Abstract
Administration of cannabinoid receptor 2 (CB2R) agonists in inflammatory and autoimmune disease and CNS injury models results in significant attenuation of clinical disease, and reduction of inflammatory mediators. Previous studies reported that CB2R signaling also reduces leukocyte migration. Migration of dendritic cells (DCs) to various sites is required for their activation and for the initiation of adaptive immune responses. Here, we report for the first time that CB2R signaling affects DC migration in vitro and in vivo, primarily through the inhibition of matrix metalloproteinase 9 (MMP-9) expression. Reduced MMP-9 production by DCs results in decreased migration to draining lymph nodes in vivo and in vitro in the matrigel migration assay. The effect on Mmp-9 expression is mediated through CB2R, resulting in reduction in cAMP levels, subsequent decrease in ERK activation, and reduced binding of c-Fos and c-Jun to Mmp-9 promoter activator protein 1 sites. We postulate that, by dampening production of MMP-9 and subsequent MMP-9–dependent DC migration, cannabinoids contribute to resolve acute inflammation and to reestablish homeostasis. Selective CB2R agonists might be valuable future therapeutic agents for the treatment of chronic inflammatory conditions by targeting activated immune cells, including DCs.
Introduction
The cannabinoid system consists of cannabinoid receptors and their ligands, including endocannabinoids, synthetic cannabinoid receptor agonists and antagonists, and phytocannabinoids. Several cannabinoid receptors have been described, that is, the classic cannabinoid receptor 1 (GPR) and GPR, the previously orphaned G-protein receptors GPR18 and GPR55, various ion channels, and intracellular peroxisome proliferator-activated receptor-γ (reviewed in Pertwee et al1 and Console-Bram2 ). The 2 classic cannabinoid receptors, CB1R and CB2R, have different distribution and functions (reviewed in Kubajewska and Constantinescu3 and Basu and Dittel4 ). CB1R is abundantly expressed on CNS and peripheral neurons and involved in neural functions. In contrast, CB2R is mostly expressed on immune cells and involved in immunoregulation. Administration of CB2R-selective agonists in models of inflammatory and autoimmune diseases such as systemic sclerosis, experimental autoimmune uveoretinitis, inflammatory bowel diseases, and experimental autoimmune encephalomyelitis (EAE) resulted in attenuation of clinical disease (reviewed in Basu and Dittel4 ). CB2R agonists also have been reported to have a beneficial effect in models of CNS injury such as cerebral infarction and spinal cord injury.5-8 In addition to effects on clinical outcome, CB2R agonists reduced the levels of inflammatory mediators in various experimental models.8-10
A possible mechanism for the anti-inflammatory effect of CB2R signaling is the direct action of CB2R agonists on immune cells. In vitro studies indicated that CB2R signaling inhibited the production of proinflammatory cytokines such as TNFα, IL-6, IL-2, and IFN-γ by activated microglia and T cells, and reduced the capacity of macrophages and dendritic cells to stimulate CD4+ T cells (reviewed in Basu and Dittel4 ). The anti-inflammatory role of CB2R signaling also may be because of a reduction in immune cell migration. Maresz et al showed that the number of encephalitogenic T cells in the CNS was significantly increased in Cnr2-deficient EAE mice, suggesting that endocannabinoids play a role in controlling T-cell migration.11 Administration of exogenous CB2R agonists reduced migration of inflammatory cells in various disease models. Decreased rolling and adhesion of leukocytes to brain microvessels has been reported in models of EAE,12 middle cerebral artery occlusion and reperfusion (MCAO/R),5 and in lipopolysaccharide-induced encephalitis.13 In the EAE model, CB2R activation was shown to reduce the total number of peripheral CD34+ hematopoietic cells in the CNS.14 In vitro reports showed that CB2R activation reduced chemotaxis of human T cells to CXCL12,15 human monocytes to CCL2/CCL3,16 murine macrophages to CCL5,17 and lipopolysaccharide-activated murine microglial cells to ADP.18
Dendritic cells (DCs), poised at the interface between innate and adaptive immunity initiate antigen-specific immune responses through their capacity to activate naive T cells. Bone marrow–derived conventional DCs or monocyte-derived inflammatory DCs migrate in response to inflammatory chemokines to sites of infection, phagocytose and process antigens, and are activated by TLR ligands. After activation, DCs mature, up-regulate MHCI and -II and costimulatory molecules, produce proinflammatory cytokines and chemokines, and acquire the ability to migrate to lymph nodes through changes in chemokine receptor expression, that is, down-regulation of CCR5 and up-regulation of CCR7 (reviewed in Steinman and Idoyaga19 ). Migration of DCs, both to the inflammatory site where they encounter the antigen and later on to the secondary lymphoid organs where they interact with and activate naive T cells, is essential to their function as innate immune sentinels and initiators of adaptive immune responses.
Matrix metalloproteinases (MMPs) are implicated in physiologic processes such as immune defense and tissue repair, as well as in cancer, cardiovascular diseases, and neurodegeneration (reviewed in Sbardella et al,20 Zitka et al,21 Manicone and McGuire,22 and Page-McCaw et al23 ). MMP-9, secreted primarily by activated DCs and macrophages, plays an essential role in immune cell migration by degrading the extracellular matrix and basement membranes through cleavage of collagen IV. The role of MMP-9 in DC migration to inflammatory sites, draining lymph nodes, and out of brain capillaries has been demonstrated previously.24-26
Similar to other immune cells, DCs express CB2R27,28 (and present study). Cannabinoids were shown to reduce the stimulatory capacity of DCs for T cells, to inhibit the production of proinflammatory cytokines (IL-6, IFN-γ, and IL-17), and chemokines (CCL2 and -3), and to up-regulate IL-10 production in DC-T cell cocultures (reviewed in Basu and Dittel4 ). To our knowledge, there are no reports of CB2R signaling effects on DC migration. In this study, we investigated the effects of CB2R agonists on the in vitro and in vivo migration of activated bone marrow–derived DCs (BMDCs). In addition to up-regulation of CCR7, migrating mature DCs up-regulate the expression of MMP-9 that is required for in vivo migration.25,29 Up to this date, there are no reports on CB2R signaling effects on either Ccr7 or Mmp9 expression in DCs. Here, we report that selective CB2R agonists reduce the migration of BMDCs through the inhibition of MMP-9 and we investigate the molecular mechanisms involved.
Methods
Mice
Six- to 8-week-old B10.A mice were purchased from The Jackson Laboratory and maintained in the Temple University School of Medicine animal facility under pathogen-free conditions. Cnr2+/+ and Cnr2−/− mice on C57BL/6 background were bred at Temple University School of Medicine by mating Cnr2+/− originally obtained from the National Institutes of Health. CB2R deficiency was confirmed by polymerase chain reaction (PCR) as described previously.30 Mice were handled and housed in accordance with the guidelines of the Temple University Animal Care and Use Committee. For the in vivo studies, 8-week-old C57BL/6 were purchased from The Jackson Laboratory.
Reagents
Prostaglandin E2 (PGE2) was purchased from Sigma. M-CSF, GM-CSF, TNF-α, IL-1β, IL-6, and CCL19 were purchased from Peprotech. GP1a, antibodies for pro-MMP-9 ELISA and MMP-9 standards were purchased from R&D Systems. CB2 agonist O-1966 was a generous gift from Anu Mahadevan (Organix). Dibutyryl-cAMP (dbcAMP) and MMP-9 inhibitor I were purchased from Calbiochem. The cAMP kit was purchased from Life Technologies. Antibodies to phospho-ERK, ERK, phospho-c-Jun, c-Jun, and c-Fos were obtained from Cell Signaling Technology and anti-GST was from Abcam.
Cell cultures
Bone marrow macrophages (BMMΦ) and BMDCs were generated in vitro from bone marrow as described previously29 in the presence of either M-CSF (10 ng/mL) or GM-CSF (20 ng/mL), respectively. For BMMΦ cultures, adherent cells were trypsinized on day 8 (CD11b+F4/80+ cells, > 85% by FACS analysis). For BMDC cultures, the nonadherent cells were harvested on day 7 and purified by immunomagnetic sorting with anti–CD11c-coated magnetic beads (CD11c+ cells, > 95% by FACS analysis).
Microglial cells were generated from neonatal mice. In brief, cerebral cortical cells from 1- to 2-day-old mice were dissociated and plated in 75-cm2 Falcon culture flasks in Dulbecco modified Eagle medium-F12 (HyClone Laboratories) supplemented with 10% heat-inactivated FBS, containing 2mM glutamine and 1× antibiotic/antimycotic (complete medium). The medium was removed and replenished with complete medium containing 10 ng/mL GM-CSF at days 5 and 10 after plating. On day 15, microglia were harvested by shaking the flasks at 350 rpm at 37°C for 25 minutes. The harvested cells were centrifuged (270g for 5 minutes) and plated at 1 × 106 cells/mL in complete medium containing 10 ng/mL GM-CSF (CD11b+F4/80+ cells, > 90% by FACS analysis).
All 3 cell types, BMMΦs, BMDCs, and microglia, were cultured at 1 × 106 cells/mL in GM-CSF supplemented complete medium and matured with TNF-α (20 ng/mL), IL-1β (10 ng/mL), IL-6 (10 ng/mL), and PGE2 (10−7M) for either 24 or 48 hours in the presence or absence of GP1a.
FACS analysis for phospho-ERK and c-Fos
Cells treated as indicated were fixed, permeabilized, and incubated with anti–mouse phospho-ERK or anti–mouse c-Fos for 40 minutes at room temperature followed by Alexa Fluor–conjugated goat anti–rabbit IgG (Invitrogen) for 30 minutes. Data were collected for 10 000 cells by FACS analysis.
MMP-9 ELISA
Purified CD11c+ DCs (1 × 106 cells/mL) were seeded in 12-well plates and treated as described in “Results.” The amounts of pro–MMP-9 released in the medium were measured by sandwich ELISA with antibodies and standards obtained from R&D Systems. The absorbance was determined using a POLARstar Optima plate reader (BMG Labtech) at a wavelength of 450 nm.
Western blot analysis
We serum-starved 3 to 6 × 106 DCs for 3 hours before treatment. Samples were prepared as described previously,31 followed by SDS-PAGE electrophoresis. Separated protein were transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories) and probed with primary antibodies against phospho-p44/p42 MAP kinase (threonine 202, tyrosine 204), total p44/p42 MAP kinase (L34F12), and phospho–c-Jun (serine 63) II, and total c-Jun (L70B11; Cell Signaling Technology) at 1:1000 dilution in 50:50 Odyssey blocking buffer:PBS (LI-COR Biosciences). Goat anti–mouse IRDye 800CW and goat anti–rabbit IRDye 680CW antibodies (LI-COR Biosciences) were used as secondary antibodies. Transferred proteins were visualized by using the Odyssey infrared imaging system (LIC-OR Biosciences).
Real-time RT-PCR
The expression of Ccr7 and Mmp9 was detected by the SYBR Green–based real-time RT-PCR technique. RNA was isolated from purified CD11c+ DCs treated as described in “Results,” and cDNA was prepared as described previously.8 The 20 μL (total volume) of the PCR mixture consisted of 4 μL of diluted cDNA, 10 μL of SYBR Green–containing PCR master mixture (2×) and 150nM of each primer. The primers for real-time RT-PCR were as follows. MMP-9: sense, 5′-AAAACCTCCAACCTCACGGA-3′ and antisense, 5′-GCGGT-ACAAGTATGCCTCTGC-3′; CCR7: sense, 5′-TTCCAGCTGCCCTACAATGG-3′ and antisense, 5′-GAAGTTGGCCACCG TCTGAG-3′; and β-actin: sense, 5′-AGCTTCTTTGCAGCTCCTTCGTTGC-3′ and antisense, 5′-ACCAGCGCAGCGATATCGTCA-3′. Real-time RT-PCR was performed using the Mx3005P (Stratagene), and the cycling conditions were 95°C for 30 seconds, 55°C for 1 minute, 72°C for 30 seconds, for 40 cycles, followed by a melting point determination or dissociation curves. The expression level of each gene was indicated by the cycle numbers needed for the cDNA to be amplified to reach a threshold. The amount of DNA was calculated from the cycle numbers by using standard curves, and the results were normalized to the housekeeping gene β-actin from the same sample.
ChIP assay
After various treatments, DCs were prepared for ChIP analysis as described previously.31 The chromatin was immunoprecipitated with anti–c-Fos, anti–c-Jun, or anti-GST as a negative control (Santa Cruz Biotechnology). After proteinase K digestion, input DNA and precipitated DNA were purified and real-time PCR-amplified with primers encompassing the MMP-9 promoter region containing the activator protein 1 (AP-1) sites (sense, GACCCTGGGAA CCGGGTCCA and antisense, CAGGGACCGGCCGTGGAAAC).
Matrigel migration assay
Matrigel migration was performed in Transwell inserts (6.5 mm) fitted with polycarbonate filters (8-μm pore size; Corning Life Sciences). The upper sides of the transwells were coated with Matrigel (BD Biosciences) diluted in PBS (100 μg/filter). CD11c+ DCs cultured with TNF-α, IL-1β, IL-6, and PGE2 with or without GP1a (5 or 1μM) for 48 hours were tested for migration to CCL19 (100 ng/mL). In some experiments, cells treated with the MMP-9 inhibitor I (10−6M) also were included. In brief, the lower chambers of the plate were filled with 500 μL of serum-free medium with or without CCL19 (100 ng/mL). DCs (1 × 105 cells in 0.1 mL) were deposited in the upper Transwell chambers and allowed to migrate for 3 hours at 37°C in 5% CO2. Migrated DCs harvested from the lower chambers were counted by FACS (60-second counts).
In vivo migration assay
BMDCs from CB2R+/+ and CB2−/− mice were treated with TNF-α, IL-1β, IL-6, and PGE2 in the presence or absence of GP1a. Forty-eight hours later, DCs were labeled with PKH-26 red fluorescent dye (Sigma) according to the manufacturer's instructions, and 106 labeled DCs were inoculated subcutaneously in the footpads of mice preinjected 24 hours earlier with 40 ng of TNF-α (subcutaneously in the footpads). At 48 hours, the numbers of labeled DCs collected from the draining popliteal lymph nodes were determined by FACS (50 000 event counts).
cAMP assay
Purified CD11c+ DCs (1 × 106 cells/mL) were seeded in 96-well plates in 100 μL of complete medium and rested overnight. The next day, cells were washed with PBS, and the medium was exchanged for cAMP assay medium (serum-free XVIVO-15 medium with 0.5mM 3-isobutyl-1-methylxanthine). After incubation for 1 hour at 37°C, the cells were treated with PGE2 in the presence or absence of the CB2R agonist. After treatment, the cAMP amounts were determined using a cAMP screening kit (Applied Biosystems) according to the manufacturer's instructions.
Statistics
Results are expressed as mean ± SD. Comparisons between multiple groups were performed by ANOVA followed by Bonferroni t test. Statistical significance was determined with P values < .05. For in vivo migration, data were analyzed using ratio paired t test in which the average of the logarithm of the ratio of treated/control is taken and tested for null hypothesis. Data were analyzed using Prism 5 software (GraphPad Software). FACS data were analyzed using CellQuest software (BD Biosciences).
Results
GP1a neither modulates DC CCR7 expression nor affects CCL19-induced chemotaxis
The chemokines CCL19 and CCL21 constitutively expressed in lymph nodes act as ligands for the chemokine receptor CCR7 expressed on mature DCs. To determine whether CB2R signaling prevents CCR7 expression, we matured purified CD11c+ BMDCs with the inflammatory cytokine cocktail consisting of TNF-α + IL-6 + IL-1β and PGE2 referred to as CCP hereafter, in the presence or absence of different concentrations of GP1a. CCP up-regulated Ccr7 mRNA expression as expected, whereas the GP1a treatment did not affect this increase (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Because cannabinoids have been previously reported to inhibit macrophage migration to CCL5 through the trans-deactivation of CCR1/CCR5 without changes in chemokine receptor expression,17 we investigated the effects of GP1a on DC chemotaxis. Mature DCs migrated in response to CCL19, and this migration was not affected by 5μM GP1a at either 24 or 48 hours (supplemental Figure 1B). A similar lack of effect was observed for 1μM GP1a (data not shown).
GP1a inhibits MMP-9 expression in myeloid-derived immune cells, including DCs
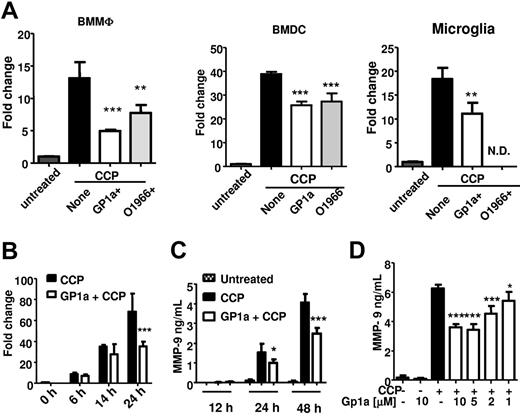
In addition to Ccr7 expression, DC migration to secondary lymph nodes requires production of MMPs to digest the extracellular matrix and basement membranes. We have reported previously that treatment of BMDCs with CCP induces high levels of MMP-9.32 To determine whether CB2R activation modulates MMP-9 expression, we treated BMMΦs, BMDCs, and primary microglia with CB2R agonist(s) during maturation with CCP. The CB2R-selective agonists inhibited expression of Mmp9 in all 3 cell types (Figure 1A). GP1a treatment did not affect cell viability in BMMΦs, BMDCs, or primary microglia cultures.
GP1a inhibits Mmp9 expression induced by CCP in myeloid immune cells. (A) BMMΦs, BMDCs, or primary microglia were treated with CCP with or without CB2R agonists GP1a (5μM) or O-1966 (5μM) for 24 hours. RNA was extracted and subjected to qRT-PCR for Mmp9. N.D. indicates not determined. (B) BMDCs were treated with CCP with or without GP1a (5μM), and RNA was extracted at different time points and subjected to qRT-PCR for Mmp9. (C) BMDCs were treated as described in panel B, and supernatants collected at various time points were subjected to MMP-9 ELISA. (D) BMDCs were treated with different concentrations of GP1a for 48 hours, and the resulting supernatants were subjected to MMP-9 ELISA. Data in panels A and B are normalized to housekeeping gene β-actin and presented as fold change compared with untreated samples. *P < .05, **P < .01, *** P < .001 compared with CCP. Data are representative of 2 (A-B) and 4 (C-D) independent experiments.
GP1a inhibits Mmp9 expression induced by CCP in myeloid immune cells. (A) BMMΦs, BMDCs, or primary microglia were treated with CCP with or without CB2R agonists GP1a (5μM) or O-1966 (5μM) for 24 hours. RNA was extracted and subjected to qRT-PCR for Mmp9. N.D. indicates not determined. (B) BMDCs were treated with CCP with or without GP1a (5μM), and RNA was extracted at different time points and subjected to qRT-PCR for Mmp9. (C) BMDCs were treated as described in panel B, and supernatants collected at various time points were subjected to MMP-9 ELISA. (D) BMDCs were treated with different concentrations of GP1a for 48 hours, and the resulting supernatants were subjected to MMP-9 ELISA. Data in panels A and B are normalized to housekeeping gene β-actin and presented as fold change compared with untreated samples. *P < .05, **P < .01, *** P < .001 compared with CCP. Data are representative of 2 (A-B) and 4 (C-D) independent experiments.
Because migration of DCs to secondary lymphoid organs is essential to initiate the adaptive immune response, we focused on the effects of the CB2R agonist GP1a on Mmp9 expression in BMDCs. CCP induced high levels of MMP-9 mRNA expression at 24 hours and Mmp9 protein secretion at 48 hours (Figure 1B-C); GP1a inhibited Mmp9 expression and secretion in a dose-dependent manner (Figure 1D). Although MMP-2 shares substrate specificity with MMP-9 and therefore has similar functions, the 2 MMPs differ in terms of expression in DCs. MMP-9 is not expressed in immature DCs and is up-regulated in DCs treated with CCP (supplemental Figure 2A). In contrast, MMP-2 is constitutively expressed in immature DCs and down-regulated on activation with CCP (supplemental Figure 2B). Although GP1a further reduces MMP-2 expression in CCP-matured DCs, we focused on its effects on MMP-9, because MMP-2 expression in activated DCs is minimal compared with MMP-9.
Inhibition of MMP-9 production by GP1a is mediated through CB2R
To confirm that wild-type BMDCs express CB2R (Cnr2), we carried out quantitative (q)RT-PCR for Cnr2 in BMDCs. Because the murine Cnr2 gene lacks introns, we included a no reverse transcriptase (no RT) control to rule out amplification of genomic DNA. BMDCs obtained from Cnr2−/− mice were analyzed to ensure the lack of Cnr2 expression. In contrast to Cnr2−/− BMDCs, wild-type DCs express CB2R (supplemental Figure 3A).
To test whether the effect of GP1a on MMP-9 expression was dependent on CB2R signaling, we generated BMDC from Cnr2+/+ and Cnr2−/− littermates and matured them with CCP for 24 or 48 hours in the presence of varying concentrations of GP1a. GP1a inhibited MMP-9 production in a concentration-dependent manner at both 24 and 48 hours in BMDC generated from Cnr2+/+ mice (supplemental Figure 3B). In contrast, GP1a had no significant effect on MMP-9 production in Cnr2−/− mice. Interestingly, lack of this receptor resulted in significantly higher levels of MMP-9 production that was not because of increased BMDC proliferation (data not shown). This observation is suggestive of modulation of MMP-9 by endogenous cannabinoids in Cnr2+/+ BMDCs.
GP1a prevents DC migration through matrigel
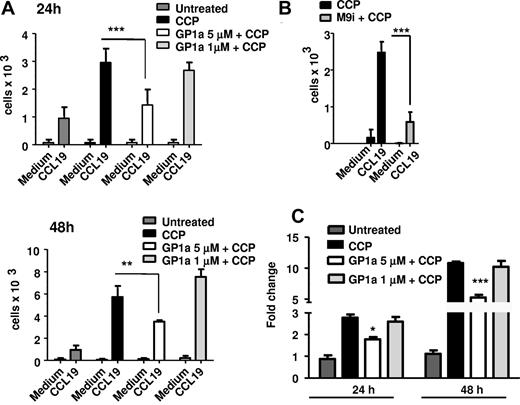
MMP-9 facilitates DC migration through digestion of the extracellular matrix and of basement membranes underlying the blood vessels. To test the functional relevance of the GP1a-induced decrease in MMP-9 levels, we subjected DCs to migration assays through transwells coated with matrigel, which contains extracellular matrix components. DCs matured for 24 or 48 hours with CCP in the presence or absence of GP1a were allowed to transmigrate in response to CCL19 through matrigel-coated transwells. DCs treated with GP1a migrated in lower numbers compared with control cells (Figure 2A). Migration through matrigel was dependent on active MMP-9 because the inclusion in the assay of an MMP-9 inhibitor abrogated the migratory capacity of control cells (Figure 2B). In agreement with the matrigel migration results, DCs treated with 5μM GP1a secreted significantly lower amounts of MMP-9 at both 24 and 48 hours (Figure 2C).
GP1a prevents DC matrigel migration. DC were treated with CCP with or without GP1a (5 μM or 1 μM) for 24 or 48h (A) or MMP-9 inhibitor (M9i) for 48h (B) and 105 cells were placed in upper Transwell chambers coated wtih Matrigel (100 μg). The bottom chambers were filled with serum free medium with or without CCL19 (100 ng/ml). 3h later migrated cells were collected from the lower chambers and counted by FACS. (C) DC were treated as in A and Mmp9 mRNA expression was determined by qRT-PCR. Data are representative of 3 independent experiments.
GP1a prevents DC matrigel migration. DC were treated with CCP with or without GP1a (5 μM or 1 μM) for 24 or 48h (A) or MMP-9 inhibitor (M9i) for 48h (B) and 105 cells were placed in upper Transwell chambers coated wtih Matrigel (100 μg). The bottom chambers were filled with serum free medium with or without CCL19 (100 ng/ml). 3h later migrated cells were collected from the lower chambers and counted by FACS. (C) DC were treated as in A and Mmp9 mRNA expression was determined by qRT-PCR. Data are representative of 3 independent experiments.
Metalloproteinases are modulated by endogenous tissue inhibitors of metalloproteinases (TIMPs). Through its interactions with pro–MMP-9, the inducible TIMP-1 inhibits cell migration. Therefore, the inhibitory effect of GP1a on DC migration could be the result of TIMP-1 up-regulation. We tested the effects of GP1a on TIMP-1 expression in DCs and although TIMP-1 was up-regulated after CCP activation, GP1a did not further increase TIMP-1 expression (supplemental Figure 2C).
GP1a-treated DCs exhibit lower migratory capacity in vivo
Our in vitro results suggest that GP1a-treated mature DCs migrate less in the matrigel assay because of the inhibition of MMP-9 production. To investigate whether GP1a-treated DCs exhibit reduced migration in vivo, we differentiated DCs from CB2R+/+ and CB2R−/− littermates and treated them with CCP in the presence or absence of GP1a. The cells were collected 48 hours later, labeled with PKH-26, and injected into the footpads of wild-type mice. Each mouse was injected with control DCs in the right footpad and with GP1a-treated DCs in the left footpad. Draining popliteal lymph nodes were harvested 48 hours later, and the numbers of labeled DCs were determined by flow cytometry. Data from different experiments were normalized by plotting the number of fluorescent cells from control legs as 100%. GP1a treatment resulted in the recovery of lower numbers of CB2R+/+ DCs. No reduction was observed for CB2R−/− DC (Figure 3A and supplemental Figure 4). In agreement with these results, CB2R+/+ DCs, but not CB2R−/− DCs treated with GP1a, secreted lower amounts of MMP-9 before inoculation into footpads (Figure 3B).
GP1a-treated DCs exhibit lower migratory capacity in vivo. (A) DCs generated from Cnr2+/+ (wt) or Cnr2−/− (ko) mice were treated with CCP with or without GP1a (5μM) for 48 hours and labeled with PKH-26. Then, 1 × 106 labeled DCs were injected subcutaneously in the footpads of wt C57BL/6 mice preinjected with 40 ng of TNF-α s.c in the footpads 24 hours earlier. Recipient mice received CCP-treated DC (control) in the right footpad and CCP + GP1a–treated DCs in the left footpad. Forty-eight hours later, cells were collected from popliteal lymph nodes, and PKH-labeled cells were analyzed by FACS. Data from 3 different experiments are normalized by plotting the number of labeled cells from the control leg as 100%. (B) Supernatants collected from DC cultures before injection were analyzed for MMP-9 via ELISA.
GP1a-treated DCs exhibit lower migratory capacity in vivo. (A) DCs generated from Cnr2+/+ (wt) or Cnr2−/− (ko) mice were treated with CCP with or without GP1a (5μM) for 48 hours and labeled with PKH-26. Then, 1 × 106 labeled DCs were injected subcutaneously in the footpads of wt C57BL/6 mice preinjected with 40 ng of TNF-α s.c in the footpads 24 hours earlier. Recipient mice received CCP-treated DC (control) in the right footpad and CCP + GP1a–treated DCs in the left footpad. Forty-eight hours later, cells were collected from popliteal lymph nodes, and PKH-labeled cells were analyzed by FACS. Data from 3 different experiments are normalized by plotting the number of labeled cells from the control leg as 100%. (B) Supernatants collected from DC cultures before injection were analyzed for MMP-9 via ELISA.
GP1a reduces PGE2-induced MMP-9 in DCs via inhibition of cAMP induction
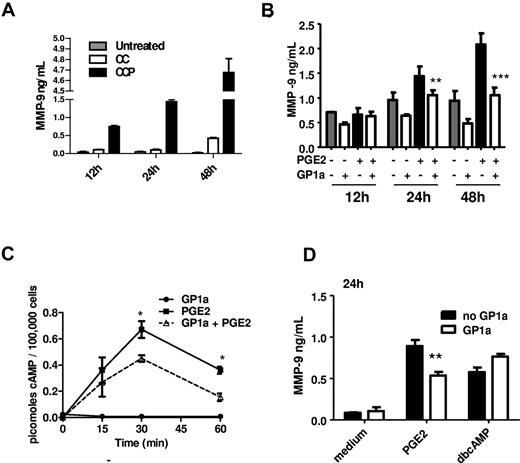
Although CCP is an excellent inducer of MMP-9 in DCs, the signaling pathways leading to MMP-9 expression are complicated by the presence of different cytokines and PGE2. In a previous study, we reported that PGE2 is the major MMP-9 inducer.29 This was confirmed by comparisons between the effects of the cytokine cocktail without PGE2 (referred to as CC) and with PGE2 (CCP; Figure 4A). Therefore, we tested the effect of GP1a on PGE2-induced MMP-9 production, and we determined that, similarly to the effects on CCP-treated DCs, GP1a reduced MMP-9 production in PGE2-treated DCs (Figure 4B).
GP1a reduces PGE2-induced MMP-9 through inhibition of cAMP induction. (A) DCs were treated with cytokine cocktail without PGE2 (CC) or cytokine cocktail + PGE2 (CCP). Culture supernatants collected at different time points were analyzed for MMP-9 by ELISA. (B) DCs were treated with PGE2 (0.1μM) with or without GP1a (5μM). Supernatant collected at different time points were analyzed for MMP-9 by ELISA. (C) DCs (1 × 105 cells) were treated as described in panel B. Cell lysates collected at different time points were analyzed for total cAMP by ELISA. (D) DCs were treated with PGE2 or dbcAMP (10μM) with or without GP1a for 24 hours. Supernatants were analyzed for MMP-9 by ELISA. Data are representative of 3 independent experiments.
GP1a reduces PGE2-induced MMP-9 through inhibition of cAMP induction. (A) DCs were treated with cytokine cocktail without PGE2 (CC) or cytokine cocktail + PGE2 (CCP). Culture supernatants collected at different time points were analyzed for MMP-9 by ELISA. (B) DCs were treated with PGE2 (0.1μM) with or without GP1a (5μM). Supernatant collected at different time points were analyzed for MMP-9 by ELISA. (C) DCs (1 × 105 cells) were treated as described in panel B. Cell lysates collected at different time points were analyzed for total cAMP by ELISA. (D) DCs were treated with PGE2 or dbcAMP (10μM) with or without GP1a for 24 hours. Supernatants were analyzed for MMP-9 by ELISA. Data are representative of 3 independent experiments.
The major PGE2 receptors expressed in BMDCs are EP2 and EP433 that signal through activation of adenylate cyclase, resulting in increased cAMP levels.34 Cannabinoid signaling through CB2R was reported to inhibit early cAMP induction in macrophages and T cells (reviewed in Kubajewska and Constantinescu3 ). To investigate whether CB2R activation in our experimental system affects cAMP levels, we activated DCs with PGE2 in the presence or absence of GP1a for various time periods and then measured cAMP levels via an ELISA assay. GP1a treatment resulted in lower levels of cAMP, reaching statistical significance at 30 and 60 minutes posttreatment (Figure 4C). These results led us to hypothesize that the effects of CB2R signaling on MMP-9 expression might be mediated through a reduction in cAMP. To test this hypothesis, we assessed the effect of GP1a in the presence of dbcAMP, a stable exogenous cAMP analog. Although GP1a reduced PGE2-induced MMP-9, it did not affect MMP-9 production induced by dbcAMP (Figure 4D). This confirms the previously described role of cAMP in MMP-9 expression in DCs,29 and suggests that the inhibitory effect of CB2R signaling is mediated through a reduction in cAMP levels.
GP1a reduces PGE2-induced ERK phosphorylation
We showed previously that MMP-9 expression induced by PGE2 is mediated through the activation of protein kinase A, a well-known cAMP target, followed by subsequent ERK1/2 phosphorylation.31 GP1a reduced PGE2-induced ERK phosphorylation as determined by flow cytometry (20 minutes posttreatment) and Western blot (Figure 5A-B).
GP1a treatment reduces PGE2 induced ERK phosphorylation. (A) DCs were treated with PGE2 with or without different concentrations of GP1a for 20 minutes. Cells were fixed, permeabilized, stained intracellular with phospho-ERK antibody, and analyzed by FACS. (B) DCs were treated with PGE2 with or without GP1a for various time periods. Cells were lysed and analyzed for phosphorylation of ERK by Western blot. Densitometric analyses are plotted in graphs normalizing phospho-ERK to total ERK. Data are representative of 2 (B) and 3 (A) independent experiments.
GP1a treatment reduces PGE2 induced ERK phosphorylation. (A) DCs were treated with PGE2 with or without different concentrations of GP1a for 20 minutes. Cells were fixed, permeabilized, stained intracellular with phospho-ERK antibody, and analyzed by FACS. (B) DCs were treated with PGE2 with or without GP1a for various time periods. Cells were lysed and analyzed for phosphorylation of ERK by Western blot. Densitometric analyses are plotted in graphs normalizing phospho-ERK to total ERK. Data are representative of 2 (B) and 3 (A) independent experiments.
GP1a decreases total c-Fos and phospho-c-Jun and subsequent binding of c-Fos and c-Jun to the AP-1 site on the MMP-9 promoter
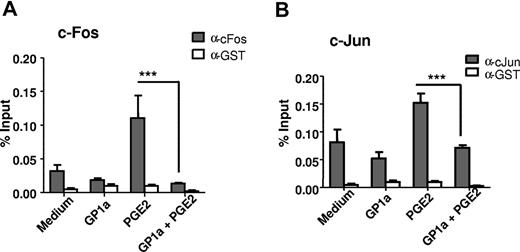
We have shown previously that PGE2 induces MMP-9 via a cAMP-dependent pathway, leading to ERK phosphorylation and subsequent activation and binding of c-Fos and c-Jun to AP-1 binding sites on the MMP-9 promoter.31 Here, we treated DCs with PGE2 in the presence or absence of GP1a, and then we measured the amounts of c-Fos via flow cytometry and of phosphorylated c-Jun via Western blot. GP1a decreased both the amount of c-Fos and the level of phospho-c-Jun (Figure 6A-B). The MMP-9 promoter has 2 AP-1 binding sites, and we have shown previously that PGE2 induces binding of c-Fos/c-Jun to the AP-1 sites on the Mmp9 promoter.31 We used ChIP assays to determine whether GP1a affects c-Fos/c-Jun binding to the Mmp9 promoter. DCs were treated with PGE2 in the presence or absence of GP1a for 1 hour, and cell lysates were immunoprecipitated with anti–c-Fos, anti–c-Jun, or anti-GST antibody as negative control, followed by real-time PCR amplification using primers encompassing the AP-1 sites in the murine Mmp9 promoter. As expected, PGE2 increased c-Fos and c-Jun binding to the AP-1 sites. In contrast, treatment with GP1a led to a significant decrease in binding of both c-Fos and c-Jun (Figure 7A-B).
GP1a treatment reduces both c-Fos induction and c-Jun phosphorylation. (A) DCs treated with PGE2 with or without GP1a for 40 minutes were fixed, permeabilized, stained intracellular with c-Fos antibody, and analyzed by FACS. (Top) Shaded area represents untreated cells. (Bottom) Black line represents GP1a + PGE2, gray line represents PGE2, and broken black line represents GP1a. (B) DCs were treated with PGE2 with or without GP1a for various time periods and analyzed for phospho-c-Jun by Western blot. Densitometric analyses are plotted in graphs normalizing phospho-c-Jun to total c-Jun. Data are representative of 2 (B) and 3 (A) independent experiments.
GP1a treatment reduces both c-Fos induction and c-Jun phosphorylation. (A) DCs treated with PGE2 with or without GP1a for 40 minutes were fixed, permeabilized, stained intracellular with c-Fos antibody, and analyzed by FACS. (Top) Shaded area represents untreated cells. (Bottom) Black line represents GP1a + PGE2, gray line represents PGE2, and broken black line represents GP1a. (B) DCs were treated with PGE2 with or without GP1a for various time periods and analyzed for phospho-c-Jun by Western blot. Densitometric analyses are plotted in graphs normalizing phospho-c-Jun to total c-Jun. Data are representative of 2 (B) and 3 (A) independent experiments.
GP1a treatment leads to decreased binding of c-Fos and c-Jun to the Mmp9 AP-1 sites. DCs were treated with PGE2 with or without GP1a for 1 hour. Cells were fixed, sonicated, and subjected to ChIP analysis using antibodies to c-Fos (A), c-Jun (B), or control IgG. Precipitated DNA was isolated and evaluated by PCR using specific primers for the AP-1 sites in the Mmp9 promoter. ***P < .001 compared with PGE2. Data are representative of 2 independent experiments.
GP1a treatment leads to decreased binding of c-Fos and c-Jun to the Mmp9 AP-1 sites. DCs were treated with PGE2 with or without GP1a for 1 hour. Cells were fixed, sonicated, and subjected to ChIP analysis using antibodies to c-Fos (A), c-Jun (B), or control IgG. Precipitated DNA was isolated and evaluated by PCR using specific primers for the AP-1 sites in the Mmp9 promoter. ***P < .001 compared with PGE2. Data are representative of 2 independent experiments.
Discussion
We reported previously the beneficial effects of selective CB2R agonists on clinical outcome in models of EAE, MCAO/R, and spinal cord injury. This was associated with a reduction in endogenous leukocyte rolling and adhesion to brain microvasculature and with reduced inflammatory cell infiltration into the CNS.5,8,12,35 Leukocyte migration and infiltration into the CNS require the up-regulation of specific adhesion molecules, chemokine receptors, and MMPs, particularly MMP-9. Here, we show that CB2R-selective agonists reduce Mmp9 expression in DCs, macrophages, and microglia. Inhibition of MMP-9 is mediated through CB2R-induced reduction in cAMP, inhibition of ERK1/2→AP-1 activation, and subsequent reduction in AP-1 binding to the Mmp9 promoter. The CB2R-mediated inhibition of MMP-9 in DCs was associated with reduced in vitro migration through matrigel. In addition, DCs treated in vitro with CB2R agonists and inoculated into mouse footpads migrated in lower numbers to the draining lymph nodes. Whether in vivo treatment of DC with CB2R agonists also results in reduced migration remains to be established.
Cannabinoids were shown to be immunoregulatory in models of inflammatory and autoimmune diseases, and significant beneficial effects were reported in neuroinflammation and neurodegeneration (reviewed in Kubajewska and Constantinescu3 , Massi et al36 , and Baker et al37 ). Several nonexclusive mechanisms play a role in the anti-inflammatory effect of cannabinoids, such as inhibition of proinflammatory cytokine and chemokine production, effects on immune cell proliferation and programmed cell death, and reduction of immune cell migration.4
In models of neuroinflammatory diseases such as EAE, cannabinoids reduced clinical outcome in conjunction with inhibition of microglial activation and immune cell migration into the CNS.11,12 In most, but not all of these studies, the effects were mediated through CB2R signaling. We reported previously that administration of selective CB2R agonists reduced endogenous leukocyte rolling and adhesion to brain microvessels in EAE and MCAO/R.5,12,35 Similar results were reported in experimental autoimmune uveoretinitis, and they were shown to correlate with a reduction in the expression of the adhesion molecules P-selectin glycoprotein ligand 1 and of lymphocyte function–associated antigen 1 on T cells.9 In contrast, we did not observe any differences in DC expression of L-selectin, ICAM-1 and lymphocyte function–associated antigen 1 after treatment with GP1a (data not shown). However, in vivo treatment of EAE mice with GP1a resulted in a decrease in CNS VCAM-1 expression (S.A., unpublished data, 2012), in agreement with previous reports indicating that CB2R signaling inhibited the up-regulation of VCAM-1 on vascular endothelial cells.38,39
In EAE, adhesion of immune cells to the brain vascular endothelium is followed by transmigration into the perivascular space and further infiltration into the CNS parenchyma. This process requires both chemokine cues and MMPs. CCL19 expressed by microglia and astrocytes, and CCL21 expressed by brain endothelial cells, serve as chemoattractants for DCs, T and B cells expressing CCR7.40 In addition, MMPs, particularly MMP-9, are required for transmigration and infiltration into CNS parenchyma of encephalitogenic T cells, macrophages, and DCs.26,41 Although both MMP-2 and -9 are produced by antigen-presenting cells and act on similar substrates, MMP-9 seems to play the crucial role in EAE. Double Mmp2/9 knockouts and young Mmp9–deficient mice are resistant to EAE, whereas Mmp2–deficient mice exhibit early onset and more severe EAE because of a compensatory increase in MMP-9 expression and activity.41-43
Among immune cells, DCs play a 2-pronged role in EAE. Their initial activity requires migration to the nearest lymph node where they act as antigen-presenting cells and activate cognate naive T cells. The second activity requires migration to the CNS to reactivate encephalitogenic T cells and to facilitate immune cell infiltration into the CNS parenchyma.44 Both activities require CCR7 and MMP-9 expression by DCs. Our results indicate that CB2R signaling does not affect Ccr7 expression or in vitro chemotaxis in response to CCL19. However, selective CB2R agonists inhibit DC MMP-9 expression and reduce both in vivo and in vitro matrigel migration.
Other than TIMPs (reviewed in Rietz and Spiers45 and Brew and Nagase46 ), little is known about endogenous inhibitors of MMP-9. We reported previously that inflammatory factors such as cytokines and PGE2 induce DC-derived MMP-9 and migration and that endogenous agents such as IFN-β inhibit DC migration partially through the transcriptional suppression of Mmp9.29,32 The present study adds CB2R ligands to the list of inhibitors for DC-derived MMP-9.
There are only a few reports on cannabinoid effects on MMPs. CB2R agonists were shown to inhibit Mmp2 expression in gliomas and Mmp9 expression in activated T cells.15,47 The inhibitory effect of cannabidiol, a non-CB1R, non-CB2R ligand, on tumor cell invasion has been attributed in some cases to the induction of TIMP1, and subsequent inhibition on MMPs.48 However, other reports show down-regulation of both Mmp2 and Timp1 in human glioma cels by CB2R agonists.47,49 Here, we found that GP1a-induced CB2R signaling in activated DCs inhibits MMP-9 expression without affecting TIMP-1.
Previously, we reported that the stimulatory effect of PGE2 on DC MMP-9 expression was mediated through the EP2/EP4→AMP→PKA/PI3K→ERK signaling pathway, leading to AP-1 activation and binding to the MMP-9 promoter.31 Here, we show that CB2R signaling interferes with the stimulatory pathway at an early point, by reducing cAMP levels and subsequent ERK and AP-1 activation. This is in agreement with the fact that CB2R are Gαi/o protein-coupled receptors whose primary function is the inhibition of adenylate cyclase.1 We should mention that factors reported to signal through pathways different from cAMP also induce Mmp9 expression in DCs.24,50 If these alternative pathways occur in vivo, the MMP-9–dependent migration of activated DCs might not be affected by CB2R signaling. However, the studies mentioned above did not address the possibility of endogenous PGE2 generation especially in an inflammatory milieu. In addition, the in vivo role of CB2R signaling is strengthened by our unpublished observations that treatment of EAE mice with CB2R agonists results in reduced MMP-9 expression in the CNS.
In conclusion, this study describes a novel mechanism for the physiologic and therapeutic function of cannabinoids, as mediated by the classic CB2R expressed primarily on immune cells. Through various actions, including the novel capacity to dampen DC production of MMP-9 and subsequent MMP-9–dependent migration described in this study, cannabinoids play a role in the resolution of acute inflammation and reestablishment of homeostasis. The fact that CB2R signaling affects proinflammatory cytokine and chemokine production in addition to immune cell migration represents a definite advantage over the therapeutic use of strictly MMP-9 inhibitors. Because of their anti-inflammatory functions targeting various immune cells, CB2R agonists could represent valuable therapeutic agents for the treatment of chronic inflammatory conditions.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases grant R01AI084065 (D.G., R.F.T.) and by National Institute on Drug Abuse training grant T32 DA07237 (S.A.) and P30 DA 13429 (Center for Substance Abuse Research, Temple University School of Medicine).
National Institutes of Health
Authorship
Contribution: S.A. designed and performed experiments, analyzed data, and wrote the manuscript; V.P.K. designed and performed ChIP assays; J.-H.Y. performed the microglial cultures and provided technical assistance; R.F.T. provided advice on the proposed studies; and D.G. contributed to the design of experiments, data analysis, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Doina Ganea, Department of Microbiology and Immunology, Temple University School of Medicine, 3500 N Broad St, Philadelphia, PA 19140; e-mail: doina.ganea@temple.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal