Abstract
Determinant factors leading from stem cells to megakaryocytes (MKs) and subsequently platelets have yet to be identified. We now report that a combination of nuclear factor erythroid–derived 2 p45 unit (p45NF-E2), Maf G, and Maf K can convert mouse fibroblast 3T3 cells and adult human dermal fibroblasts into MKs. To screen MK-inducing factors, gene expressions were compared between 3T3 cells that do not differentiate into MKs and 3T3-L1 cells known to differentiate into MKs. 3T3 cells transfected with candidate factors were cultured in a defined MK lineage induction medium. Among the tested factors, transfection with p45NF-E2/MafG/MafK lead to the highest frequency of CD41-positive cells. Adult human dermal fibroblasts transfected with these genes were cultured in MK lineage induction medium. Cultured cells had megakaryocytic features, including surface markers, ploidy, and morphology. More than 90% of MK-sized cells expressed CD41, designated induced MK (iMK). Infusion of these iMK cells into immunodeficient mice led to a time-dependent appearance of CD41-positive, platelet-sized particles. Blood samples from iMK-infused into thrombocytopenic immunodeficient mice were perfused on a collagen-coated chip, and human CD41-positive platelets were incorporated into thrombi on the chip, demonstrating their functionality. These findings demonstrate that a combination of p45NF-E2, Maf G, and Maf K is a key determinant of both megakaryopoiesis and thrombopoiesis.
Introduction
Platelets are essential for hemostatic plug formation, and platelet transfusions are widely used for patients with severe thrombocytopenia.1,2 Donor-dependent, platelet transfusions are, however, associated with practical problems, such as the limited supply because of the short storage life of platelets and the risk of bacterial infection, and with serious immune reactions. New strategies for manufacturing megakaryocytes (MKs) and subsequently platelets beginning with nondonor-dependent sources may obviate these and other platelet transfusion concerns.3
Because thrombopoietin was isolated and reported as a cytokine for primary regulation of megakaryopoiesis and thrombopoiesis,4-7 it has been used to generate enriched populations of MKs using in vitro differentiation systems. Terminally differentiated cells of the MK lineage then release platelets during thrombopoiesis.8,9 MKs and platelets have been differentiated from hematopoietic stem cells (HSCs),3 fetal liver cells,10 embryonic stem cells,11-14 and induced pluripotent stem (iPS) cells.15 Moreover, we have reported the generation of MKs and functional platelets beginning with subcutaneous adipose tissues and the preadipocyte cell line 3T3-L1.16,17 The use of HSCs as the starting material is problematic, however, because of their low capacity for in vitro expansion. For all of these various cell sources of MKs and platelets, yields are insufficient for clinical application.3,10-17 Therefore, the development of new strategies to generate platelets for transfusion is crucial.
The underlying molecular mechanisms involved in megakaryopoiesis and subsequent thrombopoiesis are only partially understood. Transcription factors as well as cytokines are involved in the lineage commitment, differentiation, and maturation of hematopoietic cells.8,9 Although the transcriptional regulation of differentiation into MK lineages has been well-studied, factors determining the induction of differentiation into MKs and platelets have yet to be identified. Identification of these factors may help to establish a new system for in vitro platelet production.
Fibroblasts are differentiated cells. Both mouse and human fibroblasts have been reprogrammed into iPS cells using 4 transcription factors.18-20 Since this development, studies to identify determinant factors for direct transdifferentiation of fibroblasts into specific cells have led to progress in both regenerative medicine and fundamental research in cell development.21-23 These reports prompted us to identify the determinant factors for MK differentiation and platelet production, based on our previous findings that MKs and platelets are generated from the preadipocyte cell line 3T3-L1, but not the fibroblast cell line 3T3, the parent cell line for 3T3-L1.17 In the present study, we first screened for MK-inducing transcription factors by comparing gene expression levels between 3T3 cells and 3T3-L1 cells, and then testing whether adult human dermal fibroblasts (HDFs) could be forced into megakaryopoiesis and subsequent platelet release by ectopic expression of candidate transcription factors.
Methods
qRT-PCR
3T3 cells and 3T3-L1 cells were maintained as described previously.17 Primary mouse low-density bone marrow mononuclear cells were obtained as described previously.24 Total RNA samples were prepared from 3T3 cells, 3T3-L1 cells, and mouse low-density bone marrow mononuclear cells using TRIzol reagent (Invitrogen). Total RNA samples also were prepared from HDFs and HDFs transfected with human transcription factors, nuclear factor erythroid–derived 2 (NF-E2) p45 unit (p45NF-E2),25-32 v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian; Maf) G31, and Maf K.31 cDNA samples were obtained by QuaniTect Reverse Transcription (QIAGEN) according to the manufacturer's protocols. Premade primers (Applied Biosystems) were used for quantitative real-time (qRT)–PCR. Gene expression levels were assessed by the threshold cycles of the target normalized to GAPDH levels.15,33
Retroviral vectors and cell culture
We prepared retroviral vectors for overexpression of the following mouse and human transcription factors: p45NF-E2, Maf G, and Maf K, as well as mouse CCAAT/enhancer binding protein (CEBP) α.34 The retroviral vectors for mouse p45NF-E2, Maf G, Maf K, and CEBPα were used for 3T3 cells. The retroviral vectors for human p45NF-E2, Maf G, and Maf K were used for HDFs. Each cDNA was subcloned into pRetroX-IRES-DsRedExpress Vector (Clontech). A CalPhos Mammalian Transfection kit (Clontech) and AmphoPack-293 cells (Clontech), as packaging cells, were used according to the manufacturer's protocol. AmphoPack-293 cells were transfected with each vector. After 48 hours of transfection, retroviral supernatants were collected. 3T3 cells infected with individual and combinations of transcription factors were cultured in maintenance medium for 3T3 cells17 for 2 days and then cultured for 8 days to differentiate into MK lineages using MK lineage induction (MKLI) medium35 composed of Iscove modified Dulbecco medium supplemented with 2mM l-glutamine, 100 U/mL penicillin G sodium, 0.1 mg/mL streptomycin sulfate, 0.5% bovine serum albumin, 4 μg/mL low-density lipoprotein cholesterol, 200 μg/mL iron-saturated transferrin, 10 μg/mL insulin, 50μM 2-β-mercaptoethanol, 20μM each nucleotide (ATP, UTP, GTP, and CTP), and 50 ng/mL thrombopoietin (a gift from Kyowa Hakko Kirin). Adult HDFs derived from the dermis of skin were purchased from Cell Applications and were maintained according to the manufacturer's protocol. HDFs infected with combinations of vectors were cultured in maintenance medium for 5 days and then cultured in MKLI medium for 12 days. Gene expression of cells at 48 hours after the infection was confirmed using qRT-PCR.
Flow cytometric analyses
Expression of cell surface markers was examined using the following directly labeled fluorescein isothiocyanate (FITC)–conjugated antibodies: anti–mouse CD41 antibody (BD Biosciences), anti–mouse CD42b antibody (EMFRET Analytics), anti–human CD41 antibody (Beckman Coulter), and anti–human CD42b antibody (Beckman Coulter). Directly labeled R-phycoerythrin (PE)–conjugated anti–human CD42b antibody (Beckman Coulter) and anti–mouse CD41 antibody (BD Biosciences) also were used. Samples to examine surface marker expression were prepared as described previously.35 Because we used a common gate for cells stained with different antibodies in the analysis, the gate was defined using unstained cells. Thus the cut-off gate for percentage of cells positive was defined using nonstained 3T3 cells and HDFs each transfected with empty vector. DNA ploidy was assessed by propidium iodide (Sigma-Aldrich) staining as described previously.35
Morphologic analyses
The ultrastructure for HDFs and p45NF-E2–, Maf G–, and Maf K–overexpressing HDFs cultured in MKLI medium was determined. These studies were done by transmission electron microscopy as described previously.36
Analyses of platelet spreading and staining
To examine the spreading of the iMK-derived platelets, these platelets were stimulated with 10μM ADP (Trinity Biotech), 10μM epinephrine (Daiichi-Sankyo), and 10μM PAR1-activating peptide (Sigma-Aldrich) simultaneously13 to spread on fibrinogen-coated glass coverslips (100 μg/mL coating concentration) into an imaging chamber.37 Imaging was obtained using an LSM710 fluorescent microscope (Carl Zeiss), and platelets were analyzed by differential interference contrast. The cells were fixed with 4% paraformaldehyde in Ca++- and Mg++-free phosphate-buffered saline for 10 minutes at room temperature and then permeabilized by 0.2% Triton X-100 in phosphate-buffered saline for 5 minutes at room temperature. The samples were stained with unlabeled anti-VWF antibody (Dako) for 60 minutes at room temperature. Samples stained with anti-VWF antibody were followed by FITC-conjugated anti–rabbit antibody. The cell samples also were stained 4,6-diamidino-2-phenylindole blue and Texas Red Phalloidin (Invitrogen). Imaging was obtained using a TCS-SP5 fluorescent microscope (Leica).
MK infusion studies
Six-week-old female immunodeficient NOD/Shi-scid/IL-2Rγnull (NOG) mice were irradiated with 2.0 Gy to induce mild thrombocytopenia.15 A week later, these NOG mice were then used for the infusion study. Large-sized cells were isolated using a 2-step density bovine serum albumin (BSA) gradient.10,38 Based on flow cytometry analysis, more than 90% of these large-sized cells, designated as induced MKs (iMKs), expressed CD41 (data not shown). We infused 5 × 105 iMKs into 20- to 23-g NOG mice. To examine whether the infused iMKs produce platelets in vivo, tail-vein blood samples were obtained from recipient NOG mice before and 5 minutes, 30 minutes, 90 minutes, 3 hours, and 6 hours after iMK infusion, and each sample was stained with FITC-conjugated anti–human CD41 antibody (Beckman Coulter) for flow cytometric analysis.
To evaluate whether the iMK-derived platelets can be incorporated into ex vivo thrombi, FITC-anti human CD41 antibody (clone SZ22)–labeled blood samples from iMK-infused mildly thrombocytopenic NOG mice 7 days after irradiation with 2.0 Gy were perfused on a type I collagen-coated chip under flow condition (1000 seconds−1) using a microchip flow-chamber system, Total Thrombus-formation Analysis System, designed to monitor platelet thrombus formation.39,40 Sodium citrate (final concentration, 3.2%) and hirudin (final concentration, 25 μg/mL) was used as anticoagulant reagents in this perfusion study.40 After perfusion for 10 minutes, the collagen-coated chip was examined by fluorescence microscopy (LSM510; Carl Zeiss) to examine iMK-derived platelets incorporation. The perfusion study was performed in the presence of 50 μg/mL (final concentration) of blocking anti–human CD42b antibody (HIP1; BD Biosciences) or an isotype control (mouse IgG1 κ; BD Biosciences).
NOG mice were purchased from the Central Institute Experimental Animals (Tokyo, Japan) and maintained in the animal care facility at Keio University. All animal experiments were performed after approval by the ethics review committee for animal experiments of Keio University.
Statistical analyses
Two-way analysis of variance with Bonferroni/Dunn test was performed to assess the difference in CD41 expression levels among 3T3 cells transfected with individual and combinations of transcription factors. Paired Student t test was used to compare the effect on CD41 expression between cells transfected with p45NF-E2, Maf G, and Maf K and cells transfected with p45NF-E2 alone and to compare CD42b expression levels between 3T3 cells transfected with an empty vector and 3T3 cells transfected with expression vectors for p45NF-E2, Maf G, and Maf K. Statistical analysis was performed using StatView (Version 5.0 for Macintosh; SAS Institute). A P value of less than .05 was considered statistically significant.
Results
Screening for MK-inducing factors
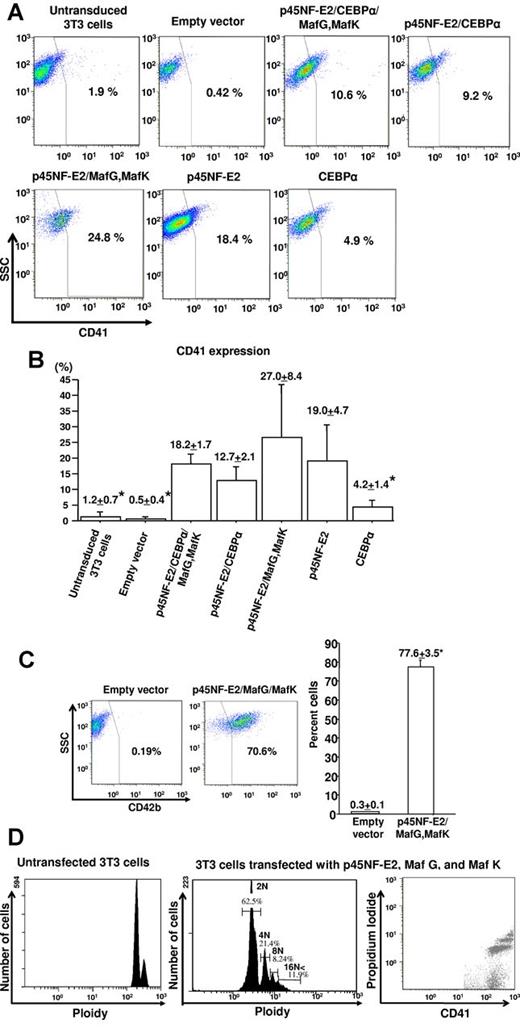
Previously, we reported that the MKs and platelets were generated from the preadipocyte cell line 3T3-L1, but not the parent fibroblast cell line 3T3.17 Gene expression of candidate transcription factors reported to regulate MK differentiation and platelet production8,9 was now compared between 3T3 cells and 3T3-L1 cells (Table 1). Determinant factors for adipocyte markers, CEBPα and peroxisome proliferator-activated receptor (PPAR)–γ, also were examined.34,41 Gene expression was assessed by threshold cycle values; thus, lower values indicate higher expression levels. Expression of p45NF-E2 and CEBPα was undetectable in 3T3 cells but detected in 3T3-L1 cells by qRT-PCR. Both cell lines expressed GATA2, FOG1, Fli1, RUNX1, and PPAR-γ, and the levels of GATA1 were undetectable (Table 1). Based on these results, we generated individual retroviruses to express mouse p45NF-E2 and its binding proteins, Maf G and Maf K,25-32 and CEBPα. The 3T3 cells transfected with different combination of these genes were differentiated into MKs. MK differentiation was assessed by CD41 expression. Representative data are shown in Figure 1A. The 3T3 cells transfected with a combination of p45NF-E2, Maf G, and Maf K had the frequency of CD41-positive cells of 27 ± 8% versus cells transfected with either CEBPα or p45NF-E2 alone (P = .003 and .19, respectively), and cells transfected with p45NF-E2 plus CEBPα (P = .028), whereas 3T3 cells transfected with vector having no insert cDNA did not react with anti–mouse CD41 antibody (Figure 1B). 3T3 cells transfected with the combination of p45NF-E2, Maf G, and Maf K had the highest frequency of CD41-positive cells. In paired Student t test, we observed that this triple combination lead to a significantly higher percentage of CD41-positive cells than transfection with p45NF-E2 alone (P = .02). Additional transfection with CEBPα along with the 3 factors did significantly further increase the number of CD41-positive cells (P = .33). Approximately 70% of p45NF-E2–, Maf G–, and Maf K–expressing cells were also positive for another MK-specific marker, CD42b (Figure 1C). Expression of CD42b on the 3T3 cells transfected with vector having no insert cDNA was not observed 8 days after culture (Figure 1C). The frequency of CD41-and CD42b-double positive cells was 27.7 ± 2.1% (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). DNA ploidy of these p45NF-E2–, Maf G–, and Maf K–expressing cells ranged from 2N to 16N (Figure 1D), the DNA ploidy of 3T3 cells on day 0 was predominantly 2N with little 4N (Figure 1D). These findings suggest that concurrent expression of p45NF-E2, Maf G, and Maf K is sufficient for the induction of polyploid MKs from 3T3 fibroblasts.
Gene expression assessed by threshold cycle values in qRT-PCR
| . | 3T3 cells . | 3T3-L1 cells . | mBMMNCs . |
|---|---|---|---|
| GATA1 | ND | ND | 8.79 ± 0.31 |
| GATA2 | 8.81 ± 0.06 | 8.08 ± 0.07 | 3.88 ± 0.37 |
| p45NF-E2 | ND | 17.91 ± 0.14 | 3.96 ± 0.01 |
| FOG1 | 9.52 ± 0.07 | 8.04 ± 0.08 | 12.00 ± 0.12 |
| Fli1 | 14.00 ± 0.2 | 13.21 ± 0.18 | 4.10 ± 0.01 |
| RUNX1 | 7.62 ± 0.07 | 8.50 ± 0.16 | 6.52 ± 0.00 |
| CEBPα | ND | 11.10 ± 0.27 | 7.04 ± 0.01 |
| PPAR-γ | 16.21 ± 0.01 | 7.14 ± 0.29 | 10.47 ± 0.61 |
| . | 3T3 cells . | 3T3-L1 cells . | mBMMNCs . |
|---|---|---|---|
| GATA1 | ND | ND | 8.79 ± 0.31 |
| GATA2 | 8.81 ± 0.06 | 8.08 ± 0.07 | 3.88 ± 0.37 |
| p45NF-E2 | ND | 17.91 ± 0.14 | 3.96 ± 0.01 |
| FOG1 | 9.52 ± 0.07 | 8.04 ± 0.08 | 12.00 ± 0.12 |
| Fli1 | 14.00 ± 0.2 | 13.21 ± 0.18 | 4.10 ± 0.01 |
| RUNX1 | 7.62 ± 0.07 | 8.50 ± 0.16 | 6.52 ± 0.00 |
| CEBPα | ND | 11.10 ± 0.27 | 7.04 ± 0.01 |
| PPAR-γ | 16.21 ± 0.01 | 7.14 ± 0.29 | 10.47 ± 0.61 |
Values (mean ± SD) are the threshold cycles of target normalized with GAPDH levels. mBMMNCs were used as a control, because expression of GATA 1 was not observed in both 3T3 cells and 3T3-L1 cells.
ND indicates not detected, that is, threshold more than 40 cycles.
Screening for MK-inducing factors. The 3T3 cells transfected with candidate transcription factors were cultured in MKLI medium. (A) Representative data of CD41 expression on 3T3 cells and 3T3 cells transfected with candidate transcription factors. (B) Mean ± SEM of percentage of cells that were CD41-positive after transfection with the empty vector or with various expression vectors (N = 3 and *P < .02 vs p45NF-E2, Maf G, and Maf K expression). (C) Representative flow data of CD42 expression on 3T3 cells transfected with either empty vector or p45NF-E2, Maf G, and Maf K vector (left). Bar graph of mean ± SEM for CD42b expression (N = 3 and *P < .02; right). (D) DNA ploidy analysis on 3T3 cells untransfected versus transfected with p45NF-E2, Maf G, and Maf K expression vector (N = 3; mean ± SEM %: 2N, 60.7 ± 2.2; 4N, 21.4 ± 3.2; 8N, 8.0 ± 0.9; and ≥ 16N, 9.6 ± 1.9).
Screening for MK-inducing factors. The 3T3 cells transfected with candidate transcription factors were cultured in MKLI medium. (A) Representative data of CD41 expression on 3T3 cells and 3T3 cells transfected with candidate transcription factors. (B) Mean ± SEM of percentage of cells that were CD41-positive after transfection with the empty vector or with various expression vectors (N = 3 and *P < .02 vs p45NF-E2, Maf G, and Maf K expression). (C) Representative flow data of CD42 expression on 3T3 cells transfected with either empty vector or p45NF-E2, Maf G, and Maf K vector (left). Bar graph of mean ± SEM for CD42b expression (N = 3 and *P < .02; right). (D) DNA ploidy analysis on 3T3 cells untransfected versus transfected with p45NF-E2, Maf G, and Maf K expression vector (N = 3; mean ± SEM %: 2N, 60.7 ± 2.2; 4N, 21.4 ± 3.2; 8N, 8.0 ± 0.9; and ≥ 16N, 9.6 ± 1.9).
p45NF-E2, Maf G, and Maf K as determinants of MK induction
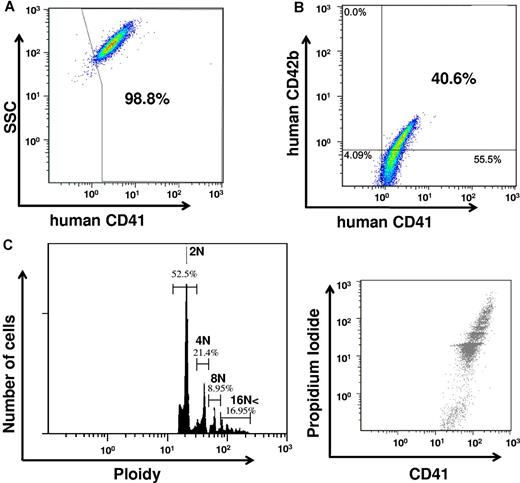
We then examined whether human adult nonhematopoietic cells could be forced to undergo megakaryopoiesis by ectopic expression of p45NF-E2, Maf G, and Maf K. Adult HDFs were transfected with these genes and then cultured in MKLI medium. We performed a 2-step BSA gradient to isolate large-sized cells10,38 and examined for MK phenotype. More than 90% of these large cells were CD41-positive (Figure 2A), with a calculated yield of 8 to 10 × 105 iMKs per 20 × 106 HDF input. Thus, ∼ 5% of HDFs were induced to become CD41-expressing MKs. Similar studies beginning with HDFs transfected with an empty vector did not result in any iMKs (supplemental Figure 2A). We examined the frequency of cells with CD41 and/or CD42b. The frequency of CD41-positive cells was 94.3 ± 3.7%. The frequency of CD42b-positive cells was 45.8 ± 7.8%. All of CD42b-positive cells were also CD41-positive (Figure 2B and supplemental Figure 2B). DNA ploidy of these p45NF-E2–, Maf G–, and Maf K–expressing cells ranged from 2N to 16N (Figure 2C), contrasting with the DNA ploidy of HDFs on day 0 that were predominantly 2N cells (supplemental Figure 2C). Furthermore, we examined gene expression of β-1-tubulin, an MK- and platelet-specific factor that is a major downstream target of p45NF-E2.42 Gene expression of β-1-tubulin as well as p45NF-E2, assessed by qRT-PCR, was detected in the iMK after 7 days of transfection, but not in HDFs (supplemental Table 1). These findings indicate that iMKs derived from HDFs transfected with human p45NF-E2, Maf G, and Maf K had intracellular as well as surface markers seen on other MK populations.
Induction of MKs from transfected adult HDFs. Adult HDFs transfected with p45NF-E2, Maf G, and Maf K were cultured in MKLI medium, and large-sized cells of the cultured cells were isolated by a 2-step BSA gradient. Representative data of CD41 (A) and CD41 and CD42b (B) expression on the large-sized cells from 3 repeats. (C) DNA ploidy analysis on HDFs transfected with p45NF-E2, Maf G, and Maf K (N = 3; mean ± SEM %: 2N, 60.7 ± 4.8; 4N, 19.5 ± 1.8; 8N, 8.7 ± 1.0; and ≥ 16N, 11.0 ± 3.0).
Induction of MKs from transfected adult HDFs. Adult HDFs transfected with p45NF-E2, Maf G, and Maf K were cultured in MKLI medium, and large-sized cells of the cultured cells were isolated by a 2-step BSA gradient. Representative data of CD41 (A) and CD41 and CD42b (B) expression on the large-sized cells from 3 repeats. (C) DNA ploidy analysis on HDFs transfected with p45NF-E2, Maf G, and Maf K (N = 3; mean ± SEM %: 2N, 60.7 ± 4.8; 4N, 19.5 ± 1.8; 8N, 8.7 ± 1.0; and ≥ 16N, 11.0 ± 3.0).
Morphologic analyses for the iMK and the iMK-derived platelets and characterization of the iMK-derived platelets
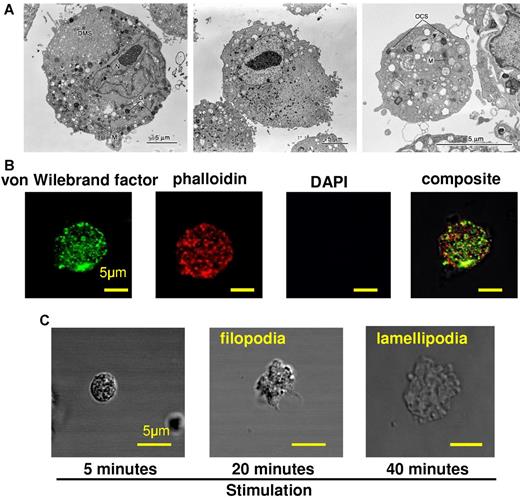
Electron microscopic observation of iMKs demonstrated typical MK organelles, including granules, a demarcation membrane system and lobulated nuclei (Figure 3A left), which differed from the cellular organization seen in HDFs before MK induction (Figure 3A middle). Electron micrograph of the iMK-derived platelet showed typical features for platelets, such as mitochondria, granules, and an open canalicular system (Figure 3A right). Granules consistent with lysosomal, dense, and α granules were seen in the iMK-derived platelet. The characteristics of the iMKs were similar to those described for MKs and platelets derived from human bone marrow CD34-positive cells and human adipose tissues.16 The iMK-derived platelets were larger (diameter, ∼ 5 μm) than peripheral platelets and thus compatible with previous reports regarding platelets produced in in vitro culture system in the absence of shear force.14,15 VWF-positive presumably α granules were observed in the iMK-derived platelets by immunostaining (Figure 3B). Isotype controls showed no positive fluorescence (supplemental Figure 3A). These immunostaining experiments were performed on iMK-derived platelets as well as peripheral platelets bound to fibrinogen-coated glass in the presence of agonists, forming filopodia and lamellipodia (Figure 3C and supplemental Figure 3B), supporting that the iMK-derived platelets were functional.
Morphologic analyses of iMKs and iMK-derived platelets and characterization of iMK-derived platelets. (A) Electron micrographs. (Left) An iMK derived from HDF cells transfected with p45NF-E2, Maf G, and Maf K. N indicates nucleus; G, granule; DMS, demarcation membrane system; and M, mitochondria. (Middle) A large adult HDF cell before the induction of MKs as in left panel. (Right) An iMK-derived platelet. M indicates mitochondria; and OCS, open canalicular system. Original magnification, ×2500. A 5-μm bar is included in each right lower corner. (B) Expression of VWF in iMK-derived platelets onto fibrinogen-coated glass in the presence of 10μM ADP, 10μM epinephrine, and 10μM PAR1-activating peptide. Pictures were taken with a 63× oil objective. A 5 μm bar is shown in each right lower corner. (C) Temporal sequence of an iMK-derived platelet spreading onto a fibrinogen-coated glass coverslip after activation. Pictures were taken with a 63× oil objective. A 5 μm bar is shown in each bottom right corner.
Morphologic analyses of iMKs and iMK-derived platelets and characterization of iMK-derived platelets. (A) Electron micrographs. (Left) An iMK derived from HDF cells transfected with p45NF-E2, Maf G, and Maf K. N indicates nucleus; G, granule; DMS, demarcation membrane system; and M, mitochondria. (Middle) A large adult HDF cell before the induction of MKs as in left panel. (Right) An iMK-derived platelet. M indicates mitochondria; and OCS, open canalicular system. Original magnification, ×2500. A 5-μm bar is included in each right lower corner. (B) Expression of VWF in iMK-derived platelets onto fibrinogen-coated glass in the presence of 10μM ADP, 10μM epinephrine, and 10μM PAR1-activating peptide. Pictures were taken with a 63× oil objective. A 5 μm bar is shown in each right lower corner. (C) Temporal sequence of an iMK-derived platelet spreading onto a fibrinogen-coated glass coverslip after activation. Pictures were taken with a 63× oil objective. A 5 μm bar is shown in each bottom right corner.
In vivo platelet release from the iMKs
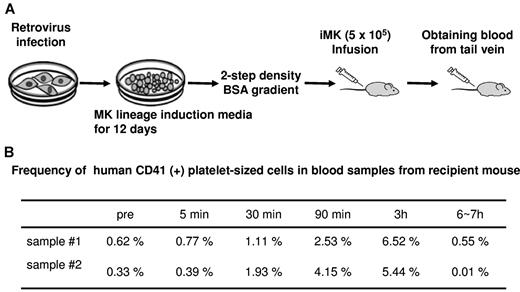
One of the hallmarks of well-developed mature MKs is the ability to undergo thrombopoiesis. To examine whether iMKs can release platelets in vivo, we infused these iMKs taking advantage of 2 observations in the recent literature: large MKs shed platelets in recipient mice10,43 and platelets derived from human MKs are most likely to survive in immunocompromised animals such as NOG mice.43 We infused 5 × 105 iMKs into NOG mice with radiation-induced thrombocytopenia (7-8 × 104/μL; Figure 4A). Tail-vein blood samples were obtained from recipient NOG mice before and after iMK infusion. The frequency of human CD41-positive platelet-sized cells increased in a time-dependent manner (Figure 4B and supplemental Figure 4). These infusion studies demonstrated that iMKs release human platelets in vivo with an estimated 5 to 10 platelets per infused iMK cell, assuming a 2-mL blood volume in the adult mice and that every infused cell released platelets.
Platelet formation in NOG mice infused with iMKs. (A) Schema of the iMK infusion study. (B) Frequency of human CD41-positive, platelet-sized cells in blood samples from recipient mice.
Platelet formation in NOG mice infused with iMKs. (A) Schema of the iMK infusion study. (B) Frequency of human CD41-positive, platelet-sized cells in blood samples from recipient mice.
iMK-derived platelets are functional
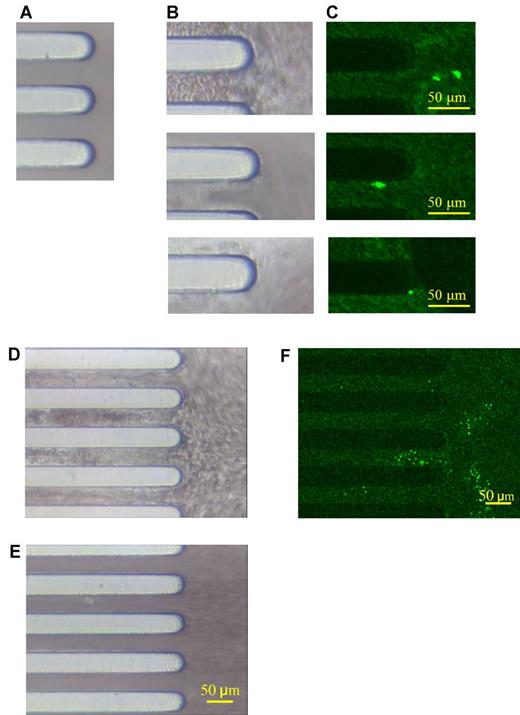
To examine whether the iMK-derived platelets are functional, we tested ex vivo thrombus formation under flow condition (Figure 5A-C). Irradiated, thrombocytopenic NOG mice with platelet counts in the range of 7 to 8 × 104/μL were infused with 5 × 105 iMKs and then phlebotomized 3 hours later. The blood samples were labeled with anti–human CD41 and then perfused on collagen-coated chips. Platelet aggregates were observed on the chip, and cells expressing human CD41 were incorporated into the thrombi on the collagen surface (Figure 5C). This incorporation of human iMK-derived platelets into thrombi was similar to that seen with 5 × 105 infused human platelets (Figure 5D-F). Thrombi were not observed using whole blood samples from irradiated and thrombocytopenic NOG mice when no infusion of human iMKs or platelets was given (Figure 5E). Based on an analysis of the blood samples pre-perfusion that had 93 ± 12) × 104 human CD41-expressing cells/mL, and the blood postperfusion that had 37 ± 5) × 104/mL, we believe that ∼ 60% of infused iMK-derived platelets were incorporated into thrombi within the collagen-coated chips.
iMK-derived platelets into thrombus formation on a collagen chip. Representative studies of FITC-anti–human CD41 antibody-labeled blood samples from iMK-infused thrombocytopenic NOG mice perfused on a collagen-coated chip under flow condition (1000 seconds−1) for 10 minutes. (A) Collagen-coated chip before perfusion. (B) Thrombi on the collagen-coated chip after the perfusion. (C) iMK-derived platelets labeled with FITC anti–human CD41 antibody are shown in bright green fluorescence and are incorporated into the thrombi. (D) Similar to panel B but after human platelets had been infused into recipient mice. (E) Whole blood samples from irradiated and thrombocytopenic NOG mice with no infused human cells were perfused on the collagen-coated chip under flow condition (1000 seconds−1) for 10 minutes. (F) Similar to panel C but for human platelets. Original magnification, ×100, and a 50 μm bar is shown in bottom right corner of images.
iMK-derived platelets into thrombus formation on a collagen chip. Representative studies of FITC-anti–human CD41 antibody-labeled blood samples from iMK-infused thrombocytopenic NOG mice perfused on a collagen-coated chip under flow condition (1000 seconds−1) for 10 minutes. (A) Collagen-coated chip before perfusion. (B) Thrombi on the collagen-coated chip after the perfusion. (C) iMK-derived platelets labeled with FITC anti–human CD41 antibody are shown in bright green fluorescence and are incorporated into the thrombi. (D) Similar to panel B but after human platelets had been infused into recipient mice. (E) Whole blood samples from irradiated and thrombocytopenic NOG mice with no infused human cells were perfused on the collagen-coated chip under flow condition (1000 seconds−1) for 10 minutes. (F) Similar to panel C but for human platelets. Original magnification, ×100, and a 50 μm bar is shown in bottom right corner of images.
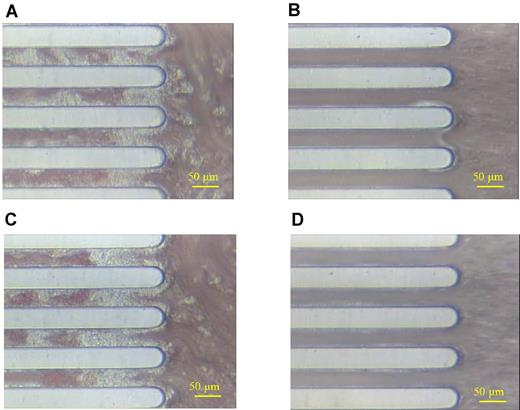
To examine whether iMK-derived platelets are passively incorporated into thrombi, the perfusion study was performed in the presence of the anti–human CD42b antibody HIP1.44 Thrombi on the collagen surface in perfusion studies with human blood were clearly observed (Figure 6A) but were absent with the inclusion of the human whole blood treated with HIP1 (Figure 6B). Thrombi were observed using human blood treated with an isotype control antibody instead of HIP1 (Figure 6C). Thrombi also formed on the chip using HIP1-treated blood from a mice infused iMK cells, but there were no human platelet-like cells incorporated (Figure 6D). These observations suggested that the iMK-derived platelets are functional, being incorporated into thrombi under flow conditions.
Active incorporation of iMK-derived human platelets into thrombi. Similar studies were done as described in Figure 5, but panels A and B are whole human blood studied on the collagen chip. Panel B has been exposed to 50 μg/mL anti–human CD42b antibody HIP1 for 20 minutes before study. (C) Similar to panel B but whole human blood has been exposed to an isotype control antibody. (D) Representative collagen chip thrombus study from NOG mice postinfusion with 5 × 105 iMKs per mouse exposed to HIP1 as in panel B. A 50 μm bar is shown in bottom right corner of each image.
Active incorporation of iMK-derived human platelets into thrombi. Similar studies were done as described in Figure 5, but panels A and B are whole human blood studied on the collagen chip. Panel B has been exposed to 50 μg/mL anti–human CD42b antibody HIP1 for 20 minutes before study. (C) Similar to panel B but whole human blood has been exposed to an isotype control antibody. (D) Representative collagen chip thrombus study from NOG mice postinfusion with 5 × 105 iMKs per mouse exposed to HIP1 as in panel B. A 50 μm bar is shown in bottom right corner of each image.
Discussion
The present study support transcription factors p45NF-E2 and its binding proteins Maf G and Maf K as determinants for megakaryopoiesis and thrombopoiesis. p45NF-E2 is a tissue-restricted subunit that forms a basic-leucine zipper heterodimeric complex with small Maf proteins that are widely expressed in many cells, a complex known as NF-E2.25-32 Although NF-E2 was originally identified in erythroid cells,25,26 p45NF-E2–deficient mice showed mild anemia but significant thrombocytopenia.32 Studies using p45NF-E2–deficient MKs suggested that the p45NF-E2 is important in terminal MK differentiation and platelet release.32 In contrast, in vitro and in vivo studies using p45NF-E2–overexpressing mouse bone marrow cells indicated that p45NF-E2 has additional roles in early megakaryopoiesis.45 The present study shows that NF-E2 drives megakaryopoiesis and thrombopoiesis from nonhematopoietic adult fibroblasts. The molecular mechanism of how iMKs are induced by the NF-E2 transcriptional complex has yet to be elucidated. Further studies are needed to elucidate the gene regulatory network by which fibroblasts are reprogrammed into iMK.
The frequency of CD41-positive cells was 94% in adult HDFs triple-transfected with p45NF-E2, Maf G, and Maf K. The frequency of CD42b-positive cells was 46%. All of CD42b-positive cells were also CD41-positive. This expression pattern was compatible with previous report.13 In contrast, triple-transfected 3T3 cells showed an unusual CD41 and CD42 expression pattern with more CD42b-positive cells than CD41: 27% were CD41-positive cells, whereas the frequency of CD42b-positive cells was 78% and double-positive cells was 27%. We observed normal expression pattern of the CD41 and CD42b expressions in primary adult bone marrow-derived mouse megakaryocytes using the antibodies used in this study (data not shown), so the antibodies used in this study works well. We propose that at least one of the components of the CD42b complex is being overexpressed in these triple-transgenic 3T3 cells but need to study this observation further.
Fibroblasts have been used to identify determinant factors for direct reprogramming into specific cells, although the use of a direct reprogramming method for regenerative medicine remained largely neglected before the establishment of iPS cells. A pioneering study by Weintraub et al in 1989 revealed that the transcription factor MyoD was sufficient for differentiation of fibroblasts into skeletal muscle cells.46 Recently, the direct reprogramming of fibroblasts into neurons,21 cardiomyocytes,22 and blood cell progenitors23 were reported. Compared with the use of iPS cells, there are some advantages of direct reprogramming for studies of specific lineage development and regenerative medicine. Direct reprogramming into specific cells does not require inducing the pluripotent state by dedifferentiation; thus, the differentiated cells can be obtained more rapidly. Also, often fewer transcription factors are needed to induce a specific cell lineage.
Platelets have been generated from human iPS cells transfected with a doxycycline-controlled c-Myc expression vector, providing 200 to 300 iPS clones from 105 HDFs.15 The 1 × 105 cultured iPS cells produced ∼ 17 × 105 MKs.15 Although it is extremely difficult to compare reprogramming efficacy between this iPS study and the present study, we obtained 8 to 10 × 105 iMKs from 20 × 106 HDFs. The number of platelets per MK was similarly low in both studies and may reflect the inefficiencies of producing human platelets by present-day strategies.
We have shown that iMK-derived platelets were detected in recipient NOG mice, and there were differences in time after infusion to peak platelet release, circulating time of released platelets, and the number of released platelets per infused MK compared with previous reports.10,47 As to peak time for platelet release, infused murine platelets were reported to result in an immediate peak count in recipient mice,10,47 whereas infused fetal liver-derived large MKs resulted in delayed platelets, with a peak at 90 minutes.10 In the present study, infused human iMK also resulted in a delay to platelet peak of 3 hours. The small difference in the delay to peak time may reflect differences in the species of MKs being infused or technical issues. Regarding the circulating time of the iMK-derived platelets, infused murine wild-type platelets showed an overall half-life of ∼ 36 hours.10 Human platelet infused into NOD/SCID mice had a reported half-life of ∼ 24 hours.47 This shorter time of circulation may in part reflect greater clearance of platelets in these mice because of the production of heterophile antibodies. Infused murine fetal liver-derived large MKs resulted in the release of platelets with an estimated half-life of ∼ 20 hours.10 Infused HDF-derived iMK had a circulating time of 6 to 7 hours in recipient NOG mice in our hands and infused human platelets had a half-life of 5 hours (data not shown). Clearly, these shorter platelet half-lives infused into NOG mice might reflect pre-existing antibodies in these immunodeficient mice to human platelets. Studies to further inhibit the immune response in the NOG mice, perhaps by suppressing macrophage functionality,48 might extend the half-life of the human platelets. At the same time, human platelets have 3 times the diameter of mice platelets and may not tolerate the high flow velocity in this small mammal.49,50 Why there were fewer iMK-derived platelets per infused cell than in that previously reported is unclear but may relate to the lower ploidy of the human MKs versus mice, the shorter half-life of the derived platelets, or poorer capacity of human MKs to shed platelets in the murine pulmonary bed than murine MKs. Clearly, too, the volume of a human platelet is 5- to 10-fold that of a mouse platelet, and this also may affect the number of platelets that can be shed per MK. Future studies will have to define megakaryopoiesis and thrombopoiesis differences based on species studied or based on the origin of the starting cells.
Finally, further studies of how p45NF-E2 can drive megakaryopoiesis and thrombopoiesis may provide insight into how these terminal differentiation events are controlled. On the practical side, these studies suggest that HDF-derived MKs may have clinical utility. HDFs transfected with p45NF-E2, Maf G, and Maf K can proliferate in culture using maintain media and then induced to become MKs. These cells may then be used to produce a large number of platelets in vitro or after infusion of the MKs into a patient. Clearly, additional studies to optimize the production of functional platelets from such cell must be done before carrying these studies to patient application.
In summary, MK-inducible factors were screened based on differences in gene expression between 3T3-L1 that can differentiate into MKs, and the parental human cell line 3T3 fibroblasts that do not differentiate into MKs. Transfecting 3T3 cells with 3 transcription factors, p45NF-E2, Maf G, and Maf K, lead to the differentiation of these cells into MKs. Adult HDF cells transfected with p45NF-E2, Maf G, and Maf K also produced MKs and were shown to be able to release functional platelets. Thus, these 3 transcription factors are sufficient to lead to platelet formation from nonhematopoietic adult fibroblasts derived from 2 different species. The generation of iMKs from fibroblasts could have important implications for studying the mechanisms of MK differentiation and platelet production and for developing of a new system for in vitro platelet production for clinical application.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Kazuya Hosokawa and Tomoko Ohnishi (Research Institute, Fujimori Kogyo) for critical advice in the perfusion study and Atsuko Igari and Kabuto Iida for technical assistance in the murine studies.
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grants-in-Aid 21591250 (to Y.M.) and 22659183 (to Y.I. and Y.M.); the Japanese Ministry of Health Labor and Welfare for intractable diseases on blood coagulation abnormalities (to M.M.); and National Institutes of Health grants U01HL099656 and P30DK090969 (to M.P.).
National Institutes of Health
Authorship
Contribution: Y.O. and Y.W. performed the described studies and interpreted data; H.S. performed the morphologic analysis with transmission electron microscopy; S.O. interpreted data and provided critical suggestions; Y.I. analyzed and interpreted data and prepared the manuscript; M.M. analyzed and interpreted data and prepared the manuscript; M.P. advised on study design, interpreted data, and prepared the manuscript; and Y.M. designed and supervised the study and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yumiko Matsubara, Department of Laboratory Medicine, Keio University School of Medicine, 35 Shina-nomachi, Shinjuku-ku, Tokyo, Japan, 160-8582; e-mail: yumikoma@sc.itc.keio.ac.jp.