Abstract
IL-21 regulates Th17 cell homeostasis, enhances the differentiation of memory B cells and antibody-secreting plasma cells, and promotes the maintenance of CD8+ T-cell responses. In this study, we investigated the phenotype, function, and frequency of blood and intestinal IL-21–producing cells in nonhuman primates that are hosts of progressive (rhesus macaques [RMs]) and nonprogressive (sooty mangabeys [SMs]) SIV infection. We found that, in both species, memory CD4+CD95+CCR6− T cells are the main IL-21 producers, and that only a small fraction of CD4+IL-21+ T cells produce IL-17. During chronic SIV infection of RMs, CD4+IL-21+ T cells were significantly depleted in both blood and rectal mucosa, with the extent of this depletion correlating with the loss of Th17 cells. Furthermore, treatment with IL-21 increased the in vivo levels of Th17 cells in SIV-infected RMs. In contrast, normal levels of CD4+IL-21+ T cells were found in SIV-infected SMs. Collectively, these data indicate that depletion of IL-21–producing CD4+ T cells distinguishes progressive from nonprogressive SIV infection of RMs and SMs, and suggest that depletion of CD4+IL-21+ T cells is involved in the preferential loss of Th17 cells that is associated with SIV disease progression. Further preclinical studies of IL-21 as a potential immunotherapeutic agent for HIV infection may be warranted.
Introduction
The pathogenesis of the immunodeficiency that occurs in HIV-infected humans and SIV-infected rhesus macaques (RMs) is the result of a complex and incompletely understood interaction between the virus and the host immune system.1 The establishment of a state of chronic, generalized immune activation is a characteristic feature of pathogenic HIV/SIV infections in humans and RMs, with many different immune cell types showing an activated/dysfunctional phenotype.1 Importantly, the level of chronic immune activation represents a strong predictor of both disease progression and poor immunologic response to antiretroviral therapy.2-4 Strong indirect support for the crucial role of immune activation in AIDS pathogenesis is provided by studies of SIV infections in African monkeys that are natural hosts for SIV, such as sooty mangabeys (SMs), in which levels of virus replication are similar or even higher to those found in HIV-infected humans, but are not sufficient to induce any signs of illness or progression to AIDS due, in part to the absence of increased levels of immune activation.5 The exact mechanisms that sustain high levels of chronic immune activation in HIV-infected humans and SIV-infected RMs, or limit them in natural hosts for SIV, are still unclear, and their elucidation is considered a key priority in contemporary AIDS research.6
CD4+ T cells, the main target of HIV and SIV, are a relatively heterogeneous population of immune cells based on phenotype, cytokine profile, and functions. CD4+ T cells can be phenotypically classified in broad subsets of naive, central memory, transitional memory, and effector memory T cells based on their differentiation status.7 In addition, T helper (Th) can be classified into subsets that include Th1, Th2, Th17, T follicular helper (Tfh), and regulatory (Treg) cells based on their cytokine profile and/or functions.8 Pathogenic HIV/SIV infections of humans and RMs are associated with major perturbations of the relative proportion of the different CD4+ T-cell subsets. Interestingly, the in vivo changes induced by HIV/SIV infections on the homeostasis of CD4+ T-cell subsets are different in natural and non-natural hosts for lentiviruses.9-11 We and others have shown that intestinal Th17 cells are preferentially depleted in pathogenic HIV/SIV infections of humans and RMs, but maintained at a healthy frequency in nonprogressive SIV infection of SMs.9,12,13 Th17 cells are essential for mucosal immunity as they respond to extracellular bacteria and fungi by recruiting neutrophils and inducing tight junctions, antibacterial defensin, and/or mucin expression, thus preserving the structural barrier of the gastrointestinal (GI) tract.14,15 Consistent with this paradigm, the depletion of Th17 cells in HIV-infected humans and SIV-infected RMs is associated with loss of mucosal integrity and signs of microbial translocation,9,13,16,17 whereas the preservation of a normal fraction of intestinal Th17 cells in SIV-infected SMs is associated with the maintenance of mucosal integrity and the absence of microbial translocation.9,17,18 The exact mechanism(s) underlying depletion of Th17 cells in pathogenic HIV/SIV infections of human and RMs, or their preservation in nonpathogenic SIV infection of SMs, are unknown, and no conclusive evidence has been generated suggesting that differences in the level of direct virus infection of Th17 cells between RMs and SMs are responsible for this observation.
IL-21, the most recently identified member of the common γ-chain, using cytokine family that includes IL-2, IL-4, IL-7, IL-9, and IL-15, is a pleiotropic cytokine mainly produced by activated CD4+ T cells, including Tfh and NKT cells.19,20 IL-21 exerts numerous immune-enhancing and immune-regulatory functions, thus playing a critical role in the initiation and control of both innate and adaptive immune responses. Immunologic roles of IL-21 include: (1) promoting the proliferation, cytolytic potential, and long-term maintenance of Ag-specific CD8+ T cells21-23 ; (2) favoring the differentiation of naive CD4+ T cells into Th17 cells, as well as Th17 cell expansion24-27 ; (3) supporting the differentiation of Ag-stimulated B cells into memory and Ab-secreting plasma cells28-30 ; and (4) regulating NK cell activation, functions, and expansion.31 Consistent with these immune functions, several in vivo studies have shown that IL-21 is an essential component of CD4+ T-cell help required to control chronic LCMV infection in mice.32-34
In this study, we hypothesized that the loss of IL-21–producing CD4+ T cells is a determinant of the preferential depletion of Th17 cells observed in SIV-infected RMs but not in SIV-infected SMs. To test this hypothesis, we have investigated the phenotype, functions, and frequency of blood and intestinal IL-21–producing CD4+ T cells in these 2 models of SIV infection. We have found that SIV infection of RMs is associated with a severe loss of circulating and intestinal CD4+IL-21+ T cells and that the observed loss of CD4+IL-21+ T cells correlates with depletion of Th17 cells, increased T-cell proliferation, and impaired overall CD4+ T-cell homeostasis. In contrast, there was no overt depletion of CD4+IL-21+ T cells in SIV-infected SMs. As such, the results presented herein suggest that reduced levels of CD4+IL-21+ cells may be a key contributor to the preferential loss of Th17 cells that is associated with mucosal immune dysfunction and disease progression in SIV-infected RMs.
Methods
Animals
A total of 59 RMs, 29 chronically SIV-infected (14 intrarectally and 15 intravenously) and 30 uninfected, and 28 SMs, 16 chronically SIV-infected and 12 uninfected, all housed at the Yerkes Nationale Primate Research Center (Atlanta, GA), were included in this study. SIV infection of RMs included SIVmac239, SIVmac251, and SIVsmE543, with length of infection between 4 and 8 months. All animal studies were approved by the Emory University Institutional Animal Care and Usage Committee.
Sample collections and processing
PBMCs were prepared from venous blood by density gradient centrifugation. Rectal (RB) and Jejunal biopsies were obtained with a rectal anoscope and a pediatric endoscope, respectively, and processed as previously described.9
Flow cytometric analysis
Fourteen-parameter flow cytometric analysis was performed on PBMC and RB-derived cells as previously described.9 Predetermined optimal concentrations were used of the following antibodies: anti-CD3–Alexa700 (clone SP34-2), anti-CD3–APC-Cy7 (clone SP34-2), anti-CD4–PE (clone L200), anti-CD8–PacBlue (clone RPA-T8), anti-CD95–PE-Cy5 (clone DX2), anti-CCR6–PE-Cy7 (clone 11A9), anti-β7–PE-Cy5 (clone FIB504), anti–IL-21–AlexaFluor-647 (clone 3A3-N2.1), anti-IFN-γ–PE-Cy7 (clone B27), (all from BD Biosciences PharMingen); anti-CD28–ECD (clone CD28.2 Beckman Coulter), anti–IL-17–AlexaFluor-488 (clone eBio64DEC17, eBioscience), and Aqua Live/Dead amine dye-AmCyan (Invitrogen). Flow cytometric acquisition was performed on at least 100 000 CD3+ T cells on an LSRII cytometer driven by the FACSDiVa Version 6.1.3 software. Analysis of the acquired data were performed using FlowJo Version 9.5.3 software (TreeStar).
Intracellular cytokine staining
PBMCs and RB-derived cells were resuspended to 1 × 106 cells/mL in complete RPMI 1640 medium. Cells were then incubated for 4 hours at 37°C in medium containing phorbol myristate acetate, A23187, and Golgi Stop. After incubation, the cells were washed and stained with surface markers followed by fixation and permeabilization. Cells were then washed and stained intracellularly for the cytokines of interest. After staining, cells were washed, fixed in PBS containing 1% paraformaldehyde, and acquired on an LSRII cytometer.
Cell sorting and quantitative PCR for SIV gag DNA
CD4+IL-21+, CD4+TNF-α+, and CD4+IFNγ+ T cells were sorted from the spleen of 4 chronically SIVsmE543-infected RMs using a FACSAria flow cytometer (BD Biosciences). Cells were initially gated based on light scatter, positive staining for CD3 (without binding to the dead cell dye) and then for CD4. Memory (CD95+) CD4+ T cells were then divided based on the production of IL-21, TNF-α, or IFN-γ. Quantification of SIVsmE543 gag DNA in sorted cells was performed by quantitative PCR as previously described.35 For cell number quantification, quantitative PCR was performed simultaneously for monkey albumin gene copy number. The sequence of the forward primer for SIVsmE543 is 5′-GGCAGGAAAATCCCTAGCAG-3′. The reverse primer sequence is 5′-GCCCTTACTGCCTTCACTCA-3′. The probe sequence is 5′-AGTCCCTGTTCRGGCGCCAA-3′.
Quantitative mRNA analysis
RNA was isolated from jejunal and colorectal biopsy derived mononuclear cells using the Qiagen RNAeasy Mini kit as per the manufacturer's instructions. The RNA was reverse transcribed using the Sigma-Aldrich enhanced avian kit. Levels of mRNA specific for rhesus IL-21 and GAPDH were quantitated using the SYBR Green real-time PCR assay using the following primer pairs: 5′-TGTGAATGACTTGGACCCTGAA-3′ and 5′-AAACAGGAAATAGCT GACCACTCA-3′ for IL-21, and 5′-GCACCACCAACTGCTTAGCAC-3′ and 5′-TCTTCTGGGTGGCAGTGATG-3′ for GAPDH. The values for IL-21 were expressed relative to the values for GAPDH, and the ratios obtained with the baseline values were used to calculate the fold increases.
Immunohistochemistry staining for IL-21
Immunohistochemistry for rabbit polyclonal anti–IL-21 (AnaSpec) was performed using a biotin-free polymer approach on 5-μm tissue sections mounted on glass slides, which were dewaxed and rehydrated with double-distilled H2O. Antigen retrieval was performed by heating sections in 10mM citrate (pH 6.0) in a pressure cooker set at 121°C for 30 seconds. Slides were stained on an IntelliPATH autostainer (Biocare Medical) with optimal conditions determined empirically that consisted of a blocking step using blocking buffer (TBS with 0.05% Tween-20 and 0.5% casein) for 10 minutes and an endogenous peroxidase block using 1.5% (volume/volume) H2O2 in TBS (pH 7.4) for 10 minutes. Primary antibody was diluted at 1:100 in blocking buffer and incubated for 1 hour at room temperature. Tissue sections were washed and detected using the Rabbit Polink-2 staining system (Golden Bridge International) according to the manufacturer's recommendations. Sections were developed with Impact 3,3′-diaminobenzidine (Vector Laboratories), counterstained with hematoxylin, and mounted in Permount (Fisher Scientific). All stained slides were scanned at 200× magnification using the ScanScope CS System (Aperio Technologies). Representative high magnification (200×) images were acquired from these whole tissue scans.
IL-21 in vivo administration
Rhesus recombinant IL-21–Fc fusion protein was produced in the Drosophila S2 system by the Resource for Nonhuman Primate Immune Reagents at Emory University with rmamuIL-21 fused to a macaque IgG2 Fc mutated to prevent binding to complement or Fc receptors.36 Three chronically SIV-infected RMs (day 380 after infection) were given 5 weekly doses of IL-21, 50 μg/kg, subcutaneously. Blood was collected 3 days after the last IL-21 dose, and the levels of Th17 cells determined by intracellular cytokine staining.
Statistical analysis
Based on the distribution of our samples, parametric t test or nonparametric Mann-Whitney test was performed to determine the significance of changes between groups. Associations between 2 parameters in the same group were assessed with the Pearson or Spearman rank correlation, based on sample distribution. All analyses were performed using Prism Version 5.0d software (GraphPad). P < .05 was considered significant.
Results
Characterization of IL-21–producing cells in SIV-uninfected RMs and SMs
The phenotype and functions of IL-21–producing cells in nonhuman primates that are natural or non-natural hosts for SIV are currently unknown. In this study, we initially determined the phenotype and frequency of IL-21–producing cells in the peripheral blood (PB) of SIV-uninfected RMs and SMs after phorbol myristate acetate/ionomycin in vitro stimulation. In both species, the CD3+CD4+ T cells were the predominant cell lineage that synthesized IL-21 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and virtually all IL-21–producing CD4+ T cells expressed CD95, a marker of memory T cells (data not shown). Of note, the percentage of cells able to produce IL-17 or IFN-γ was significantly higher within CD4+IL-21+ T cells than in their CD4+IL-21− counterpart (supplemental Figure 1).
In addition to their “signature” cytokines IL-17 and IL-22, Th17 cells may produce IL-21.37 Because Th17 cells are significantly depleted in pathogenic lentiviral infections, we determined what percentage of the total CD4+IL-21+ T cells are Th17 cells by costaining for IL-21 and IL-17. On average, < 15% of the CD4+IL-21+ T cells are IL-17+ in the PB of both RMs and SMs (Figure 1A). We next determined the fraction of IL-21−IL-17+, IL-21+IL-17−, or IL-21+IL-17+ CD4+ T cells that express the chemokine receptor CCR6 and the integrin α4β7 (determined using β7 as a surrogate for α4β7 expression38 ). These 2 receptors are critical for intestinal homing of circulating T cells and are expressed by a large fraction of Th17 cells in humans and RMs.39-41 Interestingly, in both RMs (Figure 1B) and SMs (data not shown), CD4+IL-21+IL-17− T cells appear to be distinct from IL-17–producing cells in terms of expression of these homing receptors. As shown in the graph and representative flow cytometry plot of Figure 1B, ∼ 90% of the Th17 cells (both IL-17+ and IL-17+IL-21+) in RMs express CCR6 (alone or in combination with β7), whereas less than 25% of CD4+IL-21+IL-17− T cells are CCR6+ (P = .0079). Indeed, CD4+IL-21+IL-17− T cells are significantly enriched in the CCR6−β7− subset compared with IL-17–producing cells (62% vs 4.3%; P = .0079). Taken together, these data identify memory CD3+CD4+CD95+CCR6− T cells as major producers of IL-21 in both RMs and SMs and indicate that the majority of IL-21–producing CD4+ T cells are not Th17 cells.
Phenotype of IL-21–producing cells in SIV-uninfected nonhuman primates. Characterization of IL-21–producing cells in the PB of SIV-uninfected RMs and SMs. (A) The large majority of CD4+IL-21+ T cells are not Th17 cells. Dot plot shows IL-21 by IL-17 staining in CD4+ T cells from a representative RM. The graph shows the fractions of total CD4+IL-21+ that are IL-17+ in PB of all RMs and SMs. (B) Fraction of IL-21−IL-17+, IL-21+IL-17−, or IL-21+IL-17+ CD4+ T cells expressing the chemokine receptors CCR6 and α4β7. Dot plots show CCR6 by integrin β7 staining in the 3 populations from a representative RM. The graph shows the percentages of IL-21−IL-17+, IL-21+IL-17−, or IL-21+IL-17+ CD4+ T cells expressing CCR6 and/or β7 in all RMs.
Phenotype of IL-21–producing cells in SIV-uninfected nonhuman primates. Characterization of IL-21–producing cells in the PB of SIV-uninfected RMs and SMs. (A) The large majority of CD4+IL-21+ T cells are not Th17 cells. Dot plot shows IL-21 by IL-17 staining in CD4+ T cells from a representative RM. The graph shows the fractions of total CD4+IL-21+ that are IL-17+ in PB of all RMs and SMs. (B) Fraction of IL-21−IL-17+, IL-21+IL-17−, or IL-21+IL-17+ CD4+ T cells expressing the chemokine receptors CCR6 and α4β7. Dot plots show CCR6 by integrin β7 staining in the 3 populations from a representative RM. The graph shows the percentages of IL-21−IL-17+, IL-21+IL-17−, or IL-21+IL-17+ CD4+ T cells expressing CCR6 and/or β7 in all RMs.
Quantification of blood and intestinal IL-21–producing CD4+ cells in SIV-uninfected RMs and SMs
As shown in Figure 1, IL-21–producing CD4+ T cells can be divided in 2 subsets: those producing IL-21 but not IL-17 (IL-21+IL-17−) and those producing both cytokines (IL-21+IL-17+). We next assessed the levels of these 2 subsets of CD4+IL-21+ T cells in PB and RB of SIV-uninfected RMs and SMs. In both species, the levels of circulating IL-21+IL-17− T cells were significantly higher in CD4+ (mean ± SD; RMs: 14.20 ± 6.13; SMs: 7.88 ± 3.35, P < .0001) than CD8+ (RMs: 2.57 ± 0.96; SMs: 1.93 ± 1.28) T cells (Figure 2A). Similar differences were found for the percentages of IL-21+IL-17+ T cells, which were significantly higher in CD4+ (RMs: 2.48 ± 1.06, P < .0001; SMs: 1.68 ± 3.35, P = .0018) than in CD8+ T cells (virtually undetectable in both species). Of note, the percentage of peripheral CD4+IL-21+IL-17− T cells was significantly higher (P < .0028) in SIV-uninfected RMs than in SIV-uninfected SMs, suggesting that the RM immune system may be more dependent on IL-21 availability. As the RMs included in the study were younger than the SMs, it is also possible that the observed difference is a consequence of an as yet unreported age-related decline of IL-21–producing CD4+ T cells. Studies in RB samples provided similar results, with the fraction of intestinal CD4+IL-21+IL-17− (RMs: 14.76 ± 6.12; SMs: 12.64 ± 5.63) and CD4+IL-21+IL-17+ (RMs: 2.93 ± 1.76; SMs: 2.96 ± 1.35) being significantly higher than those of CD8+IL-21+IL-17− (RMs: 2.84 ± 0.74; SMs: 4.27 ± 2.48) and CD8+IL-21+IL-17+ (RMs: 0.61 ± 0.38; SMs: 0.22 ± 0.15) T cells (Figure 2B). In contrast with data on PB samples, the fraction of CD4+IL-21+IL-17− T cells in the GI tract was not significantly different between SIV-uninfected RMs and SMs. Finally, we compared the frequency of IL-21–producing CD4+ T cells in PB and RB of each individual animal. In RMs (Figure 2C) and SMs (data not shown), the frequencies of CD4+IL-21+IL-17− (P = .0331) and CD4+IL-21+IL-17+ (P = .0439) in PB directly correlated with those in RB, suggesting that related homeostatic mechanisms may be acting in both compartments. In conclusion, these results indicate that, in SIV-uninfected RMs and SMs, IL-21–producing CD4+ T cells are distinct from Th17 cells and are present at relatively high frequencies in both blood and the intestinal mucosa.
Levels of blood and intestinal IL-21–producing cells in SIV-uninfected nonhuman primates. The percentages of CD4+ (●) or CD8+ ( ) T cells producing IL-21 but not IL-17 (IL-21+IL-17−) or both cytokines (IL-21+IL-17+) after in vitro stimulation were quantified in PB (A) and RB (B) of healthy, SIV-uninfected RMs (left panels) and SMs (right panels). (C) The percentages of CD4+IL-21+IL-17− (left panel) and CD4+IL-21+IL-17+ (right panel) T cells in PB were compared with those in RB in healthy, SIV-uninfected RMs. Statistical analyses were performed to compare the fraction of IL-21+IL-17− or IL-21+IL-17+ between CD4+ and CD8+ T cells inside each species (A-B) and to correlate the percentages of CD4+IL-21+IL-17− or CD4+IL-21+IL-17+ between PB and RB (C).
) T cells producing IL-21 but not IL-17 (IL-21+IL-17−) or both cytokines (IL-21+IL-17+) after in vitro stimulation were quantified in PB (A) and RB (B) of healthy, SIV-uninfected RMs (left panels) and SMs (right panels). (C) The percentages of CD4+IL-21+IL-17− (left panel) and CD4+IL-21+IL-17+ (right panel) T cells in PB were compared with those in RB in healthy, SIV-uninfected RMs. Statistical analyses were performed to compare the fraction of IL-21+IL-17− or IL-21+IL-17+ between CD4+ and CD8+ T cells inside each species (A-B) and to correlate the percentages of CD4+IL-21+IL-17− or CD4+IL-21+IL-17+ between PB and RB (C).
Levels of blood and intestinal IL-21–producing cells in SIV-uninfected nonhuman primates. The percentages of CD4+ (●) or CD8+ ( ) T cells producing IL-21 but not IL-17 (IL-21+IL-17−) or both cytokines (IL-21+IL-17+) after in vitro stimulation were quantified in PB (A) and RB (B) of healthy, SIV-uninfected RMs (left panels) and SMs (right panels). (C) The percentages of CD4+IL-21+IL-17− (left panel) and CD4+IL-21+IL-17+ (right panel) T cells in PB were compared with those in RB in healthy, SIV-uninfected RMs. Statistical analyses were performed to compare the fraction of IL-21+IL-17− or IL-21+IL-17+ between CD4+ and CD8+ T cells inside each species (A-B) and to correlate the percentages of CD4+IL-21+IL-17− or CD4+IL-21+IL-17+ between PB and RB (C).
) T cells producing IL-21 but not IL-17 (IL-21+IL-17−) or both cytokines (IL-21+IL-17+) after in vitro stimulation were quantified in PB (A) and RB (B) of healthy, SIV-uninfected RMs (left panels) and SMs (right panels). (C) The percentages of CD4+IL-21+IL-17− (left panel) and CD4+IL-21+IL-17+ (right panel) T cells in PB were compared with those in RB in healthy, SIV-uninfected RMs. Statistical analyses were performed to compare the fraction of IL-21+IL-17− or IL-21+IL-17+ between CD4+ and CD8+ T cells inside each species (A-B) and to correlate the percentages of CD4+IL-21+IL-17− or CD4+IL-21+IL-17+ between PB and RB (C).
Severe loss of blood and intestinal IL-21–producing CD4+ T cells after SIV infection of RMs
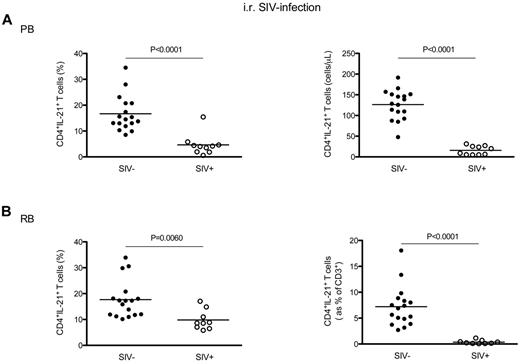
We next investigated whether and to what extent chronic SIV infection of RMs results in specific changes in the total levels of IL-21–producing CD4+ T cells. To this end, the fraction and absolute values of circulating CD4+IL-21+ T cells (without distinction between the IL-17− and IL-17+ subsets) were initially examined in 10 intrarectally SIV-infected RMs and, for comparison, in 17 uninfected animals. We observed that the percentage of CD4+IL-21+ T cells was significantly lower in SIV-infected RMs compared with uninfected animals (4.63 ± 4.10 vs 16.68 ± 6.86, P < .0001; Figure 3A left panel). The SIV-associated depletion of circulating CD4+IL-21+ T cells was even more dramatic in terms of absolute numbers, with the average number of CD4+IL-21+ T cells per microliter of blood dropping from 126.30 ± 35.54 in SIV-uninfected RM to 15.80. ± 10.65 in SIV-infected animals (P < .0001; Figure 3A right panel). We next investigated whether the loss of IL-21–producing CD4+ T cells was also present in the GI tract of SIV-infected RMs. As shown in Figure 3B (left panel), the percentage of intestinal CD4+IL-21+ T cells was significantly lower in SIV-infected RM compared with uninfected animals (9.81 ± 3.83 vs 17.70 ± 7.28, P = .0060). To further assess the SIV-associated loss of IL-21–producing CD4+ T cells in the GI tract, we next calculated the levels of CD4+IL-21+ T cells as fraction of total CD3+ T cells. This measurement accounts for the reduced fraction of IL-21–producing CD4+ T cells as well as the reduced overall frequency of intestinal CD4+ T cells after SIV infection. As shown in Figure 3B (right panel), the fraction of CD4+IL-21+ cells within the total CD3+ population was significantly lower in SIV-infected RMs compared with uninfected controls (0.36 ± 0.35 vs 7.21 ± 3.90; P < .0001). Of note, also CD4+IFN-γ+ T cells were significantly depleted in blood and GI tract of SIV-infected RMs (supplemental Figure 2). Finally, we performed a similar analysis for CD8+ T cells (supplemental Figure 3), and found that the percentage of CD8+IL-21+ T cells was overall very similar in SIV-infected and uninfected RMs, with only a slight decrease of these cells in the GI tract of SIV-infected animals (2.67 ± 1.03 vs 3.45 ± 0.86; P = .0420).
Levels of blood and intestinal CD4+IL-21+ T cells in chronic intrarectal SIV infection of RMs. (A) The percentages (left panel) and absolute numbers (cells per microliter of blood, right panel) of circulating CD4+IL-21+ T cells were compared between intrarectally SIV-infected (○) and uninfected (●) RMs. (B) The percentages (left panel) and absolute values (expressed as fraction of total CD3+ T cells, right panel) of intestinal CD4+IL-21+ T cells were compared between intrarectally SIV-infected (○) and uninfected (●) RMs. Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells between SIV-infected and uninfected animals.
Levels of blood and intestinal CD4+IL-21+ T cells in chronic intrarectal SIV infection of RMs. (A) The percentages (left panel) and absolute numbers (cells per microliter of blood, right panel) of circulating CD4+IL-21+ T cells were compared between intrarectally SIV-infected (○) and uninfected (●) RMs. (B) The percentages (left panel) and absolute values (expressed as fraction of total CD3+ T cells, right panel) of intestinal CD4+IL-21+ T cells were compared between intrarectally SIV-infected (○) and uninfected (●) RMs. Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells between SIV-infected and uninfected animals.
We next extended our study to 6 additional RMs infected with SIV intravenous and assessed before and between days 100 and 150 after infection. Consistent with our previous observations in intrarectally SIV-infected RMs, intravenously SIV-infected RMs showed a significant decrease in the absolute numbers of circulating CD4+IL-21+T cells (26.48 ± 14.00 vs 93.42 ± 39.03 cells/μL, P = .0143; Figure 4A) and in the percentage of intestinal CD4+IL-21+ cells within the total population of CD3+ T cells (0.94 ± 0.86 vs 8.86 ± 2.92 cells/μL, P = .0004; Figure 4B) compared with uninfected animals. However, the fractions of blood and intestinal CD4+IL-21+ T cells were similar between the intravenously SIV-infected and uninfected RMs (Figure 4C-D), thus indicating that, in the setting of intravenous SIV infection, the loss of IL-21–producing cells is largely the result of overall depletion of CD4+ T cells. To further confirm the reduced availability of IL-21 at mucosal sites during SIV infection of RMs, we compared the level of IL-21 mRNA in the colon and jejunum of 4 RMs before (baseline) and at 10-12 weeks after intravenous infection. This analysis revealed that SIV infection is associated with a severe reduction in the IL-21 at the mRNA level, with a fold change versus baseline of −4.75 ± 1.19 in the colon (P = .0024) and −4.27 ± 3.73 in the jejunum (P = .0664) (Figure 4E). Finally, we assessed IL-21 production in the large intestine at the protein level by immunohistochemistry in 3 SIV-uninfected and 5 intravenously SIV-infected RMs. Consistent with the flow cytometry data showing significant loss of CD4+IL-21+IL-17− cells within the total population of intestinal CD3+ T cells and the mRNA data from different gut compartments, SIV-infected RMs consistently showed a reduced number of intestinal IL-21+ cells compared with uninfected animals (Figure 4F).
Levels of blood and intestinal CD4+IL-21+ T cells in chronic intravenous SIV infection of RMs. The absolute values (A-B) and percentages (C-D) of circulating (A,C) and intestinal (B,D) CD4+IL-21+ T cells were compared between intravenously SIV-infected (○) and uninfected (●) RMs. (D) In RBs, the absolute values of IL-21–producing CD4+ T cells are expressed as fraction of total CD3+ T cells. (E) Levels of IL-21 mRNA in the colon and jejunum of 4 RMs before (baseline) and at 10-12 weeks after SIV infection. IL-21 mRNA values are expressed relative to the values for GAPDH, and the ratios obtained with the baseline values were used to calculate the fold increases. (F) IL-21 protein levels were assessed in the colon by immunohistochemistry. IL-21+ cells within the lamina propria (top panel) as well as follicular aggregates (bottom panel) are shown in 2 (of 3) representative SIV-uninfected and 2 (of 5) representative SIV-infected RMs. Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells (A-D) and of IL-21 mRNA (E) between SIV-infected and uninfected animals.
Levels of blood and intestinal CD4+IL-21+ T cells in chronic intravenous SIV infection of RMs. The absolute values (A-B) and percentages (C-D) of circulating (A,C) and intestinal (B,D) CD4+IL-21+ T cells were compared between intravenously SIV-infected (○) and uninfected (●) RMs. (D) In RBs, the absolute values of IL-21–producing CD4+ T cells are expressed as fraction of total CD3+ T cells. (E) Levels of IL-21 mRNA in the colon and jejunum of 4 RMs before (baseline) and at 10-12 weeks after SIV infection. IL-21 mRNA values are expressed relative to the values for GAPDH, and the ratios obtained with the baseline values were used to calculate the fold increases. (F) IL-21 protein levels were assessed in the colon by immunohistochemistry. IL-21+ cells within the lamina propria (top panel) as well as follicular aggregates (bottom panel) are shown in 2 (of 3) representative SIV-uninfected and 2 (of 5) representative SIV-infected RMs. Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells (A-D) and of IL-21 mRNA (E) between SIV-infected and uninfected animals.
Collectively, these data show that chronic SIV infection of RMs is associated with a significant loss of IL-21–producing CD4+ T cells in both PB and GI tract.
RM splenic CD4+IL-21+ T cells are not preferentially infected by SIV in vivo
In an effort to determine whether CD4+IL-21+ T cells were preferentially infected by SIV, we then estimated the relative in vivo frequencies of infection of highly purified (> 95%) live, memory (CD95+), CD3+CD4+CD8− T cells that produce IL-21, IFN-γ or TNF-α after phorbol myristate acetate/ionomycin in vitro stimulation (supplemental Figure 4). We used splenic cells isolated from 4 chronically SIV-infected RMs for these studies. The in vivo frequencies of SIV infection of the sorted populations were determined by quantitative PCR as the fraction of cells expressing SIVgag DNA.39 Although CD4+IL-21+ T cells can be infected by SIV in vivo, their frequency of infection is not higher than that of memory CD4+ T cells producing TNF-α or IFN-γ (Figure 5).
In vivo frequency of infection of splenic CD4+IL-21+ T cells in SIV-infected RMs. CD4+ T cells that produce IL-21, TNF-α, or IFN-γ after in vitro stimulation were sorted by flow cytometry from the spleen of 4 SIV-infected RMs. Cells were initially gated based on light scatter, followed by positive staining for CD3 without binding to the dead cell dye, and then for CD4 (without CD8 staining). Memory CD4+ T cells, gated based on characteristic expression patterns of CD28 and CD95, were then divided based on the production of IL-21, TNF-α, or IFN-γ (as shown in supplemental Figure 3). The infection frequencies of the highly purified splenic CD4+IL-21+, CD4+TNF-α+, and CD4+IFN-γ+ T cells were then determined by quantitative PCR for SIVgag DNA.
In vivo frequency of infection of splenic CD4+IL-21+ T cells in SIV-infected RMs. CD4+ T cells that produce IL-21, TNF-α, or IFN-γ after in vitro stimulation were sorted by flow cytometry from the spleen of 4 SIV-infected RMs. Cells were initially gated based on light scatter, followed by positive staining for CD3 without binding to the dead cell dye, and then for CD4 (without CD8 staining). Memory CD4+ T cells, gated based on characteristic expression patterns of CD28 and CD95, were then divided based on the production of IL-21, TNF-α, or IFN-γ (as shown in supplemental Figure 3). The infection frequencies of the highly purified splenic CD4+IL-21+, CD4+TNF-α+, and CD4+IFN-γ+ T cells were then determined by quantitative PCR for SIVgag DNA.
Loss of CD4+IL-21+ T cells is associated with depletion of Th17 cells, increased T-cell proliferation and impaired overall CD4+ T-cell homeostasis in SIV-infected RMs
To further evaluate the pathologic role of CD4+IL-21+ T-cell depletion, we next investigated potential correlations between the levels of CD4+IL-21+ T cells and the key virological and immunologic markers of disease progression in our population of SIV-infected and uninfected RMs. Consistent with the known role of IL-21 in promoting Th17 differentiation, the absolute levels of CD4+IL-21+ T cells in blood (expressed as number per microliter) significantly correlated with the fraction (Figure 6A) and absolute number (data not shown) of circulating Th17 cells (P < .0001 for both measures). Similar results were found in rectal mucosa, with the absolute levels of CD4+IL-21+ T cells (expressed as percentage of total CD3+) significantly correlated with the fraction (Figure 6B) and absolute value (data not shown) of intestinal Th17 cells (P < .0001 for both measures). Of note, these correlations in blood (P = .0127) and RB (P = .0028) remained statistically significant when only SIV-infected RMs were included in the analysis (data not shown). Furthermore, the percentage of circulating CD4+IL-21+ T cells was directly correlated to the percentage of CD4+ T cells (P = .0067; Figure 6C) and inversely correlated to the percentages of proliferating (Ki-67+) CD8+ T cells (P = .0003; Figure 6D) when both SIV-infected and uninfected RMs were included in the analysis. Of note, the correlation between CD4+IL-21+ T cells and CD8+Ki-67+ T cells remained significant when only SIV-infected animals were examined (P = .03). At the level of intestinal mucosa, we found a significant correlation between the percentages of CD4+IL-21+ T cells and those of intestinal CD4+ (P = .0063; data not shown), CD4+Ki-67+ (P < .0001; Figure 6E), and CD8+Ki-67+ (P = .0131; Figure 6F) T cells. No significant correlations were found in either blood or intestinal mucosa between CD4+IL-21+, CD4+Ki-67+, or CD8+Ki-67+ T cells and plasma viral load. To further explore the association between IL-21 production and preservation of Th17 cells, we performed a proof-of-concept study in which 3 chronically SIV-infected RMs (day 380 after infection) were treated in vivo with IL-21 (5 weekly doses of 50 μg/kg). Consistent with a role for IL-21 in promoting Th17 cell homeostasis, the frequencies of circulating IL-17–producing CD4+ T cells, and particularly those of IL-17+IL-21+ CD4+ T cells, were consistently higher after IL-21 treatment compared with baseline (Figure 6G).
Loss of CD4+IL-21+ T cells is associated with preferential depletion of Th17 cells and increased T-cell proliferation in chronically SIV-infected RMs. (A) Positive correlation between the absolute level of CD4+IL-21+ T cells (expressed as number per microliter) and the percentages of Th17 cells in blood. (B) Positive correlation between the absolute levels of intestinal CD4+IL-21+ T cells in rectal mucosa (expressed as percentage of total CD3+) and the percentages of Th17 cells in rectal mucosa. In blood, the percentage of CD4+IL-21+ T cells correlated directly with the percentage of CD4+ T cells (C) and inversely with the percentage of proliferating (Ki-67+) CD8+ T cells (D). At the level of intestinal mucosa, the percentage of CD4+IL-21+ T cells correlated negatively with the percentage of intestinal CD4+Ki-67+ (E) and CD8+Ki-67+ (F) T cells. (G) Three chronically SIV-infected RMs (day 380 after infection) were treated in vivo with 5 weekly doses of IL-21. Percentages of CD4+IL-17+ (left panel) and CD4+IL-17+IL-21+ (right panel) T cells were compared before and after IL-21 treatment. Numbers close to the symbols indicate fold increase compared to baseline.
Loss of CD4+IL-21+ T cells is associated with preferential depletion of Th17 cells and increased T-cell proliferation in chronically SIV-infected RMs. (A) Positive correlation between the absolute level of CD4+IL-21+ T cells (expressed as number per microliter) and the percentages of Th17 cells in blood. (B) Positive correlation between the absolute levels of intestinal CD4+IL-21+ T cells in rectal mucosa (expressed as percentage of total CD3+) and the percentages of Th17 cells in rectal mucosa. In blood, the percentage of CD4+IL-21+ T cells correlated directly with the percentage of CD4+ T cells (C) and inversely with the percentage of proliferating (Ki-67+) CD8+ T cells (D). At the level of intestinal mucosa, the percentage of CD4+IL-21+ T cells correlated negatively with the percentage of intestinal CD4+Ki-67+ (E) and CD8+Ki-67+ (F) T cells. (G) Three chronically SIV-infected RMs (day 380 after infection) were treated in vivo with 5 weekly doses of IL-21. Percentages of CD4+IL-17+ (left panel) and CD4+IL-17+IL-21+ (right panel) T cells were compared before and after IL-21 treatment. Numbers close to the symbols indicate fold increase compared to baseline.
Collectively, these data indicate a strong association between depletion of CD4+IL-21+ T cells and preferential loss of Th17 cells in blood and GI tract of SIV-infected RMs and suggest that loss of both cell populations contributes to the higher levels of T-cell activation and overall depletion of CD4+ T cells that is present in these animals.
Normal levels of circulating and intestinal CD4+IL-21+ T cells during nonpathogenic SIV infection of SMs
Two critical processes regulated by IL-21 (ie, Th17 cell homeostasis and maintenance of functional CD8+ T cells) are perturbed in pathogenic HIV/SIV infections of humans and RMs but preserved in nonpathogenic infection of SMs.9,42,43 As such, we hypothesized that SMs have adapted to preserve the homeostasis of IL-21–producing CD4+ T cells after SIV infection. To test this hypothesis, we investigated the levels of CD4+IL-21+ T cells in PB and RB of 16 SIV-infected and for comparison 12 uninfected SMs. In sharp contrast to the severe loss of CD4+IL-21+ T cells observed in SIV-infected RMs, SIV-infected SMs showed levels of circulating CD4+IL-21+ T cells remarkably similar to those found in uninfected animals, with no appreciable variation in the fraction (P = .9777) or absolute numbers (P = .9724) of these cells (Figure 7A). Moreover, SIV-infected SMs showed levels of intestinal CD4+IL-21+ T cells that were comparable with those found in uninfected animals (P = .6216; Figure 7B). In the current study, which included 5 SIV-infected SMs, the levels of intestinal CD4+IL-21+ T cells were not significantly different between SIV-infected and uninfected SMs (P = .1274, Figure 7B), even when expressed as fraction of total CD3+ T cells, thus accounting for the reduced level of CD4+ T cells in SIV-infected SMs.44 In addition, the levels of intestinal CD4+IL-21+ T cells were significantly higher in SIV-infected SMs (2.80 ± 1.46) than in both intrarectally (0.36 ± 0.35, P = .0004, Figure 3B) and intravenously (0.94 ± 0.86, P = .0274, Figure 4B) SIV-infected RMs.
Levels of blood and intestinal CD4+IL-21+ T cells in nonpathogenic SIV infection of SMs. The percentages (left panels) and absolute values (right panels) of circulating (A) and intestinal (B) CD4+IL-21+ T cells were compared between chronically, SIV-infected (○) and uninfected (●) SMs. In RBs, the absolute values of IL-21–producing CD4+ T cells are expressed as fraction of total CD3+ T cells (B). Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells between SIV-infected and uninfected animals.
Levels of blood and intestinal CD4+IL-21+ T cells in nonpathogenic SIV infection of SMs. The percentages (left panels) and absolute values (right panels) of circulating (A) and intestinal (B) CD4+IL-21+ T cells were compared between chronically, SIV-infected (○) and uninfected (●) SMs. In RBs, the absolute values of IL-21–producing CD4+ T cells are expressed as fraction of total CD3+ T cells (B). Statistical analyses were performed to compare the levels of CD4+IL-21+ T cells between SIV-infected and uninfected animals.
Of note, as previously shown for total and central memory CD4+ T cells,10,44,45 we found that the frequencies of intestinal CD4+IL-21+ T cells that express CCR5 is remarkably lower in SMs compared with RMs (1.41 ± 0.33 vs 38.66 ± 3.00; P < .0001; supplemental Figure 5), thus suggesting that CD4+IL-21+ T cells might be relatively resistant to SIV infection in natural host.
Discussion
In this study, we examined the frequency, phenotype, and functions of IL-21–producing cells in 2 species of nonhuman primates, SMs and RMs, which are natural and non-natural hosts for SIV, respectively. To the best of our knowledge, this is the first assessment of IL-21–producing cells in nonhuman primates before and after SIV infection. The key findings of this study are that: (1) in both RMs and SMs, memory CD4+CD95+CCR6− T cells are the main producers of IL-21; (2) the majority of CD4+IL-21+ T cells are phenotypically and functionally distinct from Th17 cells; (3) SIV infection of RMs, but not SMs, is associated with depletion of blood and intestinal CD4+IL-21+ T cells; (4) in SIV-infected RMs, the levels of CD4+IL-21+ T cells correlate with those of Th17 cells as well as with the levels of T-cell proliferation; and (5) IL-21 treatment improves the homeostasis of Th17 cells in vivo.
As expected based on human and mouse data, we found that, in these 2 species of nonhuman primates, memory CD4+ T cells are the main producers of IL-21. In addition, we observed that only a small fraction (∼ 15%) of CD4+ T cells that produce IL-21 coproduce IL-17, indicating that the large majority of CD4+IL-21+ T cells are distinct from Th17 cells. This conclusion is strengthened by the finding that, in both RMs and SMs, CD4+IL-21+ T cells also differ from Th17 cells in their expression of CCR6 and α4β7, 2 homing receptors critical for cell trafficking to the intestinal mucosa. We then investigated how SIV infection affects the levels of CD4+IL-21+ T cells. We felt that this was an important question as no data are currently available on the levels of IL-21–producing CD4+ T cells in SIV-infected nonhuman primates, and recent studies provided discordant conclusions on the levels of CD4+IL-21+ T cells in HIV-infected persons.46,47 Specifically, CD4+IL-21+ T cells were found at reduced frequencies in HIV-infected persons compared with uninfected controls in a study by Iannello et al46 but also found to be induced following infection in a second study by Yue et al.47 This discrepancy may be related to differences in the experimental protocols used48 and/or in viral load in the 2 groups of HIV-infected persons enrolled in these studies. Indeed, the findings of reduced levels of CD4+IL-21+ T cells in patients with high viral load as well as an inverse correlation between viral load and percentage of CD4+IL-21+ T cells support this view.47 A more recent study confirmed the loss of HIV-specific CD4+IL-21+ T cells in HIV-infected persons with persistent viremia and progressive disease and showed high levels of HIV-specific CD4+IL-21+ T cells in a group of HIV-1 controllers.23 Of note, these studies in HIV-infected humans largely focused on HIV-specific IL-21–producing cells, and not on the overall ability of IL-21–producing CD4+ T cells, and did not include any assessment of these cells in mucosal tissues.
Using a comparative approach, we found that CD4+IL-21+ T cells are significantly lost in blood and GI tract of SIV-infected RMs but preserved in SIV-infected SMs. In intrarectally SIV-infected RMs, both absolute numbers and percentages of IL-21–producing CD4+ T cells were significantly lower compared with uninfected animals, whereas only absolute levels of CD4+IL-21+ T cells were decreased in intravenously SIV-infected RMs. At present, the reason(s) why intrarectal, but not intravenous, SIV infection is associated with a preferential depletion of CD4+IL-21+ T cells is unknown, and a mechanistic investigation of this phenomenon is beyond the scope of the current body of work. However, the important consistent finding between both cohorts of SIV-infected RMs was the overall loss of CD4+IL21+ T cells in both blood and rectal mucosa, confirmed at both mRNA and protein levels.
As mentioned in the Introduction, IL-21 has been implicated as a key factor regulating the differentiation and maintenance of Th17 cells, a CD4+ Th subset crucial for mucosal immunity.24-27 In both humans and macaques, the HIV/SIV-induced depletion of mucosal CD4+ T cells involves preferentially Th17 cells.9,12,13,49 It is thought that Th17 depletion promotes a breakdown of the mucosal barrier, which results in the translocation of bioactive microbial products from the intestinal lumen to the systemic circulation, thus contributing to the HIV-associated chronic immune activation.14,18 In contrast, SIV-infected SMs preserve mucosal Th17 cells at frequencies comparable with those found in uninfected animals and maintain mucosal integrity and avoid microbial translocation and chronic immune activation.9,17,18 The observation that circulating and intestinal CD4+IL-21+ T cells are depleted in SIV-infected RMs, but are preserved in SIV-infected SMs, delineates a potential mechanistic link between loss of CD4+IL-21+ T cells and Th17 cell depletion in RMs, and preservations of CD4+IL-21+ T cells and of Th17 cells in SMs. The findings that (1) the levels of CD4+IL-21+ T cells directly correlated with those of Th17 cells in both blood and GI tract of SIV-infected RMs and (2) IL-21 treatment improves Th17 cell homeostasis in chronically SIV-infected RMs support this view. Because the levels of intestinal CD4+IL-21+ T cells also directly correlate with those of total intestinal CD4+ T cells and with the levels of intestinal T-cell proliferation, our data are consistent with the hypothesis that the loss of IL-21–producing CD4+ T cells is involved in 3 key phenomena (intestinal CD4+ T-cell depletion, intestinal Th17 cell depletion, and increased T-cell proliferation), which are associated with mucosal immune dysfunction and disease progression during pathogenic HIV and SIV infections.
Additional studies aimed at elucidating the observed differences in the regulation of CD4+IL-21+ T-cell homeostasis between SIV-infected RMs and SMs are needed. These comparative studies should include, for example, the determination of (1) the in vivo and in vitro frequency of infection of CD4+IL-21+ T cells, relative to other memory CD4+ T cells, in blood, gut, and lymph node; (2) the susceptibility of CD4+IL-21+ T cells to apoptosis and/or activation-induced cell death; and (3) the activation levels of the main transcription factors regulating IL-21 expression, namely, c-Maf, IRF-4, and STAT-3. It would also be important to better define the potential molecular link between CD4+IL-21+ and Th17 cells by performing longitudinal studies in which the kinetics of CD4+IL-21+ and Th17 cells are determined at the early phase of acute SIV infection, during antiretroviral therapy-mediated inhibition of virus replication, and in the phase of viral rebound that will follow antiretroviral therapy interruption. Finally, it will be important to expand our investigation of IL-21 treatment by (1) repeating the experiment in a larger number of animals, (2) starting treatment in the early infection, and (3) assessing the effects of IL-21 on Th17 cell homeostasis, immune function, and immune activation at mucosal sites. Of note, IL-21 is currently used in phase 1 and 2 clinical trials in renal cell carcinoma, melanoma, and non-Hodgkin lymphoma with an overall good safety profile,50 and a recent pilot study showed the ability of this cytokine to increase the expression of the cytotoxic molecules perforin and granzyme B in CD8+ T cells and NK cells when administered in vivo to late-stage disease SIV-infected RMs.51
In conclusion, the observation of a severe depletion of IL-21–producing CD4+ T cells during pathogenic SIV infection of RMs (but not during nonpathogenic SIV infection of SMs) defines a novel, potential mechanism involved in the SIV-associated preferential loss of Th17 cells, which is a determinant of mucosal immune dysfunction and disease progression in these animals. In addition, this study provides a rationale for further in vivo studies aimed at exploring IL-21 as a potential immune-based intervention for HIV infection and AIDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stephanie Ehnert, Christopher Souder, Tracy Meeker, and all the animal care and veterinary staff at the Yerkes Nationale Primate Research Center, and the Emory University Flow Cytometry Core as well as the Cleveland Immunopathogenesis Consortium (BBC/CLIC) for advice and helpful discussions.
This work was supported by the National Institutes of Health (NIH; grants R01-AI084836 and R56-AI087186, M.P.; grants R37-AI66998 and P01-AI 76174, G.S.), and in part by the National Cancer Institute, NIH (contract HHSN261200800001E), and the National Center for Research Resources P51RR165 (currently supported by the Office of Research Infrastructure Programs/OD P51OD11132).
The content of this publication does not necessarily reflect the official views or policies of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health or the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
National Institutes of Health
Authorship
Contribution: L.M., B.C., and Z.S.E. performed research and analyzed data; R.I.I. and E.R.-A. provided technical assistance; J.D.E. performed the IHC studies; C.V. and J.M.B. performed the frequency of infection studies; A.A.A and F.V. provided the IL-21 mRNA data; B.C., J.E., A.A.A., F.V., and S.P. contributed to design of the research; G.S., J.D.E., and J.B. contributed to design of the research and writing of the manuscript; and M.P. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mirko Paiardini, Division of Microbiology and Immunology, Yerkes National Primate Research Center, Emory University School of Medicine, 954 Gatewood Rd, Atlanta, GA 30329; e-mail: mirko.paiardini@emory.edu.
References
Author notes
L.M. and B.C. contributed equally to this study.