the insets. Note the extensive overlap of HA-SLC35D3 with TYRP1 on pigment granules (A-C) but not with STX13 (D-F) in wild-type melanocytes. In BLOC-1–deficient melan-mu cells, SLC35D3 also overlaps extensively with the mislocalized TYRP1 (G-I) on early endosomes marked by STX13 (J-L). Arrowheads in insets show examples of overlap. Scale bar indicates 10 μm.
Discussion
Platelet DGs play critical roles in coagulation and wound repair,2 and their malformation in δ-SPD results in substantial pathology. An improved understanding of DG formation may lead to novel therapies or diagnostic tools for δ-SPD. The results of the present study provide strong evidence that early endosomal domains in MKs serve as transport intermediates for DG cargoes. We have shown that SLC35D3 is up-regulated during MK differentiation and accumulates within platelets, but its absence in platelets from the δ-SPD model Slc35d3ros/ros mouse supports previous reports that SLC35D3 is required for DG biogenesis. Within MKs, SLC35D3 localizes largely to early endosomes and minimally to late endosomes, but not to lysosomes, α-granules, or mepacrine-accumulating DGs or DG precursors. SLC35D3 accumulation within platelets requires the HPS-associated protein complexes BLOC-1 and AP-3, which function on early endosomes in other cell types,12,14-16 but not BLOC-3, which functions in a distinct pathway.13 Finally, when expressed ectopically in melanocytes, a cell type that continuously generates LROs, SLC35D3 accumulates on LROs in a BLOC-1–dependent manner. These data suggest that SLC35D3 regulates DG biogenesis from early endosomes in MKs, implying that at least some DG cargoes derive directly from early endosomes.
A Slc35d3 loss-of function mutation in Slc35d3ros/ros mice causes δ-SPD,22,23 but how and where SLC35D3 functions was not known. In the present study, we show by multiple techniques, including IFM, IEM, and subcellular fractionation, and using 2 different sources of megakaryocytoid cells, the G1ME cell line and primary FLC-derived MKs, that SLC35D3 is most enriched in MK early endosomes. SLC35D3 is likely a stable resident of early endosomes in MKs and not an itinerant protein en route to lysosomes, because SLC35D3 levels in FLC-derived MKs were not altered by prolonged treatment with cycloheximide (unpublished data) and thus were not subject to rapid lysosomal degradation. This makes SLC35D3 unique among SLC35 family members, most of which reside in the Golgi and function to import sugar nucleotides for glycolipid and glycoprotein modification,24 and suggests that SLC35D3 does not have a similar function. Rather, SLC35D3 likely imports a substrate that either itself is a lumenal DG cargo or facilitates the import or retention of other DG cargos. It remains unclear whether SLC35D3 resides on DGs in platelets or regulates DG formation from endosomes. Because our Ab cross-reacts with other abundant proteins, we could not reliably define SLC35D3 localization in platelets. However, the data are consistent with SLC35D3 localization to DGs in platelets. First, SLC35D3 is absent from DG-deficient Slc35d3ros/ros platelets (Figure 6A), implying that SLC35D3 is required for DG function. Second, SLC35D3 is depleted from HPS model pearl and pallid platelets (Figure 6B-C), correlating SLC35D3 down-regulation with their DG defects and showing that AP-3 and BLOC-1 are required to properly compartmentalize SLC35D3 in platelets. Moreover, SLC35D3 depletion in HPS models correlates with the severity of the dense granule defect, because SLC35D3 was only slightly depleted in BLOC-3–deficient HPS4 model light ear platelets (Figure 6B-C), which have a more modest δ-SPD and shorter bleeding times than pearl or pallid platelets.30,43 Third, a small cohort of SLC35D3 in G1ME cells was detected in dense subcellular fractions that also contained the DG-resident MRP4 (Figure 5) and by IEM in vacuolar structures that resemble DG precursors (supplemental Figure 5A). Finally, when expressed ectopically in melanocytes, SLC35D3 localized to LROs (melanosomes) in a manner requiring BLOC-1 (Figure 7). A definitive test for DG localization requires generation of a monospecific Ab and/or analysis of platelets derived from MKs expressing epitope-tagged SLC35D3. Regardless, our data imply that early endosomal domains contact DGs as they mature, such that either SLC35D3 itself or its transport substrate influences content packaging into DGs.
Although DG precursors in MKs were identified long ago,6,44 difficulties in their visualization7,45 and a lack of DG-restricted protein markers have hampered their characterization. CD63 was proposed as a DG-restricted cargo in human platelets because it rapidly translocates to the plasma membrane on platelet activation, cofractionates with DGs, and is depleted in platelets from HPS1 patients.20,46 Based on these data and the accumulation of CD63 in MVBs of MKs, late endosomal MVBs were proposed as DG precursors.18 These data and ours can be reconciled in 1 of 2 different ways (supplemental Figure 6). First, MVBs might not be DG precursors, because CD63 is not restricted to DGs; CD63 is also present in lysosomes and to some extent in α-granules in platelets19,21,46 (and is predominantly localized to late endosomes and lysosomes in most cell types47,48 ). Therefore, the reduced CD63 levels in HPS1 platelets could potentially reflect missorting of lysosomal rather than DG proteins, given that secretion of contents from both DGs and lysosomes is reduced in HPS model platelets.43 Moreover, CD63 is expressed at normal levels in platelets from patients with δ-SPD forms other than HPS1.49 In one model from our data, DG precursors represent direct extensions of the early endosomal membrane system that mature into DGs at late stages of MK differentiation (supplemental Figure 6A and see the last paragraph). Unfortunately, we could not detect mouse CD63 in platelets or mature MKs with available reagents to test this model in our system.
In a second model, mature DGs arise from the convergence of multiple membrane sources, one derived from CD63-containing MVBs and others from SLC35D3-containing early endosomes (supplemental Figure 6B). Melanosome biogenesis provides a precedent for this model. Nonpigmented early-stage melanosomes derive from multivesicular endosomes and mature to pigmented, late-stage melanosomes through subsequent BLOC-1– and/or AP-3–dependent cargo delivery from early endosomes.42 By analogy, a CD63-containing precursor in MKs, perhaps corresponding to mepacrine-labeled structures6,7 that are malformed in BLOC-3–deficient HPS1 patients,20,46 may mature into a functional DG by the BLOC-1– and/or AP-3–dependent delivery of cargoes such as SLC35D3 during a late stage of MK differentiation (supplemental Figure 6B and see next paragraph). This model is consistent with the accumulation of SLC35D3 in early endosomes in MKs and with the requirement for BLOC-1 and AP-3 but not BLOC-3 for SLC35D3 accumulation in platelets, and would explain why platelets from HPS model mice label with mepacrine30 but lack normal DG contents43 ; they would contain the precursors, but not selected cargoes required for DG maturation.
MKs in early stages of differentiation generate DG precursors that accumulate mepacrine and nucleotides,6,7 and the numbers of DG precursors increase as MKs mature and enlarge,21,50 but the stage of MK differentiation at which DGs mature to completion is not known. The megakaryocytoid cells analyzed herein likely represent intermediate MK-differentiation states, because GATA-1–expressing G1ME cells do not form proplatelets and the FLC-derived MKs were analyzed before proplatelet formation. The enrichment of SLC35D3 within early endosomes in these cells supports a model in which DGs mature very late during MK differentiation by drawing cargoes such as SLC35D3 from early endosomes toward DG precursors (supplemental Figure 6). This model would explain why SLC35D3 localizes to melanosomes when expressed in cultured melanocytes, which are fully differentiated and continuously generate new melanosomes.42 Clarification of this model and of the 2 models for dense granule maturation will require the identification of additional DG cargoes and analyses of their localization and dynamics during MK differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cor Seinen for technical assistance; X. Long Zheng and Andrew Peden for gifts of reagents; and Joseph Italiano, Charles Abrams, Lawrence Brass, Joanna Opalinska, and Kenneth Skinner for helpful discussions.
This work was funded by the National Institutes of Health (grants R01 EY015625 from the National Eye Institute to M.S.M., grant P30 DK090969 from the National Institute of Digestive and Kidney Disorders to M.J.W., grant R21 HL096865 to M.S.M., grant P01 HL040387 to M.P., grant U01 HL099656 to M.P. and M.J.W., and National Heart, Lung, and Blood Institute training grant 2T32 HL007971 to A.S.); the National Natural Science Foundation of China (grant 30730049 to W.L.); the Leukemia & Lymphoma Society (to M.J.W.); University Medical Center Utrecht (to H.F.G.H.); Centre National de la Recherche Scientifique and Institut Curie (to GR); and the American Heart Association (postdoctoral fellowship 10POST3870044 to R.M.).
National Institutes of Health
Authorship
Contribution: R.M. participated in all aspects of the project, including experimental design, performing most of the experiments, analyzing and formatting the data, preparing the figures, and writing and editing the manuscript; Y.W., Y.Y., Z.Z., D.C.H., H.F.G.H., and A.S. performed the experiments; D.C.H. contributed to experimental design; H.F.G.H., W.L., G.R., M.J.W., and M.P. conceived parts of the project and contributed to experimental design, oversaw the experimental work, contributed to data analysis, and edited the manuscript; and M.S.M. conceived and oversaw the entire project, contributed to the experimental design, oversaw the experimental work, performed the data analysis, prepared the figures, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael S. Marks, Professor, Departments of Pathology & Laboratory Medicine and Physiology, University of Pennsylvania, Perelman School of Medicine, 513 Stellar-Chance Laboratories, 422 Curie Blvd/6100, Philadelphia, PA 19104-6100; e-mail: marksm@mail.med.upenn.edu.


![Figure 5. Endogenous SLC35D3 and STX13 cofractionate in subcellular fractions of G1ME cells. Gata-1–transduced G1ME cells were lysed by Dounce homogenization, and membranes were fractionated by Percoll gradient centrifugation. Individual fractions were analyzed by Western blotting with Abs to endogenous SLC35D3 or to MRP4 (a marker of DGs), STX13 (a marker of early endosomes [EE]) or vWF (a marker of α granules [αGs]. Note that the peak of reactivity for SLC35D3 overlaps best with STX13, supporting early endosome localization, and a small trail cofractionates with MRP-4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/2/10.1182_blood-2011-11-389551/4/m_zh89991293390005.jpeg?Expires=1767801916&Signature=NpsbT9~1FL7vQlmlak~MJeAN7LqtSWKcvVzUoCrb6ZUlojOJEoW-SLbB9JSe0EN2qtRziMPuWzle5GTV63c9T1Jq0Qy4MZRqgJaKPqrdHv1moJ8jVl2-CGEteTRQhApGXUqVYKBT0sp~A0JYFAL1-NHMEQfcNDMMnEjdgT8~C5VU6nNMBa~SBnRJxYlqoVerfTfidhwtpoG5DhjMryu1UTerwyFM75QqdM6e9t8K~~yE-EtpoCOugjQtWt9yXrTtDGw90mBJYgTOtpSlLv5Zg~5BS2WRz2qTp7397F2qaUmtFeGCqDOd2IKPmDFcGF7YhfNFR6XwIcFYxI4yWvsHLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal