Abstract
Human plasmacytoid dendritic cells (pDCs) represent a highly specialized naturally occurring dendritic-cell subset and are the main producers of type I interferons (IFNs) in response to viral infections. We show that human pDCs activated by the preventive vaccine FSME specifically up-regulate CD56 on their surface, a marker that was thought to be specific for NK cells and associated with cytolytic effector functions. We observed that FSME-activated pDCs specifically lysed NK target cells and expressed cytotoxic molecules, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and granzyme B. Elevated levels of these molecules coincided with the expression of CD56, indicative for skewing human pDCs toward an interferon-producing killer DC subset. Detailed phenotypical and functional analysis revealed that pDCs attained a mature phenotype, secreted proinflammatory cytokines, and had the capacity to present antigens and stimulate T cells. Here, we report on the generation of CD56+ human interferon producing killer pDCs with the capacity to present antigens. These findings aid in deciphering the role for pDCs in antitumor immunity and present a promising prospect of developing antitumor therapy using pDCs.
Introduction
Plasmacytoid dendritic cells (pDCs) are 1 of 2 major subsets of human DCs that circulate in the peripheral blood, and are characterized as BDCA-2+BDCA-4+CD4+CD45RA+IL-3Rα+ILT3+ILT1− CD11c− lineage− cells.1,2 On viral stimulation and subsequent TLR-mediated signaling, human pDCs produce large amounts of type I IFNs (IFN-α/β) that stimulate T-cell function of the Th1 type, stimulate NK cell cytolytic activity and promote differentiation and maturation of myeloid DCs.3-5 In addition to scavenging pathogens and presenting antigens, pDCs secrete a large array of cytokines, which are all vital for proper functioning of both the acquired and the innate immune system. pDC-derived type I IFNs are important for antiviral immunity, but also play a role in bacterial infections, allergy, and anticancer immunity.6-8 In a resting state, pDCs might induce unbiased Th, Th2, or regulatory responses, whereas their activated equivalents have stimulatory capacities and trigger Th1 responses.9,10 Salio et al demonstrated in vitro priming of naive CD8+ T cells into melanoma specific CD8+ CTLs by CD40L activated pDCs pulsed with short melanoma peptide.11 In addition, Vicari et al demonstrated murine antitumor CTL responses and tumor rejection in vivo after activating DCs with anti-interleukin (IL)–10 mAb and CpG ODN.12 Although pDCs generally circulate in the periphery, they infiltrate tissue and organs in case of infections or inflammation.13 Moreover, pDCs are also reported to infiltrate tumor lesions in head and neck cancer, lung cancer, breast cancer, ovarian cancer, and skin cancer.11,14-16 These tumor-infiltrating pDCs, which appear to be in a tolerogenic state, contribute to the suppressive tumor microenvironment via the generation of regulatory T cells16 and are correlated with poor prognosis.15,17 Nevertheless, Stary et al elegantly demonstrated that topical imiquimod treatment, a TLR7 agonist, induced the activation of human tumor-infiltrating pDCs that exhibited tumoricidal activity and led to tumor clearance.18 The existence of a hybrid cell type in mice that unifies DC and NK cell characteristics and functions was first described in 2006 by Taieb et al19 and Chan et al.20 These natural killer DCs (NKDCs) or interferon producing killer DCs (IKDCs) exert the capacity to present antigens, to secrete cytokines and to have a cytotoxic effect, largely mediated by the expression of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL).19-22 Some studies demonstrated that human DCs, under appropriate stimulation conditions, can acquire cytotoxic effector functions. However, in most cases these effector functions appear to be inferior compared with that of the more traditional cytotoxic immune cells (NK cells or cytotoxic T cells).18,22-28 Previously, we observed that commonly used preventive vaccines can activate human pDCs.29 In this study, we observed that human pDCs activated by the tick-borne encephalitis vaccine FSME specifically up-regulated CD56 surface expression, a classic marker for NK cells.30 During the last decade, CD56 expression was also reported on NKT cells,31 activated T cells, and IFNα cultivated monocytes.22,32 The functional significance of CD56 expression on immune cells remains elusive. Interestingly, expression of CD56 is often observed on immune cells with cytolytic activity. Our findings indicate that high expression of CD56 on FMSE-activated pDCs coincided with elevated levels of PD-L1, granzyme B, and TRAIL. More importantly, FSME-activated pDCs were endowed with strong tumoricidal activity. The acquisition of NK cell behavior by pDCs adds an important effector function to the diverse repertoire of pDC functions and might have major implications on the functional role of tumor-infiltrating pDCs.
Methods
Cells
Buffy coats were obtained from healthy volunteers and PBMCs were obtained from patients enrolled in ongoing clinical trials after informed consent according to institutional and international guidelines in accordance with the Declaration of Helsinki. pDCs were purified by positive isolation using anti–BDCA-4 conjugated magnetic microbeads (Miltenyi Biotec) and adjusted to 106 cells/mL in X-VIVO-15 (Lonza) supplemented with 2% human serum (Sanquin). NK cells were purified by non–NK-cell depletion (Miltenyi Biotec). NK cell and pDC purity was routinely up to 95%, as assessed by double staining with CD3/CD56 (Becton Dickinson) or BDCA-2/CD123 (Miltenyi Biotec). PDCs were activated through the addition of 10 ng/mL IL-3 (Cellgenix), 5 μg/mL ODN-CpG-C (M362; Axxora), 4 μg/mL R848 (Axxora), 10% vol/vol FSME-IMMUN (Baxter AG), 10% vol/vol BMR (mumbs-measles and rubellavaccine, Nederlands Vaccin Instituut [NVI]), 10% vol/vol Rabies (Sanofi Pasteur MSD), 10% vol/vol Act-Hib (Aventis Pasteur), and 10% vol/vol BCG (NVI). After overnight incubation FSME-pDCs were sorted based on CD56 expression and CD56high and CD56low-FSME pDCs were used in a MLR and cytotoxicy assay where indicated.
Phenotype
The phenotype of the pDC populations was determined by flow cytometry. The following primary monoclonal antibodies (mAbs) and appropriate isotype controls were used: anti–BDCA2-PE and CD123-APC (both Miltenyi Biotec); anti–HLA-ABC-PE, anti–HLA-DR/DP-FITC, anti–CD80-PE, anti–CD83-PE, anti–CD86-PE, anti–CD86-APC, anti–PD-L1-PE (all BD Bioscience Pharmingen); anti–TRAIL-PE (eBioscience); anti–Granzyme-B-PE (Sanquin); anti–ICOS-L-Alexa488 (AbD Serotec); and anti–CD40-PE (Beckman Coulter).
Mixed lymphocyte reaction
The allostimulatory capacity of the pDCs was tested in a mixed lymphocyte reaction (MLR). Allogeneic T cells were cocultured with differently matured pDCs in a 96-well round-bottom plate (pDC:T cell ratio 1:20 or 1:100 with 1 × 105 PBLs [peripheral blood leukocytes]). After 4 days of culture, 1 μCi/well of tritiated thymidine (GE Healthcare) was added for 16 hours and incorporation was measured in a β-counter.
Specific KLH responses
Cellular responses against the protein keyhole limpet hemocyanin (KLH) were measured in a proliferation assay. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples taken from patients who received vaccination with KLH-loaded mature monocyte-derived DCs (moDCs). CD4+ T cells were isolated with a CD4+ T-cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. Purified T cells were plated in a 96-well tissue culture microplate with autologous pDCs that were cultured with or without KLH and matured with IL-3, CpG-C, or FSME-IMMUN. After 4 days of culture, 1 μCi/well of tritiated thymidine was added for 16 hours and incorporation was measured in a β-counter.
Generation of CD45RA+CD8+ gp100-specific T cells
The vectors pGEM4Z-TCRα296 and pGEM4Z-TCRß296 encoding the TCR (T-cell receptor) α and β chains originating from a gp100:280-288/HLA-A2–specific CTL (cytotoxic T lymphocyte) clone were a kind gift from Dr N. Schaft (University Hospital Erlangen, Germany). Gp100-specific T cells were generated by transferring the TCR α and β chain to T cells by electroporation of mRNA, resulting in transient expression of the TCR chains as previously described.33 Briefly, the DNA vectors were linearized with SpeI enzyme and purified by phenol/chloroform extraction and ethanol precipitation, and used as DNA templates for in vitro transcription. In vitro RNA synthesis was done with T7 RNA polymerase (mMESSAGE mMACHINE T7 kit; Ambion) according to the manufacturer's instructions. After DNase treatment, RNA was purified by phenol/chloroform extraction and isopropanol precipitation. RNA concentration was measured spectrophotometrically and the RNA was stored at −20°C. RNA quality was verified by agarose gel electrophoresis.
CD8+CD45RA+ T cells were isolated from PBMCs from an HLA-A2.1–positive donor. Monocytes were removed via adherence and CD8+ T cells were isolated from the nonadherent cell population by positive isolation using FITC-conjugated anti–human CD8 (BD Bioscience) and anti-FITC microbeads (anti-FITC multisort kit; Miltenyi Biotec) according to the manufacturer's instructions. Subsequently, CD45RA+ T cells were isolated from the CD8+ T-cell fraction by negative selection using CD45RO microbeads (Miltenyi Biotec). Purity of CD8+CD45RA+ T cells was 90%-95%, as assessed by double staining using FITC-conjugated anti–human CD8 and PE-conjugated anti–human CD45RA mAbs (BD Bioscience).
For RNA electroporation, the CD45RA+CD8+ T cells were washed once with PBS and once with OptiMEM without phenol red (Invitrogen Gmbh). Ten to 12 × 106 cells were incubated for 3 minutes with 15 to 20 μg of RNA in 200 μL OptiMEM in a 4-mm cuvette (Bio-Rad). Subsequently, cells were pulsed in a Genepulser Xcell (Bio-Rad). Pulse conditions were square-wave pulse, 500 V, 5 ms. Immediately after electroporation, the cells were transferred to X-VIVO-15 medium without phenol red (Cambrex) supplemented with 2% human serum. After 4 hours incubation at 37°C, cells were washed and frozen in fetal calf serum (FCS) and 10% (dimethyl sulfoxide) DMSO in liquid nitrogen. Expression of the gp100 TCR in the T cells was verified by flow cytometry using PE-conjugated anti-TCRVβ14 mAb (Coulter Immunotech).
Gp100-specific activation of CD45RA+CD8+ T cells
PDCs from a HLA-A2.1+ donor were activated overnight with different maturation stimuli and then loaded with either specific peptide (gp100280-288) or control peptide (tyrosinase369-377) for 1 hour (1 μg/7 × 103 pDCs). PDCs (7 × 103 per well) were washed and coincubated with CD45RA+CD8+ gp100280-288–specific autologous T cells (5 × 104 per well) in round-bottom 96-well plates. After overnight incubation, CD69 expression was measured by flow cytometry using FITC-conjugated mouse anti–human CD69 (BD Pharmingen), and IFNγ production was measured using a standard sandwich ELISA. After 4 days of culture, 1 μCi/well of tritiated thymidine was added for 8 hours, and incorporation of tritiated thymidine was measured in a β-counter.
Cytokine detection
Supernatants were collected from pDC cultures after overnight stimulation, and IFNα production was analyzed with murine monoclonal capture and HRP-conjugated anti-IFNα antibodies (BenderMed Systems) using standard ELISA procedures.
To analyze the T helper cell profile, supernatants were collected after 2 days of pDC-PBL coculture. Cytokine production (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 (p70), TNFα, TNFβ, IFNγ) in the supernatant was analyzed with a human Th1/Th2 Multiplex kit (eBioscience) according to the manufacturer's instructions.
Cytotoxicity assay
Killing of target cells was determined using a flow cytometric cytotoxicity assay.34 K562, Jurkat, MEL624, Daudi, and Glioma cells were labeled with PKH67 according to the manufacturer's instructions (Sigma-Aldrich). PDCs or NK cells were cocultured with 5 × 103 PKH67-labeled K562 cells at different effector cell:target cell (E:T) ratios in 200 μL IMDM (Invitrogen) supplemented with 10% fetal bovine serum (Perbio Science) in the presence of IL-2 (Invitrogen). Positive controls consisted of 1 × 104 NK-92 cells cocultured with 1 × 104 K562 cells in the presence of 200 U/mL IL-2, negative controls of target cells without effector cells. After overnight cocultures, cell viability was measured after staining with propidium iodide (Sigma-Aldrich) and annexin-APC or annexin-V Fitc (BD Pharmingen) in annexin V–binding buffer (BD). Flow cytometric measurements of target cell viability were performed on a FACSAria flow cytometer (BD) or FACSCalibur (BD) and analyzed using FlowJo Version 9.2 software (TreeStar). Specific killing of target cells was depicted relatively to the viability of target cells without effector cells, according to the formula34 :
Cytotoxicity mechanism studies
Cytotoxicity blocking experiments were performed as described, except that effector cells (FSME-pDCs) were preincubated at 37°C with either neutralizing CD56 mAbs (20 μg/0.5 × 106 pDCs), TRAIL mAbs (20 μg/0.5 × 106 pDCs; R&D Systems), or the perforin/granzyme-B inhibitor concanamycin A (100nM/ 0.5 × 106 pDCs; Tocris Bioscience) before start of coculture with PKH67-labeled K562 target cells. Control experiments were run in parallel using TRAIL isotype control mAbs (mouse IgG1; R&D Systems). K562 cells were added at a final E/T ratio of 20:1. In addition, K562 cells were incubated with FSME-pDCs supernatant. Furthermore, 5-μm pore size polycarbonate membranes (Costar) were placed on an aliquot of 600 μL RPMI medium containing 5 × 103 K562 cells. A total of 1 × 105 FSME-pDCs in 100 μL culture medium were seeded in the upper compartment and incubated for 18 hours to assess killing of K562 cells by soluble factors.
Statistics
All experiments were performed at least 3 times and results are shown as the mean ± SEM. datasets were tested by 1-way ANOVA followed by the post-hoc test Tukey multiple comparison test or Dunnett test.
Results
FSME-IMMUN activates human pDCs
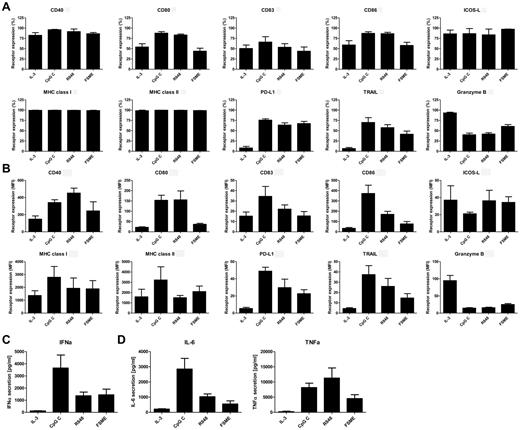
Previously, we demonstrated that human pDCs can be stimulated by the preventive vaccine FSME.29 Here we studied in more depth how FSME stimulation differs from the known pDCs stimuli IL-3, as well as the TLR7 and TLR9 agonists, R848, and CpG-C C, respectively. In accordance with literature,28,35 we showed that pDC maturation was associated with high expression of CD40, CD80, CD83, CD86, PD-L1, TRAIL, ICOS-L, MHC class I and II, and low expression of granzyme B (Figure 1A-B). IL-3 stimulated pDCs had high expression of granzyme B, lower levels of CD40, CD80, CD83, CD86, and were negative for PD-L1 and TRAIL (Figure 1A-B). Human pDCs activated by FSME up-regulated the costimulatory molecules CD40, CD80, and CD86 and the maturation marker CD83 to comparable levels as IL-3 stimulated pDCs (Figure 1A-B). Moreover, FSME-pDCs as well as TLR-activated pDCs showed up-regulated levels of PD-L1 and TRAIL (Figure 1A-B). On the other hand, granzyme B expression was higher in FSME-stimulated pDCs compared with TLR-activated pDCs, and lower compared with IL-3-pDCs (Figure 1A-B). Furthermore, pDC activation with the TLR agonists or FSME led to the secretion of type I IFN (Figure 1C), IL-6, and TNFα (Figure 1D). Taken together, FSME, a preventive vaccine for tick-borne encephalitis virus infections, activates human pDCs.
FSME induces pDC maturation. Human pDCs were incubated overnight with IL-3, CpG-C, R848, or FSME and the expression of various molecules was measured by flow cytometry. The graphs show (A) the percentage and the mean fluorescence intensity (MFI), and (B) expression levels of surface molecules CD40, CD80, CD83, CD86, PD-L1, TRAIL, ICOS-L, MHC class I and II expression, and intracellular granzyme B. The data are mean values ± SEM of at least 5 independent experiments with different donors. Supernatants of pDC cultures after incubation with IL-3, CpG-C, R848, or FSME were analyzed for the presence of (C) IFNα, (D left) IL-6, and (D right) TNFα. Data shown are mean values of at least 5 independent experiments ± SEM.
FSME induces pDC maturation. Human pDCs were incubated overnight with IL-3, CpG-C, R848, or FSME and the expression of various molecules was measured by flow cytometry. The graphs show (A) the percentage and the mean fluorescence intensity (MFI), and (B) expression levels of surface molecules CD40, CD80, CD83, CD86, PD-L1, TRAIL, ICOS-L, MHC class I and II expression, and intracellular granzyme B. The data are mean values ± SEM of at least 5 independent experiments with different donors. Supernatants of pDC cultures after incubation with IL-3, CpG-C, R848, or FSME were analyzed for the presence of (C) IFNα, (D left) IL-6, and (D right) TNFα. Data shown are mean values of at least 5 independent experiments ± SEM.
Activated pDCs are potent stimulators of T-cell responses
T-cell expansion and activation is a crucial step in generating effective immune responses. All activated pDCs displayed a mature phenotype (Figure 1); therefore, we determined the immunogenic capacity of the differently activated pDCs. CpG-C-pDCs induced stronger allogeneic T-cell responses than both IL-3–cultured and FSME-pDCs (Figure 2A). Interestingly, lower numbers of FSME-pDCs (ratio 1:100) induced lower T-cell responses than higher numbers (ratio 1:20; Figure 2A). Furthermore, both FSME-pDCs and CpG-C-pDCs were capable of stimulating antigen-specific autologous T cells as demonstrated by the KLH-specific proliferation of T cells from patients who had been previously vaccinated with KLH-loaded DCs (Figure 2B).36-38 To investigate whether the stimulated pDCs induced a Th1-type effector response, we analyzed the cytokines in supernatants of the KLH response. FSME-pDCs and CpG-C-pDCs induced the secretion of high levels of IFNγ and TNFα by PBLs (Figure 2C). Differences between antigen-specific T-cell cytokine production after pDC stimulation with FSME or CpG-C were observed for IL-5 and IL-2. The production of IL-5 was slightly increased, whereas IL-2 was decreased on stimulation with FSME-pDCs compared with CpG-C-pDCs (Figure 2C). Taken together, activated pDCs exhibit the capacity to induce antigen-specific Th1 responses.
Activated pDCs are potent stimulators of both CD4+ and CD8+ T cells. Proliferation of T cells was measured by 3H-Thymidine incorporation and depicted as proliferation in counts per minute. (A) Peripheral blood leucocytes (1 × 105) were stimulated for 4 days with either 5 × 103 or 1 × 103 allogeneic pDCs. IL-3–pDCs evoke lower T-cell proliferation compared with pDCs activated with either CpG-C or FSME vaccine. The left graph shows the pDC:PBL ratio 1:20 and the right graph 1:100. Data are the mean values ± SEM of 3 independent experiments with different donors. (B) Patient-derived (previously vaccinated with KLH) CD4+ T cells (1 × 105) were stimulated with 5 × 103 KLH loaded (black bars) and unloaded (blank bars) autologous pDCs. We loaded pDCs with KLH using serum that contained KLH specific antibodies. KLH loaded pDCs were highly capable of inducing proliferative KLH-specific recall responses. Data shown are the mean values ± SEM of 1 representative experiment. (C) Cytokine production by autologous T cells after stimulation was analyzed by a cytokine bead array and production is depicted in pg/mL. (D-F) Naive CD45RA+CD8+ T cells specific for gp100280-288 were cocultured with autologous pDCs activated through CpG-C or FSME vaccine and loaded with either gp100280-288 (black bars) or the irrelevant tyrosinase peptide (gray bars). After 16 hours of coculture, antigen-specific activation of CD8+ T cells was analyzed by measurement of CD69 (D) surface expression and by secretion of IFNγ in the supernatant (E). Antigen-specific T-cell proliferation was measured after 4 days of culture (F). Data shown are mean values ± SEM of 1 representative experiment of 2 independent experiments performed with different donors.
Activated pDCs are potent stimulators of both CD4+ and CD8+ T cells. Proliferation of T cells was measured by 3H-Thymidine incorporation and depicted as proliferation in counts per minute. (A) Peripheral blood leucocytes (1 × 105) were stimulated for 4 days with either 5 × 103 or 1 × 103 allogeneic pDCs. IL-3–pDCs evoke lower T-cell proliferation compared with pDCs activated with either CpG-C or FSME vaccine. The left graph shows the pDC:PBL ratio 1:20 and the right graph 1:100. Data are the mean values ± SEM of 3 independent experiments with different donors. (B) Patient-derived (previously vaccinated with KLH) CD4+ T cells (1 × 105) were stimulated with 5 × 103 KLH loaded (black bars) and unloaded (blank bars) autologous pDCs. We loaded pDCs with KLH using serum that contained KLH specific antibodies. KLH loaded pDCs were highly capable of inducing proliferative KLH-specific recall responses. Data shown are the mean values ± SEM of 1 representative experiment. (C) Cytokine production by autologous T cells after stimulation was analyzed by a cytokine bead array and production is depicted in pg/mL. (D-F) Naive CD45RA+CD8+ T cells specific for gp100280-288 were cocultured with autologous pDCs activated through CpG-C or FSME vaccine and loaded with either gp100280-288 (black bars) or the irrelevant tyrosinase peptide (gray bars). After 16 hours of coculture, antigen-specific activation of CD8+ T cells was analyzed by measurement of CD69 (D) surface expression and by secretion of IFNγ in the supernatant (E). Antigen-specific T-cell proliferation was measured after 4 days of culture (F). Data shown are mean values ± SEM of 1 representative experiment of 2 independent experiments performed with different donors.
Next we investigated the capacity of FSME-pDCs to induce CD8+ T-cell responses. Therefore, pDCs were loaded with gp100280-288 peptides and cocultured with naive autologous CD45RA+CD8+ T cells expressing the gp100280-288–specific T-cell receptor. PDCs activated by IL-3, CpG-C, and by FSME all demonstrated the ability to induce antigen-specific T-cell responses, as illustrated by the up-regulation of the early T-cell activation marker CD69 (Figure 2D), and the secretion of IFNγ (Figure 2E) 24 hours after activation. Interestingly, although less IFN-γ was produced in cocultures of gp100–specific T cells with autologous FSME-pDCs compared with CpG-C-pDC, FSME-pDCs demonstrated the ability to induce stronger gp100-specific proliferative T-cell responses than pDCs activated by CpG-C (Figure 2F). These data demonstrate that pDCs have the capacity to evoke antigen-specific naive CD8+ T-cell responses.
FSME activated pDCs express CD56
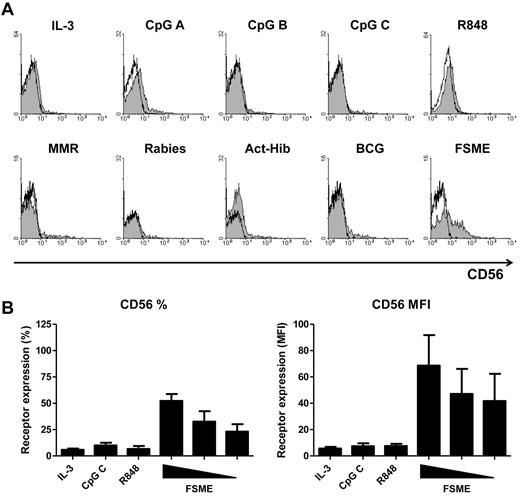
Interestingly, as we studied the expression level of CD56 on activated human pDCs we found that 53.1% ± 5.8% of FSME-pDCs acquired de novo CD56 expression (Figure 3A-B). Furthermore, we demonstrated that FSME-induced up-regulation of CD56 was dose-dependent (Figure 3B). To our knowledge this is a new finding, as up to now CD56 expression was described to be present on NK cells, NKT cells, IFN-activated moDCs, and a small DC subset circulating in the blood, but so far never on pDCs.22,39 Therefore, we sought to determine whether other stimuli known to induce pDC activation also induced CD56 receptor expression. Neither IL-3 nor the known TLR agonists, CpG-A, CpG-B, CpG-C, or R848, induced CD56 expression (Figure 3A-B). Moreover, also vaccines that previously induced pDC activation,29 BMR, rabies, Act-Hib, and BCG, did not induce CD56 expression (Figure 3A). Thus, FSME activation specifically induced the up-regulation of CD56 expression on human pDCs.
FSME induces CD56 expression on human pDCs. Human pDCs were incubated with various stimuli as indicated and the expression of CD56 was studied by flow cytometry. (A) The gray filled histograms show the expression of CD56 on pDCs after overnight stimulation compared with isotype control (black lined histograms). Data shown are of 1 representative experiment of 3 performed. (B) The graphs show the percentage (left graph) and MFI (right graph) of CD56 expression on human pDCs after overnight stimulation. ; overnight FSME stimulation with 10% vol/vol, 5% vol/vol, and 2.5% vol/vol. Data shown are mean values ± SEM of at least 4 independent experiments performed with different donors.
FSME induces CD56 expression on human pDCs. Human pDCs were incubated with various stimuli as indicated and the expression of CD56 was studied by flow cytometry. (A) The gray filled histograms show the expression of CD56 on pDCs after overnight stimulation compared with isotype control (black lined histograms). Data shown are of 1 representative experiment of 3 performed. (B) The graphs show the percentage (left graph) and MFI (right graph) of CD56 expression on human pDCs after overnight stimulation. ; overnight FSME stimulation with 10% vol/vol, 5% vol/vol, and 2.5% vol/vol. Data shown are mean values ± SEM of at least 4 independent experiments performed with different donors.
High CD56 expression coincides with higher levels of PD-L1, TRAIL, granzyme B, and effector functions
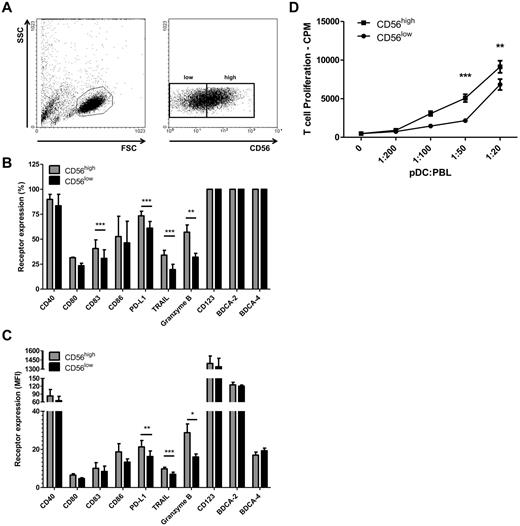
The finding that FSME up-regulated CD56 prompted us to study the phenotype of CD56high and CD56low FSME-pDCs (Figure 4A). Both populations showed an almost identical phenotypical pattern as we did not observe a difference in the expression of BDCA-2, BDCA-4, MHC class I and II, CD123, CD40, CD80, CD86, and ICOS-L (Figure 4B-C and data not shown). However, the percentage of CD83-expressing cells was slightly higher in the CD56high population (Figure 4B-C). Interestingly, CD56high pDCs expressed significant higher levels of TRAIL and granzyme B (Figure 4B-C). These data suggest that CD56high pDCs are endowed with the capacity to kill granzyme B and TRAIL-sensitive target cells. Next we analyzed whether CD56high pDCs are functionally different from CD56low pDCs. Previous studies showed that pDCs produce IFNα within 12 hours after activation, and then become refractory to any further stimulation,40-42 therefore isolated pDCs were activated for 6 hours with FSME followed by intracellular cytokine staining. We did not detect differences in IFNα secretion nor in TNFα secretion between the 2 populations (data not shown). Furthermore, after overnight activation FSME-pDCs were sorted based on CD56 expression. Subsequent analysis revealed that CD56high pDCs are more potent inducers of allogeneic T-cell responses (Figure 4D). Taken together, these findings demonstrate that CD56high FSME-pDCs acquire strong effector functions.
High CD56 expression coincides with higher levels of PD-L1, TRAIL, granzyme B, and effector functions. (A) The dot plots show the gating strategy used to identify and distinguish CD56low and CD56high expressing FSME-pDCs. Based on the gating strategy, phenotypical characterization was performed to study the percentage (B) and MFI (C) of the various molecules expressed by FSME-pDCs. (D) Peripheral blood leucocytes (1 × 105) were stimulated for 4 days with either 5 × 103, 2 × 103, 1 × 103, or 0.5 × 103 allogeneic CD56high or CD56low FSME-pDCs. (B-D) Data shown are mean values ± SEM of at least 3 independent experiments performed with different donors (*P < .05, **P < .01, ***P < .001).
High CD56 expression coincides with higher levels of PD-L1, TRAIL, granzyme B, and effector functions. (A) The dot plots show the gating strategy used to identify and distinguish CD56low and CD56high expressing FSME-pDCs. Based on the gating strategy, phenotypical characterization was performed to study the percentage (B) and MFI (C) of the various molecules expressed by FSME-pDCs. (D) Peripheral blood leucocytes (1 × 105) were stimulated for 4 days with either 5 × 103, 2 × 103, 1 × 103, or 0.5 × 103 allogeneic CD56high or CD56low FSME-pDCs. (B-D) Data shown are mean values ± SEM of at least 3 independent experiments performed with different donors (*P < .05, **P < .01, ***P < .001).
FSME activated pDCs abrogate tumor cell growth
To determine whether our FSME-pDCs also exhibit tumoricidal activity, cells were cocultured for 18 hours or 3 days with K562 cells at different pDC:K562 ratios. Only FSME-stimulated pDCs were able to specifically lyse K562 cells up to 59% ± 4.7% (ratio 20:1) after 18 hours and up to 71% ± 6.6% (ratio 20:1) after 3 days (Figure 5A). Moreover, the absolute number of K562 cells that was detected after 18 hours or 3 days of coculture with FSME-pDCs was strongly decreased (Figure 5B). Although, we could not detect specific lysis of K562 cells by IL-3 pDCs or R848 pDCs, we did observe that they inhibited malignant-cell replication on 3-day coculture (Figure 5B). Furthermore, CD56high pDCs were equally efficient in killing K562 cells compared with CD56low pDCs (data not shown). Plasmacytoid DCs activated with the other vaccines did not induce specific lysis. Next to FSME, also the MMR vaccine endowed pDCs with tumoricidal capacities (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
FSME activated pDCs are potent killer pDCs. NK cells were stimulated with IL-2 and human pDCs were activated overnight with various stimuli as indicated and cocultured with PKH-labeled K562 cells. After 18 hours or 3 days of coculture specific lysis was determined by studying the expression of annexin V and propidium iodide (A) and the absolute number (B) of K562 cells. Data shown are mean values ± SEM of at least 3 independent experiments performed with different donors (*P < .05, **P < .01). (C) FSME activated pDCs were cocultured with PKH67-labeled K562 target cells at an E/T ratio of 20:1 in the presence of either anti-CD56, anti-TRAIL blocking mAb, or the granule exocytosis inhibitor concanamycin A. Parallel experiments were performed using TRAIL isotype-matched control mAbs. Furthermore, K562 cells were cultured in the presence of supernatant derived FSME-pDC cultures or in a transwell assay. (D) PDCs were activated overnight with FSME and cocultured with PKH-labeled K562, Jurkat, MEL624, Daudi, and Glioma cells. (C-D) Specific lysis of target cells was determined after 18 hours of incubation. Data shown are mean values ± SEM of at 3 independent experiments performed with different donors at least in duplo.
FSME activated pDCs are potent killer pDCs. NK cells were stimulated with IL-2 and human pDCs were activated overnight with various stimuli as indicated and cocultured with PKH-labeled K562 cells. After 18 hours or 3 days of coculture specific lysis was determined by studying the expression of annexin V and propidium iodide (A) and the absolute number (B) of K562 cells. Data shown are mean values ± SEM of at least 3 independent experiments performed with different donors (*P < .05, **P < .01). (C) FSME activated pDCs were cocultured with PKH67-labeled K562 target cells at an E/T ratio of 20:1 in the presence of either anti-CD56, anti-TRAIL blocking mAb, or the granule exocytosis inhibitor concanamycin A. Parallel experiments were performed using TRAIL isotype-matched control mAbs. Furthermore, K562 cells were cultured in the presence of supernatant derived FSME-pDC cultures or in a transwell assay. (D) PDCs were activated overnight with FSME and cocultured with PKH-labeled K562, Jurkat, MEL624, Daudi, and Glioma cells. (C-D) Specific lysis of target cells was determined after 18 hours of incubation. Data shown are mean values ± SEM of at 3 independent experiments performed with different donors at least in duplo.
Because FMSE-activated pDCs expressed TRAIL and granzyme B, we evaluated the contributions of these pathways to the cytotoxic effect of pDCs on K562 cells. We performed cytotoxicity blocking experiments using anti-TRAIL neutralizing antibodies and concanamycin A, a selective inhibitor of vacuolar-type H+ ATPase that prevents acidification and degranulation of perforin/granzyme-containing cytotoxic granules. Because cytotoxicity of FSME-pDCs against K562 cells was most prominent at high E:T ratios, we chose the E/T ratio of 20:1 for blocking experiments. As shown in Figure 5C, neutralization of TRAIL, granzyme B, or the combination of TRAIL and granzyme B activity revealed that these cytotoxic molecules did not participate in the observed cytotoxicity. It has been suggested in literature that the expression of CD56 might promote effector-target cell interaction.43 Therefore, we performed additional blocking experiments where FSME-pDCs were preincubated with anti-CD56 antibodies. We demonstrate that blocking CD56 on FSME-pDCs did not result in diminished specific lysis (Figure 5C). Furthermore, FSME-pDCs specifically killed the MHC class I negative tumor cell lines K562 and Daudi, but minimally the MHC class I positive tumor cell lines GliomaX, Jurkat, and MEL624 (Figure 5D). In addition, K562 cells could not be killed by supernatant from FSME-pDC cultures or in a transwell assay, indicating that the killing capacity of FSME-pDCs is cell-cell contact dependent (Figure 5C). Similarly, supernatants derived from FSME-pDC cultures did not affect Daudi cell viability (data not shown). Taken together, our data indicate that on FSME activation human pDCs gain cytotoxic effector functions and specifically lyse NK target tumor cell lines in a cell-cell contact dependent manner.
Discussion
Here we show that human pDCs after FSME-induced activation attain a wide range of immunostimulatory and inhibitory molecules, secrete pro-inflammatory cytokines, present antigens and activate T cells, and display tumoricidal activity. Moreover, to the best of our knowledge, we identified for the first time a stimulus that specifically induces the expression of CD56, a molecule predominantly associated with NK cells. The implication of expression of CD56 by the FSME-activated pDCs is very intriguing yet elusive as its exact function on NK cells is not known. Importantly, we demonstrated that high CD56 expression coincided with the expression of cytotoxic molecules. Evidence is emerging that on proper activation DC subsets can express and secrete molecules associated with cytotoxic effector functions.18,22-28 Just recently, Kalb et al demonstrated that pDC-based antitumor immunity is mediated by IFNα and that imiquimod induced TRAIL expression.44 These findings reveal that FSME as a stimulus turns freshly isolated pDCs in cells that share key features with previously described murine IKDCs19,20 and human CD56+ DCs with myeloid-lineage orientation. CD56–expressing cell types are generally equipped with cytotoxic functions, suggesting that hematopoietic expression of CD56 is confined to cells exhibiting cytotoxic properties. We therefore hypothesize that the expression of CD56 has a role in the special effector functions acquired by human pDCs on FSME stimulation. As a cell adhesion molecule, it could help the pDCs to bind their targets, or as a homophilic binding protein it might promote cross-talk with other CD56-expressing cells, such as NK cells or pDCs themselves.45 Recently the group of Thurnher et al demonstrated that CD56+ DCs interact with and activate NK cells.46,47 Furthermore, studies that used CD56-expressing NK cells and T cells, showed that CD56 may potentiate the cytotoxic activity of these cells by promoting effector–target cell interaction.43 Our findings suggest that a role for CD56 in the observed cytotoxicity can be excluded. However, whether CD56 is involved in the bidirectional crosstalk between different cells of the immune system remains to be elucidated.
We observed a cytotoxic effect, up to 50% specific lysis after 18 hours, of FSME and MMR-pDCs on leukemic K562 cells. Interestingly, this effect was much stronger compared with IL-3, R848, and other vaccine activated pDCs. In line with the report from Matsui et al, differently activated pDCs inhibited malignant cell replication in a 3-day coculture.26 Interestingly, MMR-induced activation did not lead to the expression of CD56; however, these cells did obtain tumoricidal activity. Previous reports demonstrated that virus-activated pDCs can acquire cytolytic function that is mediated by the expression of TRAIL.23-25 On FSME stimulation human pDCs up-regulated TRAIL expression. However, in our hands the tumoricidal activity of FSME-pDCs could not be blocked by TRAIL-blocking antibodies (Figure 5C). In addition, Jahrsdorfer et al demonstrated that pDC-derived granzyme B impaired allogeneic T-cell proliferation, which is in line with our observation where IL-3 and FMSE pDCs induce lower T-cell proliferation compared with CpG-C pDCs (Figure 2A).28 Although FMSE-pDCs expressed granzyme B, it was not involved in the specific lysis of K562 cells (Figure 5C). Although concurrent blocking of TRAIL and granzyme B did not diminish the tumoricidal activity, the possibility that other pDC-derived cytotoxic molecules act in synergy to exert their cytotoxic function cannot be excluded. However, we did demonstrate that FSME-pDCs required cell-contact–dependent mechanisms to directly lyse target cells. Furthermore, the need of long incubation for detectable tumoricidal effects of IL-3 and R848 pDCs hints toward the involvement of slow inducers of apoptosis, such as granzyme B (perforin-independent), TNF-α, or FasL.48 Moreover, we demonstrated that FSME-pDCs specifically lysed MHC class I negative NK target tumor cell lines. Underscoring that FSME-pDCs acquired NK cell effector functions.
A remaining question is the immunologic relevance of killer tumor-infiltrating pDCs. It is generally accepted that macrophages and DCs outnumber the classic killers (NK and CTLs) in tumor tissues.27 By their ability to secrete large amounts of type I IFN pDCs were already shown to mediate cross-talk with other immune cells, such as NK cells or T cells leading to superior antitumor immunity.6,49 The here described cytotoxic effector function provides a new insight for a role of tumor-infiltrating pDCs in tumors. Properly activated tumor-infiltrating pDCs might not only directly lyse cancer cells, but might also take up the released tumor-associated antigens and present them to T cells. Altogether, killer pDCs might represent a potential target for the development of new strategies to eliminate cancer. This property has already been underscored by Stary et al, who demonstrated that activation of human tumor-infiltrating pDCs resulted in tumor clearance.18 Moreover, just recently Drobits et al described that imiquimod-activated tumor-infiltrating pDCs not only killed tumor cells in mice, but also that this was independent of the adaptive arm of the immune system.50
Besides the acquired cytotoxic effector function, phenotypic characterization of human pDCs activated overnight with FSME revealed that pDCs acquired a phenotype that did not completely resemble TLR7/9-induced activation nor IL-3 stimulation. Where FSME-pDCs displayed similar expression of CD40, TRAIL, and PD-L1 and secretion of IL-6, TNFα, and IFNα as TLR7/9-activated pDCs, they more resembled IL-3–pDCs when considering expression levels of CD80, CD83, CD86, and granzyme B. Strikingly, based on their ability to activate antigen-specific naive CD8+ T cells, FSME-pDCs resemble TLR-activated pDCs, whereas FSME-pDCs induced similar allogeneic T cell and mixed antigen specific Th-cell responses as IL-3–pDCs. As the FMSE vaccine contains the inactivated TBEV (ssRNA virus), it is probably acting as a TLR7 agonist, although it is not completely mimicking the effects of the TLR7 agonist R848 on pDCs. For the CpG-ODN classes it has already been described that different CpG-ODNs trigger TLR9 in different endosomal compartments resulting in functional differences. Whether FSME behaves similar to CpG-ODNs and triggers TLR7 in specific endosomal compartments or even triggers a yet unidentified receptor remains to be elucidated. Notwithstanding, based on the present dataset we conclude that human FSME activated pDCs are inducers of antigen-specific T-cell responses.
In conclusion, the data presented here unequivocally demonstrates that human pDCs can acquire CD56 expression and become killer cells. For the first time we reported on CD56-expressing IFN-producing killer pDCs that are equipped with an antigen presentation machinery.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The Netherlands Organization for Scientific Research (NWO ZonMW; Vidi grant 91776363 to I.J.M.d.V.). E.L.S. as a postdoctoral researcher and S.A. as a PhD fellow are funded by the Research Foundation Flanders (FWO Vlaanderen). This work was supported in part by a research grant of the FWO Vlaanderen.
Authorship
Contribution: J.T. and E.L.S. designed and performed research, analyzed data, and wrote the paper; S.A., R.N.J., and C.G.F. contributed to experimental design and writing of the paper; and I.J.M.d.V. supervised the study, and contributed to experimental design and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. Jolanda M. de Vries, Dept of Tumor Immunology, Radboud University Nijmegen Medical Centre, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: j.devries@ncmls.ru.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal