Abstract
Natural killer (NK) cells secrete lytic granules to directly kill virus-infected or transformed cells and secrete cytokines to communicate with other cells. Three-dimensional super-resolved images of F-actin, lytic granules, and IFN-γ in primary human NK cells stimulated through different activating receptors reveal that both IFN-γ and lytic granules accumulated in domains where the periodicity of the cortical actin mesh at the synapse opened up to be penetrable. Ligation of some activating receptors alone (eg, CD16 or NKG2D) was sufficient to increase the periodicity of the actin mesh, but surprisingly, ligation of others (eg, NKp46 or CD2) was not sufficient to induce cortical actin remodeling unless LFA-1 was coligated. Importantly, influenza virus particles that can be recognized by NK cells similarly did not open the actin mesh but could if LFA-1 was coligated. This leads us to propose that immune cells using germline-encoded receptors to directly recognize foreign proteins can use integrin recognition to differentiate between free pathogens and pathogen-infected cells that will both be present in blood. This distinction would not be required for NK cell receptors, such as NKG2D, which recognize host cell–encoded proteins that can only be found on diseased cells and not pathogens.
Introduction
Natural killer (NK) cells are innate lymphocytes whose responses are controlled through the balance of signals from germline-encoded activating and inhibitory receptors.1 Integration of signals occurs across a structured interface, termed the immune synapse, between NK cells and target cells.2,3 The spatial and temporal organization of the synapse is important for coordinating interactions between immune cell receptors, kinases, phosphatases, and adaptors as well as for directing the interaction between cells.4-6 When signals downstream of activating receptors dominate, a cytolytic NK cell synapse can be assembled across which lytic molecules are secreted toward the target cell.7-9
One of the best characterized NK cell activating receptors is NKG2D, which recognizes stress-induced ligands, such as MHC class I chain-related protein A (MICA), and triggers NK cells to stop migrating, spread symmetrically, and activate cytoskeletal reorganization.8,10,11 Super-resolution microscopy revealed that remodeling of cortical actin occurs in domains within the central region of the synapse establishing secretory domains where lytic granules dock.12,13 It is also established that cytokines can be secreted directionally across immune synapses.14 In particular, IFN-γ has been demonstrated to be directionally secreted in vitro from T cells to antigen-presenting cells and in vivo between T cells and brain cells.15-18
NK cell–mediated IFN-γ secretion is important for shaping the Th1 immune response, modulating dendritic cell and macrophage activation and stimulating antiproliferative effects in virus-infected or transformed cells.19-22 NK cell expression of IFN-γ can be induced after exposure to stimulatory cytokines (eg, IL-12 and IL-18) or through engagement of activating receptors (eg, NKG2D).23-25 There is evidence that assembly of a structured synapse occurs for directed secretion of cytokine by NK cells, but the relationship between formation of the synapse and cytokine secretion has been little studied.22,26 It is not known, for example, whether or not cortical actin remodeling occurs at the synapse for cytokine secretion. To address this here, we used super-resolution imaging to study IFN-γ secretion at the NK cell synapse.
Many activating receptors can control NK cell responses.27 These include the natural cytotoxicity receptors NKp46, NKp44, and NKp30 and the Fc receptor CD16,28 which mediates antibody-dependent cellular cytotoxicity.29 The natural cytotoxicity receptors can trigger lytic activity against some, but not all, tumor cell lines, but their ligands on tumors have yet to be identified.30 There is evidence that NKp46 and NKp44 can bind the influenza virus molecule hemagglutinin.31,32 Mice with genetic differences that include a lack of NKp46 surface expression can fare better or worse with influenza infection,33,34 and so it is likely that further details remain to be uncovered. In any case, a strategy for NK cells to directly detect viral protein in mice is very well established for the cytomegalovirus protein m157, which is recognized by mouse activating NK cell receptor Ly49H.35-38 This presents the problem of understanding how immune cells expressing innate germline-encoded receptors for viral protein are able to distinguish virus-infected cells from viral particles. A cytolytic response must be directed against influenza-infected cells and not influenza viral particles. Different synergies between NK cell receptors revealed here by super-resolution microscopy provide a solution to this.
Methods
Cells and virus
Daudi transfectants were maintained in RPMI 1640 supplemented with 20% FCS, 100 μg/mL streptomycin, 100 μg/mL penicillin, 100 μg/mL l-glutamine, and 100 U/mL IL-2 (all Invitrogen; complete media). Primary human NK cells were isolated by negative magnetic selection and cultured as previously described.39 All donors were healthy and gave informed consent for their blood to be used in accordance with the Declaration of Helsinki (with ethics approved by the National Research Ethics Service; 05/Q0401/108). Unless otherwise indicated, isolated NK cells were stimulated with 150 U/mL human recombinant IL-2 (Roche Diagnostics) and experiments carried out 6 days later (cultured pNK cells). Influenza A/PR8 (H1N1) virus particles were a kind gift from O. Mandelboim (Hebrew University, Jerusalem, Israel).
Preparation of coated slides
Glass coverslips were prepared as previously described.12 mAbs and recombinant proteins were used at 3.0 μg/mL and influenza virus particles at 4000 hemagglutinin units (HU)/mL, unless otherwise indicated. mAbs to NKG2D (clone 149810, R&D Systems), CD16 (clone 3G8, BD Biosciences PharMingen), CD2 (clone RPA-2.10, BD Biosciences PharMingen), NKp46 (clone 461-G1, IgG1, a kind gift from O. Mandelboim, Hebrew University Hadassah Medical School, Jerusalem, Israel), or murine IgG1 isotype control (Jackson Immunology), recombinant MICA-Fc (R&D Systems), recombinant ICAM-1 (R&D Systems, 2.5 μg/mL) or recombinant CD58 (R&D Systems) or influenza virus particles were used.
Microscopy
IL-2–cultured pNK cells were added in complete media supplemented with 25mM HEPES to antibody-, ligand-, or virus-coated slides then fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were incubated on slides for 6 minutes before fixing unless indicated otherwise. For NK/cell target cell conjugate formation, freshly isolated or IL-2–cultured pNK cells were mixed in a 1:1 ratio with target cells for 90 minutes unless indicated otherwise. Conjugates were fixed in 4% paraformaldehyde and permeabilized with Triton X-100 (Sigma-Aldrich) before staining with anti–IFN-γ mAb (cloneB27, BD Biosciences PharMingen) followed by Alexa-488–conjugated donkey antimouse antibody (Invitrogen) and phalloidin-Atto647N (Sigma-Aldrich).
To visualize F-actin, cells were stained with 2 U/mL phalloidin–AlexaFluor-488 (Invitrogen) or phalloidin-Atto647N (Sigma-Aldrich). To visualize IFN-γ, cells were stained with anti-IFN-γ mAb (clone B27, BD Biosciences PharMingen) followed by AlexaFluor-488–conjugated donkey antimouse antibody (Invitrogen), or for super-resolution structured microscopy, cells were stained with directly labeled mAb: AlexaFluor-488–labeled IgG2B anti-IFN-γ mAb (R&D Systems) or AlexaFluor-488–labeled IgG1 anti-IFN-γ mAb (clone B27, BD Biosciences PharMingen). To visualize lytic granules, cells were stained with antiperforin mAb (clone δG9, BD Biosciences) followed by AlexaFluor-594–conjugated goat antimouse antibody (Invitrogen). Bright-field, fluorescence, and internal reflection microscopy (IRM) images were obtained with a confocal microscope (Leica SP5 RS) with a 63× water immersion lens (NA 1.2). Three-dimensional SI microscopy was performed (OMX, Applied Precision) using a 100×, 1.4 NA oil objective (Olympus).
Image analysis
Structured illumination images were analyzed using our program written in Matlab (Mathworks) as previously described.12 The area of contact between NK cells and coated surfaces was obtained using ImageJ (National Institutes of Health), and quantification of IFN-γ staining was analyzed using the ImageJ particle analysis tool. Cell symmetry was determined using our previously described program written in G (Labview Version 9, National Instruments).10
Statistical analysis
Column statistics were performed with GraphPad software (Prism Version 5). Mean values and SEM are shown unless indicated otherwise. Two sample groups were analyzed by t test. Groups larger than 2 were analyzed by 1-way ANOVA. For parametric data, a Bonferri posttest was applied, and for nonparametric data a Kruskal-Wallis test was carried out followed by Dunn multiple comparison posttest. In Figure 7, the odds ratio was calculated as follows:
Results
Ligation of NKp46 by mAb requires LFA-1 coligation to induce reorganization of cortical actin
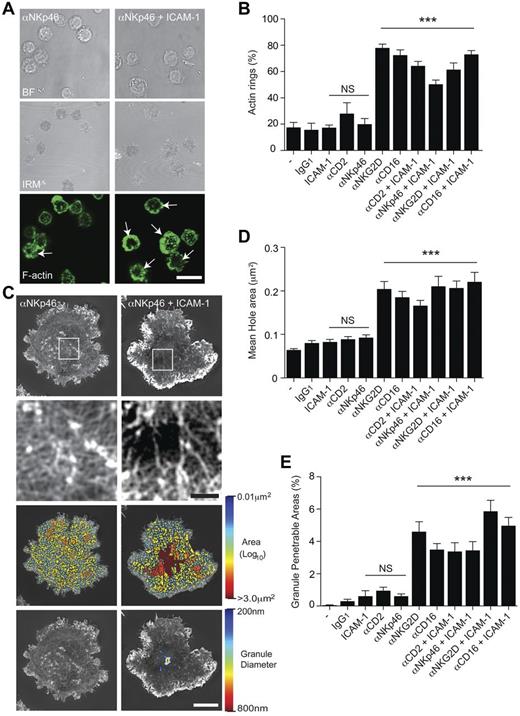
We first tested whether or not the formation of a ring of F-actin at the synapse periphery is a common early step in cytolytic synapse formation. Human primary NK cells (pNK), expanded in IL-2 but left to rest for 6 days before testing (cultured pNK cells), were stimulated on surfaces coated with monoclonal antibody (mAb) against the natural cytotoxicity receptor NKp46 (3.0 μg/mL), with or without ICAM-1 (2.5 μg/mL). pNK cells formed a distinct ring of F-actin at the synapse periphery, marked with phalloidin, considerably more frequently when slides were coated with anti-NKp46 mAb and ICAM-1 compared with anti-NKp46 mAb alone (Figure 1A). The percentage of pNK cells that formed a peripheral ring of F-actin was quantified for cells stimulated on glass surfaces coated with (3.0 μg/mL) mAb against different NK cell activating receptors, CD2, NKp46, NKG2D, or CD16, with or without costimulation of LFA-1 using (2.5 μg/mL) ICAM-1 (Figure 1B). In contrast to cells stimulated through NKG2D or the Fc receptor CD16, ligation of CD2 or NKp46 did not trigger formation of this F-actin ring. However, peripheral rings of actin were frequently formed when NK cells were stimulated on surfaces coated with mAb against CD2 or NKp46 plus the integrin ICAM-1.
Ligation of NKp46 by mAb requires coligation of LFA-1 to induce reorganization of cortical actin. (A) Bright-field, IRM, and fluorescence images of pNK cells stimulated on surfaces coated with mAb against NKp46, with or without ICAM-1. Symmetric rings of F-actin at the synapse periphery (indicated by white arrows) were frequently observed on slides coated with both αNKp46 and ICAM-1 (bottom right panel). Bar represents 20 μm. (B) The proportion of pNK cells that form a F-actin ring at the synapse periphery when stimulated on poly-L-lysine–coated surfaces (−) with isotype–matched mAb (IgG1) or activating mAbs (Isotype IgG1) against αNKG2D, αCD16, αCD2, or αNKp46, each with or without ICAM-1. Graph represents mean ± SEM, from 3 independent experiments counting 45-65 cells per condition. (C) Representative images obtained by SI microscopy of F-actin at the interface between pNK cells and surfaces coated with αNKp46 (left) or αNKp46 and ICAM-1 (right). The center of the synapse is enlarged in the panels below. Bar represents 1 μm. Third-row panels: The holes between actin filaments in the central region of the synapse as heat maps, with the smallest holes shown in blue (0.01 μm2) and largest (> 3.0 μm2) in red. Bottom panels: Regions are shown within the actin network through which a particle (such as a lytic granule) of diameter 200 nm (blue) to 800 nm (red) could fit. Bars represent 5 μm. (D) Quantification of the average size of holes in the actin mesh at the pNK synapse center for cells stimulated as for panel B. Graph represents mean ± SEM; n = 21-65 cells per condition. (E) Quantification of the proportion of the synapse penetrable by a granule of 250 nm diameter for cells stimulated as in panel B; graph represents mean ± SEM; n = 21-65 cells per condition. In all panels, data are from 3 experiments. ***P < .0001. NS indicates not significant, data compared by 1-way ANOVA.
Ligation of NKp46 by mAb requires coligation of LFA-1 to induce reorganization of cortical actin. (A) Bright-field, IRM, and fluorescence images of pNK cells stimulated on surfaces coated with mAb against NKp46, with or without ICAM-1. Symmetric rings of F-actin at the synapse periphery (indicated by white arrows) were frequently observed on slides coated with both αNKp46 and ICAM-1 (bottom right panel). Bar represents 20 μm. (B) The proportion of pNK cells that form a F-actin ring at the synapse periphery when stimulated on poly-L-lysine–coated surfaces (−) with isotype–matched mAb (IgG1) or activating mAbs (Isotype IgG1) against αNKG2D, αCD16, αCD2, or αNKp46, each with or without ICAM-1. Graph represents mean ± SEM, from 3 independent experiments counting 45-65 cells per condition. (C) Representative images obtained by SI microscopy of F-actin at the interface between pNK cells and surfaces coated with αNKp46 (left) or αNKp46 and ICAM-1 (right). The center of the synapse is enlarged in the panels below. Bar represents 1 μm. Third-row panels: The holes between actin filaments in the central region of the synapse as heat maps, with the smallest holes shown in blue (0.01 μm2) and largest (> 3.0 μm2) in red. Bottom panels: Regions are shown within the actin network through which a particle (such as a lytic granule) of diameter 200 nm (blue) to 800 nm (red) could fit. Bars represent 5 μm. (D) Quantification of the average size of holes in the actin mesh at the pNK synapse center for cells stimulated as for panel B. Graph represents mean ± SEM; n = 21-65 cells per condition. (E) Quantification of the proportion of the synapse penetrable by a granule of 250 nm diameter for cells stimulated as in panel B; graph represents mean ± SEM; n = 21-65 cells per condition. In all panels, data are from 3 experiments. ***P < .0001. NS indicates not significant, data compared by 1-way ANOVA.
To assess whether or not coligation of LFA-1 by ICAM-1 allowed NKp46 to trigger remodeling of cortical actin, super-resolution structured illumination (SI) microscopy was used to image F-actin, marked by phalloidin, in pNK cells incubated on slides coated with anti-NKp46 mAb (3.0 μg/mL) with or without ICAM-1 (Figure 1C). pNK cells stimulated on anti-NKp46 alone had a dense network of cortical branching actin across the NK cell surface interface, similar to that observed in unstimulated pNK cells. In contrast, cells stimulated with anti-NKp46 and ICAM-1 showed a dense network of F-actin at the synapse periphery and a much less dense mesh of branching F-actin in the central region of the synapse (Figure 1C top panels). Gaps between actin filaments were mapped across the immune synapse (Figure 1C third-row panels) using a custom MatLab program,12 and analyzed to reveal holes that would be predicted to be large enough to allow a particle of diameter 200-800 nm, to pass through (Figure 1C bottom panels).
These analyses were applied to cultured pNK cells from multiple donors, stimulated on surfaces coated with poly-L-lysine or poly-L-lysine with isotype-matched mAb (IgG1) or activating mAbs against CD2, NKp46, NKG2D, or CD16, all with or without ICAM-1. This revealed that, for cells stimulated with anti-CD2 or anti-NKp46 mAb, the mean area of holes between actin filaments in the central synapse were < 0.075 μm2 and not significantly different from pNK cells on surfaces coated with poly-L-lysine alone or poly-L-lysine plus control IgG1 or ICAM-1. However, the hole size was dramatically increased to > 0.15 μm2 when cells were activated by ligation of NKG2D or CD16, or when CD2 or NKp46 were coligated with LFA-1 (Figure 1D).
Importantly, very little or none of the actin mesh would be penetrable to granules with a diameter of 250 nm in pNK cells when NKp46 or CD2 were ligated unless LFA-1 was coligated (Figure 1E). When both NKp46 and LFA-1 were ligated, 3.5% ± 0.5% of the central synaptic region would be penetrable by a lytic granule of 250 nm, similar to that seen for NKG2D ligation alone (4.6% ± 0.6%). Thus, cortical actin remodeling can occur for many types of NK cell activation, which differ in their requirements for LFA-1 coligation.
Opening of the actin mesh is not augmented by increasing ligation of NKp46 or NKG2D
A simple explanation of these initial observations would be that we had not ligated enough NKp46 to trigger a rearrangement of cortical actin. The anti-NKp46 mAb used induces effective target cell lysis in standard assays of redirected lysis.40 So it certainly can activate through NKp46, and it was therefore important to determine whether higher concentrations could mediate NK cell activation and cortical actin remodeling in the absence of other target cell ligands. In addition, it was interesting to test whether or not the extent of cortical actin remodeling scaled with the amount activating receptor ligation, or if instead, the size of the region in which the actin mesh opens is fixed and it just does or does not open.
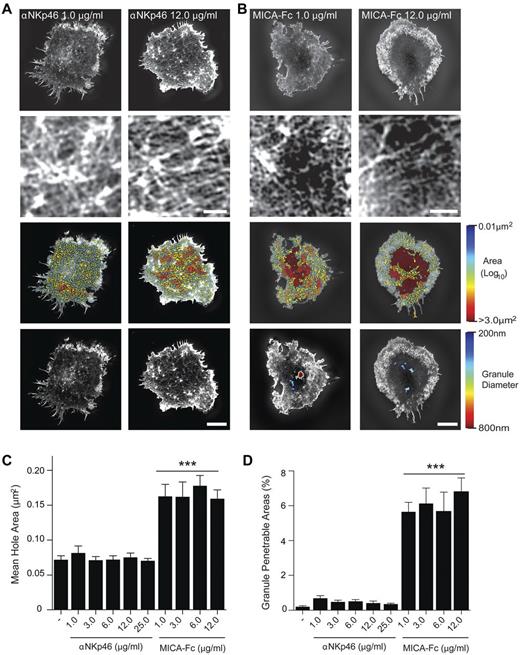
Cultured pNK cells were stimulated on surfaces coated with 1-12 μg/mL of NKp46 mAb (Figure 2A) or 1-12 μg/mL of the NKG2D ligand, MICA-Fc (Figure 2B). Cortical actin was detected by super-resolved SI imaging of F-actin. No differences in cortical actin structure could be observed between cells stimulated with 1 or 12 μg/mL of anti-NKp46 (Figure 2A bottom panels). For pNK cells stimulated on either 1 or 12 μg/mL of MICA-Fc small domains opened up in the central region of the pNK cell synapse (Figure 2B bottom panels).
Opening of the cortical actin mesh is not augmented by increasing NKG2D or NKp46 ligation. (A) Top panels: Representative SI images of F-actin in pNK cells stimulated on surfaces coated with 1.0 or 12.0 μg/mL of αNKp46 mAb. The center of the synapse is enlarged in the panels directly below. Bar represents 1 μm. Bottom center panel images: Holes within the actin mesh as heat maps related to hole area with the smallest holes shown in blue (0.01 μm2) and largest (> 3.0 μm2) shown in red. Bottom panels: The domains within the cortical F-actin mesh through which lytic granules of diameters 200 nm (blue) to 800 nm (red) could penetrate. (B) Top panels: Representative SI images of F-actin in pNK cells stimulated on surfaces coated with the NKG2D ligand MICA (1.0 μg/mL, 12.0 μg/mL). As in panel A, the center of the synapse is enlarged in the panels below. Bar represents 1 μm. Bottom middle panels: Heat maps of actin mesh hole areas. Bottom panels: The domains through which lytic granules with diameters ranging from 200 nm to 800 nm may penetrate. Bars represent 5 μm. (C) The mean area of holes within the central region of the pNK cell synapse for cells stimulated as indicated. (D) The proportion of the NK cell synapse predicted to be penetrable by a granule with a diameter of 250 nm for cells stimulated as indicated. Graphs represent mean ± SEM; n = 30 per condition using cells from 3 independent donors. ***P < .0001, compared with poly-L-lysine controls (−) by 1-way ANOVA.
Opening of the cortical actin mesh is not augmented by increasing NKG2D or NKp46 ligation. (A) Top panels: Representative SI images of F-actin in pNK cells stimulated on surfaces coated with 1.0 or 12.0 μg/mL of αNKp46 mAb. The center of the synapse is enlarged in the panels directly below. Bar represents 1 μm. Bottom center panel images: Holes within the actin mesh as heat maps related to hole area with the smallest holes shown in blue (0.01 μm2) and largest (> 3.0 μm2) shown in red. Bottom panels: The domains within the cortical F-actin mesh through which lytic granules of diameters 200 nm (blue) to 800 nm (red) could penetrate. (B) Top panels: Representative SI images of F-actin in pNK cells stimulated on surfaces coated with the NKG2D ligand MICA (1.0 μg/mL, 12.0 μg/mL). As in panel A, the center of the synapse is enlarged in the panels below. Bar represents 1 μm. Bottom middle panels: Heat maps of actin mesh hole areas. Bottom panels: The domains through which lytic granules with diameters ranging from 200 nm to 800 nm may penetrate. Bars represent 5 μm. (C) The mean area of holes within the central region of the pNK cell synapse for cells stimulated as indicated. (D) The proportion of the NK cell synapse predicted to be penetrable by a granule with a diameter of 250 nm for cells stimulated as indicated. Graphs represent mean ± SEM; n = 30 per condition using cells from 3 independent donors. ***P < .0001, compared with poly-L-lysine controls (−) by 1-way ANOVA.
Specifically, the mean hole areas between actin filaments for a range of concentrations of anti-NKp46 mAb between 1 and 25 μg/mL were < 0.075 μm2 (Figure 2C), and the proportion of the synapse predicted to be penetrable by a lytic granule of 250 nm in diameter was < 0.5% (Figure 2D). In contrast, 1.0 μg/mL MICA-Fc was sufficient to induce a significant increase in mean hole size within the central synapse (> 0.15 μm2) and led to > 5% of the synapse being predicted to be penetrable by lytic granules. Interestingly, the extent of cortical actin remodeling was not significantly changed by increasing concentrations of MICA-Fc. This is consistent with a model in which cortical actin remodeling occurs to the same extent in individual cells once a threshold for NK cell activation has been reached.
Influenza-induced NK cell spreading and F-actin polymerization requires coligation of LFA-1
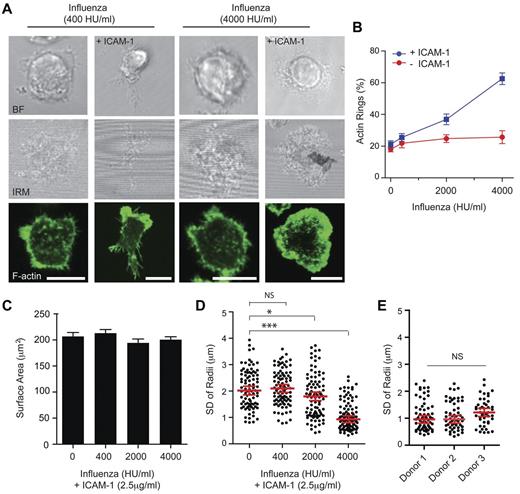
NKp46 and the other natural cytotoxicity receptor family members, NKp44 and NKp30, play a role in NK cell antiviral immunity.41 Here we tested whether or not influenza virus particles would lead to human NK cell activation and actin remodeling and whether or not this required LFA-1 coligation. Slides were coated with PR8 (H1N1) influenza virus particles (at 400 or 4000 HU/mL) and ICAM-1 (2.5 μg/mL) to determine the concentration of virus particles required to activate cultured pNK cells. Symmetric spreading and polymerization of a peripheral ring of F-actin were frequently observed when 4000 HU/mL was used but cells remained with an asymmetric morphology and a polarized distribution of F-actin when on slides coated with a lower number (400 HU/mL) of virus particles (Figure 3A). The percentage of cultured pNK cells that formed a peripheral ring of F-actin was determined after stimulation on surfaces coated with a range of influenza virus particle concentrations (0-4000 HU/mL) with or without ICAM-1 (2.5 μg/mL; Figure 3B). In the absence of ICAM-1, an increasing concentration of influenza particles had no effect on NK cell activation assessed by the frequency of symmetric spreading and F-actin ring formation. However, in the presence of coligation of LFA-1, formation of a peripheral ring of F-actin increased from 21.2% ± 2.2% to 62.5% ± 3.7%, when NK cells were stimulated with increasing concentrations of influenza virus particles (up to 4000 HU/mL).
Coligation of LFA-1 is required for influenza viral particles to trigger NK cell symmetric spreading. (A) Representative bright-field, IRM, and fluorescence images of pNK cells, stained with phalloidin–AlexaFluor-488, stimulated on surfaces coated with a low (400 HU/mL; left panels) or high (4000 HU/mL; right panels) concentrations of influenza virus particles, with or without ICAM-1. Bars represent 10 μm. (B) Graph represents the proportion of pNK cells that form a peripheral ring of F-actin when influenza virus particles were titrated onto glass surfaces coated with poly-L-lysine with or without ICAM-1. Graph represents mean ± SEM, from 4 independent experiments counting > 70 cells per condition. (C) Quantification of the pNK cell surface contact areas determined by IRM on slides coated with ICAM-1 and increasing concentrations of influenza virus particles (0-4000 HU/mL). Data are mean ± SEM for n > 300 cells per condition from 4 independent experiments. (D) The symmetry of the contact interface for cells stimulated on slides coated with ICAM-1 and increasing concentrations of influenza virus particles is represented as the SD of the radial distances. Data represent the distance from the NK cell center to the circumference measured at 360 radii for n = 87-99 cells, from 3 independent experiments. Graphs represent the median ± SE of the median for each dataset. ***P < .0001. *P < .01. NS indicates not significant, analyzed by 1-way ANOVA. (E) The SD of radial distances was compared for pNK cells, from 3 independent donors, stimulated on surfaces coated with 4000 HU/mL influenza virus particles and ICAM-1. Each data point on the graph represents the SD of radii for a single cell, and the median ± SE of the median is shown for n = 45-63 cells per donor. NS indicates not significant, analyzed by 1-way ANOVA.
Coligation of LFA-1 is required for influenza viral particles to trigger NK cell symmetric spreading. (A) Representative bright-field, IRM, and fluorescence images of pNK cells, stained with phalloidin–AlexaFluor-488, stimulated on surfaces coated with a low (400 HU/mL; left panels) or high (4000 HU/mL; right panels) concentrations of influenza virus particles, with or without ICAM-1. Bars represent 10 μm. (B) Graph represents the proportion of pNK cells that form a peripheral ring of F-actin when influenza virus particles were titrated onto glass surfaces coated with poly-L-lysine with or without ICAM-1. Graph represents mean ± SEM, from 4 independent experiments counting > 70 cells per condition. (C) Quantification of the pNK cell surface contact areas determined by IRM on slides coated with ICAM-1 and increasing concentrations of influenza virus particles (0-4000 HU/mL). Data are mean ± SEM for n > 300 cells per condition from 4 independent experiments. (D) The symmetry of the contact interface for cells stimulated on slides coated with ICAM-1 and increasing concentrations of influenza virus particles is represented as the SD of the radial distances. Data represent the distance from the NK cell center to the circumference measured at 360 radii for n = 87-99 cells, from 3 independent experiments. Graphs represent the median ± SE of the median for each dataset. ***P < .0001. *P < .01. NS indicates not significant, analyzed by 1-way ANOVA. (E) The SD of radial distances was compared for pNK cells, from 3 independent donors, stimulated on surfaces coated with 4000 HU/mL influenza virus particles and ICAM-1. Each data point on the graph represents the SD of radii for a single cell, and the median ± SE of the median is shown for n = 45-63 cells per donor. NS indicates not significant, analyzed by 1-way ANOVA.
The cell surface contact areas were similar for pNK cells stimulated on surfaces coated with ICAM-1 alone or ICAM-1 plus influenza virus particles at different concentrations (Figure 3C). However, differences in cell morphology were apparent and were revealed quantitatively by comparing distances from the cell perimeter to the centroid of the contact interface along 360 radii (Figure 3D). NK cells stimulated on ICAM-1–coated slides or slides coated with low influenza virus particle concentrations had a more asymmetric morphology (ie, higher variability in radii, 2.09 ± 0.08 μm) and NK cells stimulated on 4000 HU/mL of influenza virus particles had a more symmetric morphology (ie, low variability in radii, 0.91 ± 0.07 μm). The activation of pNK cells by influenza virus particles (4000 HU/mL) and ICAM-1 (2.5 μg/mL), as assessed by the extent of symmetric spreading, showed no significant variability across 3 different healthy donors (Figure 3E). Thus, slides coated with influenza virus particles were only able to stimulate symmetric spreading of NK cells and a peripheral ring of F-actin when LFA-1 was coligated.
Coligation of LFA-1 is required for influenza virus to trigger rearrangement of NK cell cortical actin
To test whether or not NK cell activation through different ligands would result in cortical actin remodeling, cultured pNK cells were stimulated on glass surfaces coated with influenza virus particles or CD58, both with or without ICAM-1, and F-actin was imaged using SI microscopy. For both conditions, the periodicity of cortical actin could only be observed to increase in the central region of the synapse in cells in which LFA-1 was coligated (example images shown in Figure 4A).
LFA-1 coligation is required for influenza virus to trigger rearrangement of NK cell cortical actin. (A) Top panels: SI images of F-actin in pNK cells stimulated on surfaces coated with influenza virus particles (4000 HU/mL) with or without ICAM-1 (2.5 μg/mL), or the CD2 ligand, CD58 (5.0 μg/mL), with or without ICAM-1. The center of the synapse is enlarged in the panels directly below. Bars represent 1 μm. Bottom middle panels: Holes between actin filaments are represented as heat maps with the smallest holes (0.01 μm2) shown in blue and largest (> 3.0 μm2) shown in red. Bottom panels: The domains within the actin mesh at the NK cell surface interface through which a granule of 200-800 nm in diameter could fit through. Bars represent 5 μm. (B) Surface areas of pNK cells stimulated on coverslips coated with poly-L-lysine (−) and the CD2 ligand CD58, influenza virus particles, or the NKG2D ligand MICA, all with or without ICAM-1. (C) The mean area of holes within the NK cell synapse center for NK cells stimulated as in panel B. (D) The proportion of the NK cell synapse predicted to be penetrable by a granule with a diameter of 250 nm for cells stimulated as indicated. Graphs represent mean ± SEM for n = 30-75 cells across 3 independent experiments. ***P < .0001. **P < .001. *P < .01. NS indicates not significant, analyzed by 1-way ANOVA and compared with poly-L-lysine–coated slides.
LFA-1 coligation is required for influenza virus to trigger rearrangement of NK cell cortical actin. (A) Top panels: SI images of F-actin in pNK cells stimulated on surfaces coated with influenza virus particles (4000 HU/mL) with or without ICAM-1 (2.5 μg/mL), or the CD2 ligand, CD58 (5.0 μg/mL), with or without ICAM-1. The center of the synapse is enlarged in the panels directly below. Bars represent 1 μm. Bottom middle panels: Holes between actin filaments are represented as heat maps with the smallest holes (0.01 μm2) shown in blue and largest (> 3.0 μm2) shown in red. Bottom panels: The domains within the actin mesh at the NK cell surface interface through which a granule of 200-800 nm in diameter could fit through. Bars represent 5 μm. (B) Surface areas of pNK cells stimulated on coverslips coated with poly-L-lysine (−) and the CD2 ligand CD58, influenza virus particles, or the NKG2D ligand MICA, all with or without ICAM-1. (C) The mean area of holes within the NK cell synapse center for NK cells stimulated as in panel B. (D) The proportion of the NK cell synapse predicted to be penetrable by a granule with a diameter of 250 nm for cells stimulated as indicated. Graphs represent mean ± SEM for n = 30-75 cells across 3 independent experiments. ***P < .0001. **P < .001. *P < .01. NS indicates not significant, analyzed by 1-way ANOVA and compared with poly-L-lysine–coated slides.
For quantitative analysis across multiple cells, the contact area was compared for cells on slides coated with poly-L-lysine or activated through CD58, influenza virus particles, or MICA-Fc, all with or without ICAM-1 (Figure 4B). Influenza virus particles did not increase the contact area of NK cells compared with poly-lysine controls, but an increase in contact area could be observed when LFA-1 was coligated by ICAM-1. Ligation of CD2 with CD58 could increase the cell contact area independent of LFA-1 ligation. Interestingly, ligation of LFA-1 or CD2 alone resulted in a similar NK cell surface area, 205 ± 9 μm2 and 214 ± 14 μm2, respectively, but coligation resulted in a significant increase in cell surface area, 266 ± 10 μm2, the sum of their individual effects.
Analysis of the 3D-SI microscopy images revealed that the average size of the gaps between F-actin filaments within the central synapse was not significantly changed for pNK cells stimulated on ICAM-1, 0.08 ± 0.006 μm2, CD58, 0.09 ± 0.005 μm2, or influenza virus particles, 0.1 ± 0.005 μm2, compared with unstimulated cells (Figure 4C). In contrast, regions of the actin mesh structure had opened up in cells stimulated on MICA-Fc to be on average 0.16 ± 0.01 μm2, or cells stimulated with ICAM-1 plus CD58 or influenza virus particles, with average hole sizes of 0.15 ± 0.007 μm2 and 0.17 ± 0.01 μm2, respectively. Small domains predicted to be penetrable by a granule of 250 nm in diameter only opened up across > 4.5% of the pNK cell synapse when LFA-1 was coligated with CD58 or influenza virus particles. Thus, stimulation of NK cells by influenza virus particles requires coligation of LFA-1 to mediate changes in the periodicity of cortical actin.
Polarization of IFN-γ in NK cells stimulated through NKG2D and LFA-1
The strategies NK cells use to protect against viral infection and tumor transformation are not limited to the secretion of cytolytic granules. NK cells are also a major source of chemokines and cytokines, including being a major source for IFN-γ. Unstimulated or IL-2–cultured primary human NK cells do not express detectable IFN-γ mRNA or IFN-γ protein, but expression can be induced after exposure to stimulatory cytokines (eg, IL-12 and IL-18) or through engagement of activating receptors (eg, NKG2D).23-25,42-44 It has been shown that stimulating NK cells with cross-linked NKG2D ligand or tumor cell lines expressing NKG2D ligands was sufficient to induce IFN-γ secretion by human peripheral blood NK cells.25,45,46
Thus, we set out to characterize the organization of IFN-γ protein in cultured or freshly isolated pNK cells incubated with the B-cell line Daudi, transfected to express MICA (Daudi/MICA) or transfected to express β2-microglobulin (Daudi/β2M; Figure 5A). We have previously shown that Daudi/MICA activates NK cells whereas Daudi/β2M inhibits.47 IFN-γ was not detectable in conjugates formed between cultured or freshly isolated pNK and Daudi/β2M but was detected in a proportion of conjugates between cultured or freshly isolated pNK and Daudi/MICA. The percentage of conjugates expressing IFN-γ peaked within 90 minutes of initial stimulation (Figure 5B). Interestingly, the frequency of IFN-γ positive cells was similar for cultured or freshly isolated pNK at 20.1% ± 0.3% and 22.9% ± 0.8%, respectively. IFN-γ was no longer detectable after 6 hours consistent with the majority of IFN-γ having been secreted by that time.45
Polarization of IFN-γ in NK cells stimulated through NKG2D and LFA-1. (A) Images of conjugates between cultured pNK cells and Daudi/MICA or Daudi/β2M. Cells were coincubated for 30 or 90 minutes and stained for F-actin (red) and IFN-γ (green). Bars represent 10 μm. (B) The proportion of pNK cells expressing IFN-γ in conjugates formed between fresh or cultured pNK cells and Daudi/MICA for the times indicated. Graph represents mean ± SEM from 3 experiments; n = 76-170. (C) Images of F-actin (green) and IFN-γ (red) in cultured pNK cells stimulated for 90 minutes on surfaces coated with MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL). Bar represents 20 μm. (D) The proportion of pNK cells expressing IFN-γ after stimulation as in panel D for 5-180 minutes. Graph represents mean ± SEM from 5 donors; n > 200 cells. (E) Images of cultured pNK cells stimulated as in panel E. Right panels: Orthogonal (XZ, YZ) views, perpendicular to the planes marked with dotted lines in the XY image. (F) The proportion of IFN-γ staining in 4 quadrants of the cell, denoted by their relative distance from the coverslip (0 at the coverslip, 1 the top of the cell). Graph represents data for cells incubated on surfaces coated as in panel C for 1.5 or 3 hours as mean ± SEM (n = 60 or 30). (G) The surface area of pNK cells that express IFN-γ (IFN-γ+), or are IFN-γ negative (IFN-γ−), after incubation on surfaces coated as in panel C. (H) The symmetry of pNK cells that either express IFN-γ or are IFN-γ− after stimulation on slides coated with MICA-Fc and ICAM-1 for 90 minutes is represented as the SD of the radial distances for n = 50 or 80 cells. Graphs represent mean ± SEM; n = 50-100. ***P < .0001 (t test). **P < .001 (t test).
Polarization of IFN-γ in NK cells stimulated through NKG2D and LFA-1. (A) Images of conjugates between cultured pNK cells and Daudi/MICA or Daudi/β2M. Cells were coincubated for 30 or 90 minutes and stained for F-actin (red) and IFN-γ (green). Bars represent 10 μm. (B) The proportion of pNK cells expressing IFN-γ in conjugates formed between fresh or cultured pNK cells and Daudi/MICA for the times indicated. Graph represents mean ± SEM from 3 experiments; n = 76-170. (C) Images of F-actin (green) and IFN-γ (red) in cultured pNK cells stimulated for 90 minutes on surfaces coated with MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL). Bar represents 20 μm. (D) The proportion of pNK cells expressing IFN-γ after stimulation as in panel D for 5-180 minutes. Graph represents mean ± SEM from 5 donors; n > 200 cells. (E) Images of cultured pNK cells stimulated as in panel E. Right panels: Orthogonal (XZ, YZ) views, perpendicular to the planes marked with dotted lines in the XY image. (F) The proportion of IFN-γ staining in 4 quadrants of the cell, denoted by their relative distance from the coverslip (0 at the coverslip, 1 the top of the cell). Graph represents data for cells incubated on surfaces coated as in panel C for 1.5 or 3 hours as mean ± SEM (n = 60 or 30). (G) The surface area of pNK cells that express IFN-γ (IFN-γ+), or are IFN-γ negative (IFN-γ−), after incubation on surfaces coated as in panel C. (H) The symmetry of pNK cells that either express IFN-γ or are IFN-γ− after stimulation on slides coated with MICA-Fc and ICAM-1 for 90 minutes is represented as the SD of the radial distances for n = 50 or 80 cells. Graphs represent mean ± SEM; n = 50-100. ***P < .0001 (t test). **P < .001 (t test).
Across all donors tested (n = 10), only a subset of fresh or cultured pNK cells that formed conjugates with Daudi/MICA expressed IFN-γ. Interestingly, in these IFN-γ–positive conjugates between NK cells and Daudi/MICA, only 1 of 71 target cells appeared to be lysed, as indicated by blebbing, even though Daudi/MICA have been shown to be efficiently lysed by pNK in cytotoxicity assays.12 This is consistent with a specific subset of NK cells mediating IFN-γ expression.
To test whether or not NKG2D engagement directly stimulates IFN-γ, cultured pNK cells were incubated on glass surfaces coated with MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL) and imaged by confocal microscopy (Figure 5C). Within 45 minutes, expression of IFN-γ protein could be detected in a small proportion of pNK cells, 2.2% ± 0.2% and this peaked 90 minutes after stimulation, when 18.6% ± 0.6% expressed the cytokine (Figure 5D). No detectable IFN-γ protein expression was seen in cultured pNK cells stimulated on ICAM-1 (2.5 μg/mL) for 90 or 180 minutes (data not shown).
At all times tested, IFN-γ staining was focused toward the interface formed between pNK cells and MICA-Fc– and ICAM-1–coated surfaces (Figure 5E-F). Comparison of the area of the cell surface interface formed between pNK cells and ligand-coated surfaces revealed that cells expressing IFN-γ formed a larger contact area (> 180 μm2) than cells in which no IFN-γ staining could be detected (Figure 5G). Indeed, IFN-γ+ cells had a similar surface area to that measured for cells which had spread symmetrically and form a polymerized ring of F-actin when pNK cells were activated for shorter times on MICA-Fc–coated surfaces (Figure 2C). However, analysis of cell morphology revealed that, in contrast to the symmetric spreading observed during cytolytic synapse formation, IFN-γ–expressing cells tended to have a more asymmetric morphology compared with pNK cells in which IFN-γ expression could not be detected (Figure 5H).
Taken together, these data show that a subset of pNK cells up-regulate IFN-γ protein expression after NKG2D–mediated NK cell activation, and this up-regulation is not dependent on IL-2 costimulation. Furthermore, our data show that at least a fraction, if not all, IFN-γ expressed after NKG2D and LFA-1 costimulation, is polarized to the NK cell synapse.
The cortical actin mesh opens up in pNK cells that express IFN-γ
To determine whether changes could be observed in the cortical actin structure in the subset of pNK that expressed IFN-γ, 2-color SI microscopy was used to image F-actin and IFN-γ. Cultured pNK cells were stimulated for 90 minutes on surfaces coated with ICAM-1 (2.5 μg/mL) or MICA-Fc (2.5 μg/mL) plus ICAM-1 (2.5 μg/mL), and the cell surface contact area was imaged (Figure 6A). In cells stimulated on ICAM-1, a dense network of cortical actin could be observed across the cell surface interface (Figure 6A upper panels), and IFN-γ staining was not detected. In cells stimulated on MICA-Fc and ICAM-1, in which IFN-γ could be detected, regions within the cortical actin mesh had opened up to form small domains with diameters of 200-800 nm (Figure 6A bottom panels). Measurement of these changes in actin structure revealed that the mean hole areas across the cell surface interface were significantly increased to 0.18 ± 0.007 μm2 compared with cells stimulated on ICAM-1 alone, where mean hole areas were 0.074 ± 0.004 μm2 (Figure 6B). This resulted in small domains that had diameters of > 200 nm opening in discrete regions across 5.3% ± 0.2% of the cell surface interface of cells near which IFN-γ could be detected (Figure 6C).
The cortical actin mesh opens up in NK cells expressing IFN-γ. (A) Left panels: Super-resolution images of F-actin and IFN-γ in pNK cells stimulated for 90 minutes on coverslips coated with ICAM-1 (2.5 μg/mL) or MICA-Fc (2.5 μg/mL) with ICAM-1 (2.5 μg/mL). Bars represent 5 μm. Center panels: Holes in the actin mesh as a heat map. Middle right panels: The domains in the actin mesh, which have opened up to be penetrable by a spherical particle of diameter 200-800 nm, and the regions indicated by the white square are enlarged in the far right panels. (B) Quantification of the mean areas of holes in the actin mesh for pNK cells stimulated for 90 minutes on surfaces coated as in panel A. (C) The proportion of the synapse where the actin mesh has opened up to produce domains penetrable by a particle of diameter 200-800 nm in pNK cells stimulated on surfaces coated as in panel A. Graphs represent mean ± SEM from 3 experiments; n = 30. ***P < .0001 (t test). (D) Bright-field and 2-color SI images of perforin (red) and IFN-γ (green) in pNK cells stimulated for 90 minutes on surfaces coated with MICA-Fc and ICAM-1. Bars represent 10 μm. (E) Quantification of the number of lytic granules, stained with α-perforin mAb, in the whole volume of each pNK cell after stimulation for 6 (white) or 90 minutes (black) on slides coated with poly-L-lysine or MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL). At 90 minutes, when IFN-γ could be detected, IFN-γ+ and IFN-γ− cells are plotted separately. Bars represent the median from 4 experiments; n > 30. ***P < .0001 (analyzed by 1-way ANOVA). (F) Comparison of the number of granules per NK cell for 4 independent donors whose cells were stimulated for 6 minutes on slides coated with MICA-Fc and ICAM-1. Each data point represents a single cell. Bars represent the median for each dataset.
The cortical actin mesh opens up in NK cells expressing IFN-γ. (A) Left panels: Super-resolution images of F-actin and IFN-γ in pNK cells stimulated for 90 minutes on coverslips coated with ICAM-1 (2.5 μg/mL) or MICA-Fc (2.5 μg/mL) with ICAM-1 (2.5 μg/mL). Bars represent 5 μm. Center panels: Holes in the actin mesh as a heat map. Middle right panels: The domains in the actin mesh, which have opened up to be penetrable by a spherical particle of diameter 200-800 nm, and the regions indicated by the white square are enlarged in the far right panels. (B) Quantification of the mean areas of holes in the actin mesh for pNK cells stimulated for 90 minutes on surfaces coated as in panel A. (C) The proportion of the synapse where the actin mesh has opened up to produce domains penetrable by a particle of diameter 200-800 nm in pNK cells stimulated on surfaces coated as in panel A. Graphs represent mean ± SEM from 3 experiments; n = 30. ***P < .0001 (t test). (D) Bright-field and 2-color SI images of perforin (red) and IFN-γ (green) in pNK cells stimulated for 90 minutes on surfaces coated with MICA-Fc and ICAM-1. Bars represent 10 μm. (E) Quantification of the number of lytic granules, stained with α-perforin mAb, in the whole volume of each pNK cell after stimulation for 6 (white) or 90 minutes (black) on slides coated with poly-L-lysine or MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL). At 90 minutes, when IFN-γ could be detected, IFN-γ+ and IFN-γ− cells are plotted separately. Bars represent the median from 4 experiments; n > 30. ***P < .0001 (analyzed by 1-way ANOVA). (F) Comparison of the number of granules per NK cell for 4 independent donors whose cells were stimulated for 6 minutes on slides coated with MICA-Fc and ICAM-1. Each data point represents a single cell. Bars represent the median for each dataset.
These rearrangements in cortical actin were similar in magnitude to those observed in the center of the cytolytic synapse (Figure 4C-D). To compare the location of lytic granules and IFN-γ, 2-color 3D-SI microscopy was used to obtain super-resolution images of the whole cell volume. Cultured pNK cells were stimulated on surfaces coated with MICA-Fc and ICAM-1 for 90 minutes, and cells were imaged for perforin marked by a mAb (IgG2b) followed by AlexaFluor-594–conjugated antimouse IgG2b antibody and for IFN-γ using an mAb (IgG1) directly labeled with AlexaFlour-488 (Figure 6D). In all cells, IFN-γ was detected close to the cell surface interface, and perforin and IFN-γ intracellular staining did not colocalize. This is consistent with previous studies demonstrating that IFN-γ and lytic granules are secreted via different pathways.48
Strikingly, for pNK cells in which IFN-γ staining was detected, perforin staining was frequently observed at only very low levels or not at all. The total number of detectable perforin-stained lytic granules within the whole cell volume was quantified from SI images of pNK cells (Figure 6E). The number of granules per cell ranged from 5 to 99, median 39 ± 3 granules/cell, for unstimulated NK cells and was similar for cells that did not show IFN-γ expression on stimulation on MICA-Fc– and ICAM-1–coated surfaces for 6 minutes or 90 minutes. The number of granules per cell was significantly lower in pNK cells that expressed IFN-γ, from 0 to 38 and median 12 ± 2 granules/cell. Granule numbers were consistent between different donors (Figure 6F). Thus, pNK cells that up-regulated IFN-γ protein expression was either constitutively low in the number of lytic granules they contained or they had lost perforin at an early time point. Either way, IFN-γ secretion and directed secretion of lytic granules were not occurring concurrently, suggesting that the cortical actin rearrangements observed at the cell surface interface in IFN-γ–expressing pNK cells are probably important for cytokine secretion.
Intracellular compartments containing IFN-γ preferentially localize to regions where the actin mesh has opened up
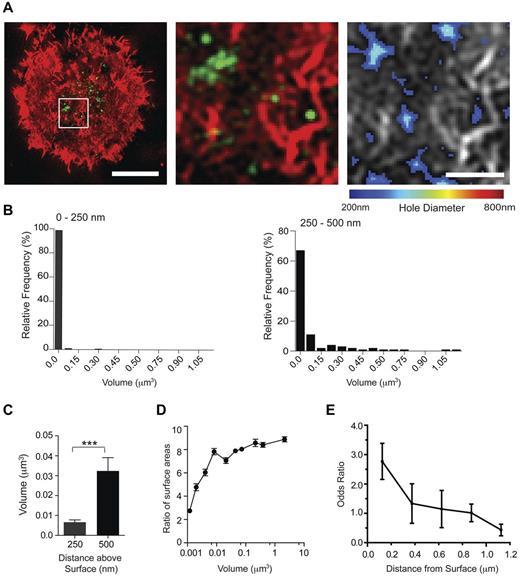
We next used 2-color 3D-SI microscopy to directly compare the location of IFN-γ and openings within the actin mesh. IFN-γ staining could be detected in multiple compartments either colocalized or in close proximity to where the cortical actin mesh had opened (Figure 7A center and right panels). IFN-γ did not have a uniform size or morphology (Figure 7B), and compartments within 250 nm of the coverslip had a smaller distribution of volumes, 0.001-0.3 μm3, and lower average volume, 0.006 ± 0.001 μm3, compared with those 250-500 nm above the interface, which had volumes ranging from 0.001 to 1.12 μm3, and an average volume of 0.03 ± 0.006 μm3 (Figure 7C). To describe the shape of the IFN-γ compartments, the surface area of the compartment was compared as a ratio to the surface area of a perfect sphere with a volume equal to that of the measured compartment volume. This meant that a compartment that was a sphere would give a value of 1; and the higher the number, the more irregular was the shape (ie, less spherical). Interestingly, a relationship could be observed between the size of an IFN-γ–stained compartment and its sphericity (Figure 7D): IFN-γ compartments that were large were also less spherical. Together, these data establish that IFN-γ localized within the synaptic actin mesh accumulated in smaller, more spherical, structures compared with IFN-γ further from the synapse.
Intracellular compartments containing IFN-γ preferentially localize to regions where the actin mesh has opened up. (A) Right panel: Two-color SI images of F-actin (red) and IFN-γ (green) in a pNK cell activated on a surface coated with MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL) for 90 minutes. Bar represents 5 μm. White box represents the region of the immune synapse that is enlarged in the center panel. Right panel: The domains within the enlarged region that have opened up in the cortical actin mesh to be 200-800 nm in diameter. Bar represents 1μm. (B) Relative frequencies of the volumes of IFN-γ stained compartments located at 0-250 nm (left) or 250-500 nm (right) up into the cell away from the contact with the surface. See main text for precise numeric quantification of this data. (C) The average volume of IFN-γ compartments located within 250 nm or 250-500 nm of the cell surface interface. Graphs represent mean ± SEM from 3 experiments; n = 250. ***P < .0001. (D) The ratio of surface area of compartments containing IFN-γ to surface area of a sphere with an equal volume is plotted against the measured volume of the compartment. Graph represents mean ± SEM from 3 experiments; n = 473. (E) Odds ratios for IFN-γ to be localized on regions within the cortical actin mesh in which holes 200-800 nm in diameter have opened are plotted as mean ± SEM at axial distances for IFN-γ located 0-1 μm above the surface of slides coated with MICA-Fc and ICAM-1. Graph represents n = 10 cells from 3 experiments.
Intracellular compartments containing IFN-γ preferentially localize to regions where the actin mesh has opened up. (A) Right panel: Two-color SI images of F-actin (red) and IFN-γ (green) in a pNK cell activated on a surface coated with MICA-Fc (2.5 μg/mL) and ICAM-1 (2.5 μg/mL) for 90 minutes. Bar represents 5 μm. White box represents the region of the immune synapse that is enlarged in the center panel. Right panel: The domains within the enlarged region that have opened up in the cortical actin mesh to be 200-800 nm in diameter. Bar represents 1μm. (B) Relative frequencies of the volumes of IFN-γ stained compartments located at 0-250 nm (left) or 250-500 nm (right) up into the cell away from the contact with the surface. See main text for precise numeric quantification of this data. (C) The average volume of IFN-γ compartments located within 250 nm or 250-500 nm of the cell surface interface. Graphs represent mean ± SEM from 3 experiments; n = 250. ***P < .0001. (D) The ratio of surface area of compartments containing IFN-γ to surface area of a sphere with an equal volume is plotted against the measured volume of the compartment. Graph represents mean ± SEM from 3 experiments; n = 473. (E) Odds ratios for IFN-γ to be localized on regions within the cortical actin mesh in which holes 200-800 nm in diameter have opened are plotted as mean ± SEM at axial distances for IFN-γ located 0-1 μm above the surface of slides coated with MICA-Fc and ICAM-1. Graph represents n = 10 cells from 3 experiments.
In synapses where IFN-γ accumulated, holes within the actin mesh appeared somewhat more diffuse within the central region of the synapse compared with granule-penetrable areas where lytic granules accumulate at synapses. This was confirmed by the average distance from openings in the actin mesh to the centroid of the cell being 2.1 μm ± 0.1 in synapses where lytic granules accumulated (n = 15 cells from 3 experiments),12 and 3.6 μm ± 0.1 in synapses where IFN-γ accumulated. However, the significance of this remains unclear. To compare the location of IFN-γ with domains where the cortical actin mesh had remodeled, super-resolved z-stacks were taken through the first 1.0 μm into the cell above the contact with the activating surface, and images were analyzed using a custom MatLab program. We analyzed how frequently IFN-γ compartments were localized precisely on the domains that had opened up. This analysis revealed that compartments containing IFN-γ within 250 nm of the interface frequently localized to domains that were predicted to be penetrable by particles of 200-800 nm diameter (indicated by an odds ratio of 2.76 ± 0.6; Figure 7F). Further away from the cell surface membrane, in compartments located deeper inside the NK cell, IFN-γ did not have a tendency to locate in line with these small domains. Thus, in a subset of pNK cells stimulated through NKG2D and LFA-1, IFN-γ is detected at specific domains where the periodicity of the cortical actin structure has increased.
Discussion
Studies of the key steps during NK cell activation that lead to lytic granule or cytokine secretion have been hampered by the resolution limit of conventional light microscopy. Here we used super-resolution microscopy to image the actin cytoskeleton at the NK cell immune synapse during cytolytic synapse formation and during secretion of the cytokine IFN-γ. We show that ligation of NKG2D or the Fc receptor CD16 was sufficient to induce remodeling of the NK cell cortical actin structure to produce discrete nanometer-scale domains within the synapse center sufficient for individual lytic granules to pass through. In contrast, these rearrangements in cortical actin were only observed after ligation of NKp46 or CD2 when LFA-1 was coligated. Similarly, influenza virus particles were also unable to trigger an opening within synaptic actin unless LFA-1 was coligated. Integrin-mediated adhesion is therefore important for NK cells to respond to influenza.
These data are important in demonstrating that NK cells do not open the actin mesh in response to viral particles without coligation of nonviral protein. We suggest that broadly, innate germline-encoded receptors that are capable of recognizing foreign or virus particles could use LFA-1 ligation of ICAM-1 to differentiate between virally infected cells and free virus particles. This distinction would not be required for NK cell–activating receptors, such as NKG2D, which recognize host cell proteins that are induced in response to infection or disease.
Directed secretion of cytokines is a well-established function of the immune synapse,14 and the directed secretion of IFN-γ has been reported between T cells and antigen-presenting cells and specifically, T cells and B cells.15-17 Here we found that ∼ 20% of human peripheral blood NK cells up-regulate IFN-γ expression after ligation of the NK cell activating receptor NKG2D. This frequency was similar for freshly isolated NK cells and those cultured in IL-2, indicating that this subset is not selectively expanded by IL-2, as is known to be the case for other subsets of NK cells.49 Expression of IFN-γ did not occur concurrently with target cell lysis, and the subset of NK cells expressing IFN-γ had very low levels of perforin. This could be because this subset was constitutively low in lytic granules or because they had degranulated at an earlier time.
IFN-γ protein was targeted to the immune synapse in these cells, which suggests that at least a fraction of the IFN-γ protein is destined for directed secretion. This might be an important mechanism for directly stimulating antiviral responses in infected target cells without unwanted effects on neighboring cells. In summary, we have demonstrated that cortical actin remodeling plays a role in different types of intercellular secretion and is triggered by NK cell receptors with various requirements for integrin coligation, consistent with different strategies for immune surveillance and facilitating discrimination between pathogens and pathogen-infected cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Spitaler and S. Rothery in the Facility for Imaging by Light Microscopy, Imperial; E. Wegel and G. Ball in the Micron Advanced Bioimaging Unit, Oxford, M. Mehrabi for cell culture; and H. Evans for discussions.
This work was supported by the Medical Research Council, the Biotechnology and Biologic Sciences Research Council, Woflson Royal Society (Research Merit Award), Lister Institute (Prize Fellowship), Marie Curie Fellowship, the Wellcome Trust, Fell Fund (grant), and the Edward Penley Abraha Cephalosporin Fund.
Authorship
Contribution: A.C.N.B. and D.M.D. conceived and designed the experiments and wrote the manuscript; A.C.N.B. and I.M.D. performed experiments; A.C.N.B. and J.-M.A. analyzed the data; and I.M.D. and I.D. helped establish instrumentation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel M. Davis, Division of Cell and Molecular Biology, Sir Alexander Fleming Building, Imperial College London, SW7 2AZ, United Kingdom; e-mail: d.davis@imperial.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal