Abstract
Acquired von Willebrand syndrome is described in patients with Waldenström macroglobulinemia (WM). Assessment of ristocetin cofactor activity (VWF:RCo) and von Willebrand factor (VWF) antigen (VWF:Ag) in 72 consecutive patients with WM showed a negative relation between VWF levels < 130 U/dL and both monoclonal immunoglobulin M concentration (mIgMC) and viscosity. Ten patients with VWF:RCo < 50 U/dL (< 40 for patients with blood group O) fulfilled the acquired von Willebrand syndrome criteria. They had higher mIgMC and viscosity. Reduction in mIgMC was associated with increase in VWF levels. The low VWF:RCo/VWF:Ag ratio suggested that high viscosity might be associated with increased shear force and cleavage of multimers. Surprisingly, 43 patients (59%) presented with high VWF:Ag (> 110 U/dL). They had higher bone marrow microvessel density and vascular endothelial growth factor expression on bone marrow mast cells. Five-year survival rates of patients with VWF:Ag < 110, between 110 and 250, and more than 250 U/dL were 96%, 71%, and 44%, respectively (P < .0001). High VWF:Ag was also a significant adverse prognostic factor for survival after first-line therapy (P < .0001), independently of the international scoring system. These results support systematic assessment of VWF in patients with WM. The adverse prognostic value of high VWF levels raises issues on interactions between lymphoplasmacytic cells, mast cells, and endothelial cells in WM.
Introduction
Waldenström macroglobulinemia (WM) is a lymphoproliferative disorder, characterized by production of serum monoclonal immunoglobulin M (mIgM) and tumoral bone marrow infiltration by lymphoplasmacytic cells (LPCs).1 Most symptoms (cytopenia, organomegaly, and hyperviscosity syndrome) presumably reflect the tumor burden, whereas other manifestations are caused by the immunologic (autoimmune disorders) or physicochemical characteristics of the mIgM (cryoglobulinemia with temperature-dependent elevation of viscosity).1,2 Among these manifestations, the occurrence of acquired von Willebrand syndrome (AVWS) is a rare event, characterized by bleeding related to the decrease of von Willebrand factor (VWF) activity, without any personal or family history of bleeding, by contrast with congenital von Willebrand disease.3–6
VWF, an adhesive glycoprotein produced by the vascular endothelium and megakaryocytes, plays a key role in primary hemostasis. VWF derives from the pro-VWF, a large 360-kDa precursor. After dimerization and glycosylation in the endoplasmic reticulum and the Golgi apparatus, the pro-VWF reaches the trans-Golgi network where it undergoes multimerization and finally cleavage into mature VWF and a smaller molecule, the VWF propeptide (VWFpp).7 VWF is stored as ultralarge multimers in endothelial Weibel-Palade bodies. In cultured endothelial cells, VWF exocytosis from Weibel-Palade bodies is induced by multiple receptor agonists, including histamine,8 thrombin, and vascular endothelial growth factor (VEGF).9,10 On plasma release from endothelial cells, these ultralarge multimers are temporarily retained on the endothelium and cleaved by the plasmatic protease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin domain 13) into smaller forms, released in plasma.11 The physiologic VWF proteolytic activity of ADAMTS13 is increased by exposure to shear stress.12 VWFpp is cosecreted from endothelial cells on an equimolar basis with VWF. However, VWFpp is cleared much more rapidly than VWF, resulting in distinct VWFpp/VWF antigen (VWF:Ag) ratio under steady state conditions.13 Hence, the VWFpp/VWF:Ag ratio can reflect either VWF clearance14 or endothelial cell disorders.15 Multiple pathogenic mechanisms are described in AVWS, including selective VWF adsorption on tumoral cells, increased VWF proteolysis, and presence of both neutralizing or nonneutralizing anti-VWF antibodies.4,6 In case reports on AVWS occurring in patients with WM, either IgG anti-VWF activity6,16,17 or complex interaction between VWF and the mIgM18 have been described. To better investigate the presentation and outcome of AVWS in patients with WM, we assessed the VWF profile in a series of 72 consecutive patients.
Methods
Objectives of the study
The aims of this clinical study were, first, to describe the distribution of VWF ristocetin cofactor activity (VWF:RCo) and VWF:Ag levels in patients with WM and thus to estimate the frequency of AVWS in these patients; second, to assess the putative underlying mechanisms involved in AVWS; and, third, to evaluate the clinical and biologic significance of changes in VWF levels observed in WM.
Inclusion criteria
According to the second international workshop on WM, inclusion criteria were as follows. (1) Diagnosis was based on the detection of serum mIgM along with bone marrow tumoral LPC infiltrate higher than 20% either on bone marrow smears or on trephine biopsy.19 (2) Treatment was initiated only in symptomatic patients, defined by the presence of constitutional symptoms, cytopenia, symptomatic hepatomegaly, symptomatic splenomegaly, bulky and/or symptomatic lymphadenopathy, mIgM-related symptoms, or hyperviscosity syndrome.20 (3) Informed consent was obtained according to the protocol recommendations approved by the institution review board of the Schaffner Hospital, and this study was conducted in accordance with the Declaration of Helsinki.
From January 2004 to February 2010, 72 consecutive patients with WM seen at the Hematology Department, Hôpital Schaffner, Lens, France, were included for clinical features assessment along with the determination of VWF levels, serum viscosity, cryoglobulinemia, blood group, complete blood count, C-reactive protein, fibrinogen, and serum mIgM concentration (obtained from the densitometry tracing of the serum electrophoretic pattern). Plasma was collected for VEGF measurement at the same time in 30 patients.
At the time of inclusion, median age was 70 years (range, 39-91 years) and sex ratio (M/F) was 1.6; 53 patients had never received therapy and 19 patients were relapsing more than 6 months after 1 previous treatment. Overall, 17 patients were asymptomatic and 55 were symptomatic and eligible for therapy. None of the former patients and 19 of the latter patients had received prior therapy. Main clinical and treatment characteristics are summarized in supplemental Table 1 and supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Median overall survival and median survival after first-line therapy were estimated at 108 and 79 months, respectively, with a median follow-up of 54 months in patients alive at stopping date.
Blood collection, serum viscosity, VEGF, and hemostasis assays
At study inclusion, plasma was collected in 0.129M buffer citrate and stored at −70°C. Plasma level of VWF:Ag was measured by immunoturbidimetry with the sue of VWF:Ag reagent (Siemens, Healthcare Diagnostics) on STAR (Stago). VWF:RCo was measured by aggregometry with the use of the BC von Willebrand Reagent (Siemens, Healthcare Diagnostics). A VWF:RCo/VWF:Ag ratio more than 0.7 was considered as normal. AVWS was suspected when VWF:RCo level was lower than 50 U/dL (40 U/dL for patients with blood group O). If so, the levels of VWF:RCo and VWF:Ag were monitored during the entire follow-up. The multimeric structure of plasma VWF was analyzed by electrophoresis with 0.1% sodium dodecyl sulfate and 1.5% agarose gel, and the percentage of the highest molecular weight multimers (> 15 mers) was determined after densitometric scanning, as previously described.21 A pool of normal platelet-poor plasma was used as a reference in each gel electrophoresis. The presence of anti-VWF antibody was assessed by ELISA as previously described.17 The VWFpp level was measured by ELISA (GTI Diagnostics) in a subgroup of 21 patients with (7) or without (14) AVWS criteria. A VWFpp/VWF:Ag ratio higher than 2.8 (blood group O) or higher than 2.4 (other blood group) indicates an increased clearance of mature VWF.
VEGF level was determined by ELISA on plasma collected on EDTA and stored at −70°C, using the Human VEGF Quantikine kit (R&D Systems), according to the manufacturer's protocol. This assay determines the level of the most abundant plasma VEGF isoform (165 kDa) whose normal value remains < 115 pg/mL.
Serum viscosity (η) was assessed with a Ubbelhode viscosimeter (Schott Instruments GmbH) with suspending ball-level for the determination of kinematic viscosity, under atmospheric pressure, at 37°C, by measuring the flow time t with the following equation: η = t.K, where K is the viscosimeter constant, given by the manufacturer and checked at regular intervals. η was expressed in centi-Stoke, and normal values range from 1.08 to 1.22. Kinematic viscosity is the ratio of the viscous force, estimated by dynamic viscosity (in centipoise) to the inertial force, characterized by serum density.
Immunohistochemical analyses of bone marrow von Willebrand factor, microvessel density, and VEGF
Four percent formalin-fixed, paraffin-embedded bone marrow biopsy specimens were decalcified with RDO (Eurobio). New sections 3 μm thick were obtained from each block and stained with hematoxylin and eosin for histologic study. Immunohistochemical analysis of von Willebrand factor expression was performed with the automated immunostainer (Dako) with polyclonal rabbit anti–human von Willebrand factor antibody (Dako), and a standard labeled streptavidin–biotin–peroxidase complex technique, according to the instructions provided by the manufacturer (Kit LSAB2 peroxydase; Dako). Revelation was performed with 3-amino-9-ethylcarbazole. Immunohistochemical analysis of VEGF expression was performed with the automated immunostainer BENCHMARK XT (Ventana Medical Systems) and mouse monoclonal anti-VEGF antibody (dilution 1/50; Santa Cruz Biotechnology). Revelation was performed with Ultraview Universal DAB Detection Kit (Ventana Medical Systems) in combination with antigen retrieval pretreatment with pH 8 CC1 buffer.
Bone marrow biopsy was available for immunohistochemical analyses in 23 patients (3 patients with AVWS and 20 with normal or high VWF levels). To ensure completely blinded analyses, VWF and VEGF immunostainings were performed in 2 independent pathology laboratories (Lens and Clermont Ferrand) on adjacent sections from each bone marrow biopsy and assessed in duplicate by S.P. and P.M. and by C. Charpy. and O.T., respectively, who were also blinded to both clinical and hemostasis data.
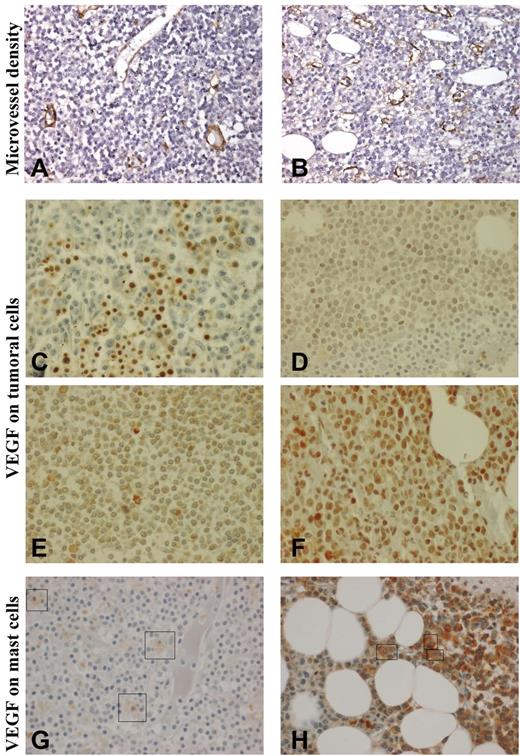
Anti-VWF antibody stained endothelial cells that normally act as internal positive control in all cases. A positivity in less than 10% of cells of interest (LPCs) was considered as nonsignificant. Microvessel density (MVD) was evaluated by means of VWF immunostaining. Each bone marrow biopsy specimen was first totally scanned at low magnification (×100) to identify 3 hot spot zones. MVD was then estimated at high magnification (×400) within hot spot zones with the use of a semiquantitative method that was based on the following scale: grade 0, vascularity similar to normal control; grade 1, slightly increased MVD (Figure 1A); grade 2, moderately increased MVD; and grade 3, markedly increased MVD (Figure 1B).
Immunohistochemistry in bone marrow biopsies. Microvessel density (MVD; Nikon Eclipse 80i and Sony DFW-X700 camera, ×400): (A): MVD graded 1 (slightly increased); (B) MVD graded 3 (markedly increased). Vascular endothelial growth factor (VEGF) expression (Olympus BX-51 microscope and DP50 camera, ×600) on bone marrow lymphoplasmacytic cells (LPCs): (C) strong staining on erythrocytes, no staining on LPCs; (D) grade 1 (weak) staining of 10%-50% of LPCs; (E) grade 2 (moderate) staining of more than 50% of LPCs; (F) grade 3 (strong) staining of more than 50% of LPCs. VEGF expression on mast cells: (G) weak staining (+) and (H) moderate staining (++). Because mast cells are rare and scattered compared with LPCs, they have been indicated by a box.
Immunohistochemistry in bone marrow biopsies. Microvessel density (MVD; Nikon Eclipse 80i and Sony DFW-X700 camera, ×400): (A): MVD graded 1 (slightly increased); (B) MVD graded 3 (markedly increased). Vascular endothelial growth factor (VEGF) expression (Olympus BX-51 microscope and DP50 camera, ×600) on bone marrow lymphoplasmacytic cells (LPCs): (C) strong staining on erythrocytes, no staining on LPCs; (D) grade 1 (weak) staining of 10%-50% of LPCs; (E) grade 2 (moderate) staining of more than 50% of LPCs; (F) grade 3 (strong) staining of more than 50% of LPCs. VEGF expression on mast cells: (G) weak staining (+) and (H) moderate staining (++). Because mast cells are rare and scattered compared with LPCs, they have been indicated by a box.
On the basis of both staining intensity and percentage of stained cells, VEGF expression on LPCs was graded as follows: O, no or rare staining (< 10% of LPCs; Figure 1C); 1, weak staining (10%-50% of LPCs; Figure 1D); 2, moderate staining in more than 50% of LPCs (Figure 1E); and 3, strong staining in more than 50% of LPCs (Figure 1F). Cytoplasmic staining of VEGF on mast cells was graded as follows: −, no staining; +, weak staining (Figure 1G); and ++, moderate staining (Figure 1H). VEGF staining on endothelial cells was graded as follows: −, no staining; and +, presence of stained endothelial cells.
Statistical analyses
Nonlinear regression analyses with transformation of the original variables with the use of smoothing spline function, when needed, and Spearman rank correlation coefficients were used for assessing the relations between VWF levels and other quantitative characteristics, including serum IgM concentration, serum viscosity, and plasma VEGF.
Descriptive statistics of patients with suspected AVWS or other abnormalities of VWF included all clinical and demographic characteristics.
Repeated simultaneously evaluations of VWF:Ag, VWF:RCo, and mIgM concentration were available in patients with AVWS. The correlation coefficients between VWF:Ag and VWF:RCo levels and serum mIgM concentration were estimated and tested with the linear mixed model.22
Overall survival, survival after inclusion, and survival after first-line therapy were calculated from the date of diagnosis, the date of inclusion, and the date of initiation of first-line therapy, respectively, to the stopping date, the date of last follow-up, or the date of death if they occurred earlier. The validity of the proportional hazard assumption was checked by the distribution of Schoenfeld residuals and the test proposed by Therneau and Grambsch.23 Survival and standard deviation were estimated by the method of Kaplan and Meier and were compared by use of the log-rank test. The prognostic significance of VWF abnormalities was explored on the whole series with the following analyses. (1) Recursive partitioning analysis of overall survival with VWF:Ag as covariate was used to identify cutoff values. (2) VWF:Ag was introduced as a binary covariate in a Cox model for survival after first-line therapy along with the presence of AVWS or intermediate or high international scoring system (ISSWM) risk or each of the adverse prognostic characteristics included in the ISSWM.24 Only bivariate Cox models were fitted because of the limited number of events. (3) Martingale residuals were used to assess the functional form of VWF:Ag in a Cox model of overall survival. Finally, the relations between VWF level and bone marrow MVD, between VWF level and bone marrow VEGF expression, and between bone marrow MVD and bone marrow VEGF expression were evaluated by the Mann-Whitney test and the 2-sided Fisher test. Because of the limited sample size for the immunohistochemical studies, bootstrap resampling (1000 replicates) was used. All statistical analyses were conducted with the SAS 8.0 (SAS Institute) and the S-PLUS 6.2 (Insightful Corp) software programs.
Results
Inverse relations between VWF levels less than 130 U/dL and both mIgM concentration and viscosity
Median levels of VWF:Ag and VWF:RCo were 132 U/dL (range, 9-804 U/dL) and 112 U/dL (range, 13-800 U/dL), respectively. No significant difference was observed in VWF:Ag and VWF:RCo levels between asymptomatic patients, symptomatic patients without prior therapy, and patients with symptomatic relapse (P = .73 and P = .52, respectively). Increased VWF levels were observed in a significant proportion of patients with WM. The relations between VWF levels and both serum mIgM concentration and viscosity were not linear. Indeed, smoothing spline analyses showed linear relations between serum mIgM concentration and both VWF:RCo and VWF:Ag only for levels < 130 U/dL (supplemental Figure 1). For patients with VWF levels less than 130 U/dL, the Spearman rank correlation coefficients (R) confirmed the negative relations between serum mIgM concentration and VWF:RCo (R = −0.55, P = .009) and VWF:Ag (R = −0.60, P = .007).
Similarly, smoothing spline analyses showed negative linear relations between serum viscosity and VWF:RCo and VWF:Ag only for levels below 130 U/dL (supplemental Figure 1, R = −0.60, P = .0014 and R = −0.66, P = .0009, respectively). No other clinical characteristic was found associated with VWF values.
Characteristics of patients with suspected AVWS
AVWS was suspected in 10 patients with symptomatic WM. In these patients, VWF:RCo ranged from 13 to 45 U/dL and VWF:Ag from 9 to 69 U/dL. Their main characteristics are shown in Table 1. Compared with patients with normal or high VWF concentrations, patients with suspected AVWS had higher serum mIgM concentration (median, 42 vs 13.2 g/L; P = .0002 Mann-Whitney test) and higher serum viscosity (median, 3.25 vs 1.33 g/L; P < .0001 Mann-Whitney test), and cryoglobulinemia was more frequently recorded (5 of 10 patients vs 7 of 62; P = .008).
Characteristics of patients with suspected acquired von Willebrand syndrome (10 patients)
| Unique patient no. . | ABO blood group . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | VWF:RCo/VWF:Ag ratio . | VWFpp/VWF:Ag ratio* . | Anti-VWF antibody . | IgM, g/L . | Serum viscosity, cSt . | Survival after first treatment, mo† . | Age at treatment initiation, y . | International scoring system24 . | Clinical features and treatment . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 296 | A | 64 | 21 | 0.33 | 1.84 | Absent | 42 | 3.46 | 72+ | 67 | High | Epistaxis, 4 plasma exchanges (Figure 3A) |
| 314 | O | 9 | 14 | 1.56 | 20.3 | Absent | 78 | NA | 123+ | 73 | High | Gingivorrhagia, epistaxis. 7 plasma exchanges; desmopressin before bone marrow biopsy (Figure 3B) |
| 333 | A | 47 | 45 | 0.96 | 2.06 | Absent | 36 | 2 | 76 | 77 | High | Epistaxis (Figure 3C) |
| 520 | O | 48 | 31 | 0.65 | 6.85 | NA | 47 | 3.25 | 15+ | 62 | Intermediate | Epistaxis |
| 467 | A | 69 | 43 | 0.62 | NA | NA | 39 | 3.5 | 170 | 63 | Unknown | Epistaxis; human VWF before insertion of device for plasma exchanges (7) |
| 572 | NA | 24 | 22 | 0.92 | NA | NA | 65 | 5.75 | 8+ | 42 | Low | Recombinant factor VIII before insertion of device for plasma exchange (2) |
| 577 | A | 22 | 21 | 0.95 | 4.18 | Absent | 54 | 3.72 | 6+ | 60 | Intermediate | Human VWF before bone marrow biopsy |
| 351 | O | 36 | 13 | 0.36 | 8.33 | Absent | 34 | 2.4 | 184 | 61 | Unknown | Hematoma of wrist; surgery without hemostatic therapy (Figure 3D) |
| 337 | B | 40 | 28 | 0.70 | 2.85 | Absent | 32 | 3.1 | 176 | 39 | Low | Surgery without hemostatic therapy |
| 566 | O | 38 | 32 | 0.84 | NA | NA | 50.3 | 3.18 | 8+ | 59 | Low | 3 plasma exchanges |
| Unique patient no. . | ABO blood group . | VWF:Ag, U/dL . | VWF:RCo, U/dL . | VWF:RCo/VWF:Ag ratio . | VWFpp/VWF:Ag ratio* . | Anti-VWF antibody . | IgM, g/L . | Serum viscosity, cSt . | Survival after first treatment, mo† . | Age at treatment initiation, y . | International scoring system24 . | Clinical features and treatment . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 296 | A | 64 | 21 | 0.33 | 1.84 | Absent | 42 | 3.46 | 72+ | 67 | High | Epistaxis, 4 plasma exchanges (Figure 3A) |
| 314 | O | 9 | 14 | 1.56 | 20.3 | Absent | 78 | NA | 123+ | 73 | High | Gingivorrhagia, epistaxis. 7 plasma exchanges; desmopressin before bone marrow biopsy (Figure 3B) |
| 333 | A | 47 | 45 | 0.96 | 2.06 | Absent | 36 | 2 | 76 | 77 | High | Epistaxis (Figure 3C) |
| 520 | O | 48 | 31 | 0.65 | 6.85 | NA | 47 | 3.25 | 15+ | 62 | Intermediate | Epistaxis |
| 467 | A | 69 | 43 | 0.62 | NA | NA | 39 | 3.5 | 170 | 63 | Unknown | Epistaxis; human VWF before insertion of device for plasma exchanges (7) |
| 572 | NA | 24 | 22 | 0.92 | NA | NA | 65 | 5.75 | 8+ | 42 | Low | Recombinant factor VIII before insertion of device for plasma exchange (2) |
| 577 | A | 22 | 21 | 0.95 | 4.18 | Absent | 54 | 3.72 | 6+ | 60 | Intermediate | Human VWF before bone marrow biopsy |
| 351 | O | 36 | 13 | 0.36 | 8.33 | Absent | 34 | 2.4 | 184 | 61 | Unknown | Hematoma of wrist; surgery without hemostatic therapy (Figure 3D) |
| 337 | B | 40 | 28 | 0.70 | 2.85 | Absent | 32 | 3.1 | 176 | 39 | Low | Surgery without hemostatic therapy |
| 566 | O | 38 | 32 | 0.84 | NA | NA | 50.3 | 3.18 | 8+ | 59 | Low | 3 plasma exchanges |
VWF:Ag indicates von Willebrand factor (VWF) antigen; VWF:RCo, ristocetin cofactor activity; VWFpp, propeptide; IgM, monoclonal immunoglobulin M concentration (densitometry); and NA: not available.
A VWF:pp/VWF:Ag ratio > 2.8 in case of blood group O or > 2.4 otherwise denotes an increase in the clearance of mature VWF. Cryoglobulinemia was found in patients 314, 351, 467, 572, and 577.
Censoring is denoted with +.
In 4 patients with AVWS a low VWF:RCo/VWF:Ag ratio was found, which could indicate a decreased proportion of the high molecular weight multimers. VWF multimeric distribution was assessed in 2 patients with AVWS, but the presence of the mIgM precluded quantitative studies (supplemental Figure 2). The high VWFpp/VWF:Ag ratio, found in 5 of the 7 evaluated patients, suggested that in most cases there was an increase in the clearance of mature VWF (Figure 2). However, anti-VWF antibody was found in none of the 6 tested patients, and immunohistochemical staining on bone marrow trephine biopsy found no significant presence of VWF on LPCs in 3 patients (Table 2).
The propeptide (VWFpp) to von Willebrand factor antigen (VWF:Ag) ratio in Waldenstrom macroglobulinemia (WM). *The VWFpp/VWF:Ag ratio is significantly higher in 7 patients with WM with suspected acquired von Willebrand syndrome (AVWS), compared with 14 patients without suspected AVWS. The latter patients were stratified according to VWF:Ag values identified by the recursive partitioning analysis. Solid line indicates the median.
The propeptide (VWFpp) to von Willebrand factor antigen (VWF:Ag) ratio in Waldenstrom macroglobulinemia (WM). *The VWFpp/VWF:Ag ratio is significantly higher in 7 patients with WM with suspected acquired von Willebrand syndrome (AVWS), compared with 14 patients without suspected AVWS. The latter patients were stratified according to VWF:Ag values identified by the recursive partitioning analysis. Solid line indicates the median.
Histological findings, microvessel density, and vascular endothelial growth factor (VEGF) expression in bone marrow biopsy of 23 patients with Waldenström macroglobulinemia
| Unique patient no. . | VWF:Ag, U/dL . | VWFpp/ VWF:Ag ratio . | Pathologic pattern . | Microvessel density . | VEGF expression . | ||
|---|---|---|---|---|---|---|---|
| Lymphoplasmacytic cells . | Mast cells . | Endothelial cells . | |||||
| 520 | 48 (AVWS) | 6.85 | Diffuse | 2 | 0 | − | − |
| 333 | 47 (AVWS) | 2.06 | Nodular | 2 | 1 | + | − |
| 296 | 64 (AVWS) | 1.84 | Nodular paratrabecular | 1 | 1 | − | − |
| 308 | 69 | NA | Diffuse | 2 | 0 | − | + |
| 357 | 74 | NA | Interstitial paratrabecular | 1 | 0 | − | − |
| 526 | 97 | NA | Nodular | 1 | 0 | + | − |
| 529 | 71 | NA | Diffuse | 2 | 1 | − | − |
| 311 | 107 | 0.76 | Diffuse | 2 | 3 | − | − |
| 321 | 111 | 1.31 | Diffuse | 1 | 0 | − | − |
| 482 | 137 | NA | Interstitial paratrabecular | 1 | 3 | − | + |
| 319 | 150 | NA | Paratrabecular | 1 | 0 | − | − |
| 524 | 199 | NA | Interstitial | 1* | 2 | − | + |
| 501 | 197 | 0.89 | Diffuse | 2 | 3 | − | +/− (localized) |
| 505 | 213 | NA | Diffuse | 2 | 1 | − | − |
| 327 | 150 | 0.67 | Diffuse paratrabecular | 3 | NA* | − | − |
| 368 | 158 | NA | Diffuse | 3 | 1 | + | - |
| 401 | 246 | 1.07 | Interstitial paratrabecular | NA | 2 | ++ | NA |
| 523 | 271 | NA | Diffuse | 2 | 3 | − | + |
| 318 | 322 | NA | Diffuse | 2 | 0 | + | − |
| 307 | 364 | NA | Interstitial paratrabecular | 3 | 0 | + | − |
| 440 | 552 | NA | Diffuse | 3 | 1 | ++ | − |
| 531 | 565 | NA | Diffuse | 3 | 3 | + | +/− (localized) |
| 329 | 804 | NA | Diffuse | 3 | 0 | − | − |
| Unique patient no. . | VWF:Ag, U/dL . | VWFpp/ VWF:Ag ratio . | Pathologic pattern . | Microvessel density . | VEGF expression . | ||
|---|---|---|---|---|---|---|---|
| Lymphoplasmacytic cells . | Mast cells . | Endothelial cells . | |||||
| 520 | 48 (AVWS) | 6.85 | Diffuse | 2 | 0 | − | − |
| 333 | 47 (AVWS) | 2.06 | Nodular | 2 | 1 | + | − |
| 296 | 64 (AVWS) | 1.84 | Nodular paratrabecular | 1 | 1 | − | − |
| 308 | 69 | NA | Diffuse | 2 | 0 | − | + |
| 357 | 74 | NA | Interstitial paratrabecular | 1 | 0 | − | − |
| 526 | 97 | NA | Nodular | 1 | 0 | + | − |
| 529 | 71 | NA | Diffuse | 2 | 1 | − | − |
| 311 | 107 | 0.76 | Diffuse | 2 | 3 | − | − |
| 321 | 111 | 1.31 | Diffuse | 1 | 0 | − | − |
| 482 | 137 | NA | Interstitial paratrabecular | 1 | 3 | − | + |
| 319 | 150 | NA | Paratrabecular | 1 | 0 | − | − |
| 524 | 199 | NA | Interstitial | 1* | 2 | − | + |
| 501 | 197 | 0.89 | Diffuse | 2 | 3 | − | +/− (localized) |
| 505 | 213 | NA | Diffuse | 2 | 1 | − | − |
| 327 | 150 | 0.67 | Diffuse paratrabecular | 3 | NA* | − | − |
| 368 | 158 | NA | Diffuse | 3 | 1 | + | - |
| 401 | 246 | 1.07 | Interstitial paratrabecular | NA | 2 | ++ | NA |
| 523 | 271 | NA | Diffuse | 2 | 3 | − | + |
| 318 | 322 | NA | Diffuse | 2 | 0 | + | − |
| 307 | 364 | NA | Interstitial paratrabecular | 3 | 0 | + | − |
| 440 | 552 | NA | Diffuse | 3 | 1 | ++ | − |
| 531 | 565 | NA | Diffuse | 3 | 3 | + | +/− (localized) |
| 329 | 804 | NA | Diffuse | 3 | 0 | − | − |
VWF:Ag indicates von Willebrand factor antigen; VWFpp, VWF propeptide; AVWS, acquired von Willebrand syndrome; and NA, not available.
Grades for semiquantitative assessment of immunostainings for microvessel density are as follows: 0, similar vascularity in comparison with normal control; 1, slightly increased; 2, moderately increased; and 3, markedly increased. Grades for VEGF on lymphoplasmacytic cells are as follows: 0, no significant staining (< 10% of cells); 1, staining in 10%-50% of cells; 2, moderate staining in more than 50% of cells; and 3, strong staining in more than 50% of cells. Cytoplasmic staining of VEGF in mast cells is as follows: −, absent; +, weak; and ++, moderate. Cytoplasmic staining of endothelial cells is as follows: −, absent; and +, present.
Material was dissociated and/or hemorrhagic.
Six patients presented with mild bleeding (mainly epistaxis). Eight patients underwent surgical or invasive procedure: 4 received preventive treatment, including desmopressin (1 patient), factor VIII (1 patient), or VWF concentrates (2 patients); and 4 patients did not need hemostatic treatment for the insertion of plasmapheresis device or after long-term response to WM therapy (Table 1).
All patients with suspected AVWS received WM treatment, and VWF levels were monitored during the follow-up. Management of hyperviscosity required plasma exchanges in 5 patients (2-7 plasma exchanges over 2-15 days). Plasma exchanges were followed by normalization of VWF:RCo levels within 10 days after the initiation of plasmapheresis in all patients but 1 (VWF:RCo level rose from 10 to 32 U/dL after the fourth exchange). Short-term and long-term reductions of IgM level observed after plasma exchanges and WM treatment were associated with an increase in both VWF:Ag and VWF:RCo levels (linear mixed model: R = −0.377, P = .0047 and R = −0.46, P = .0023, respectively). Figure 3 displays the long-term changes observed in 4 patients as examples of this association.
Evolution of ristocetin cofactor activity (VWF:RCo) and Willebrand factor antigen (VWF:Ag) and serum monoclonal immunoglobulin M (IgM) concentration over time in 4 patients with acquired von Willebrand syndrome. The solid line indicates the evolution of serum IgM concentration, the dashed line indicates VWF:Ag, and the line of alternating dashes and dots indicates the VWF:RCo. Arrows indicate that plasma exchanges have been performed. (A) Patient 296, (B) patient 314, (C) patient 333, and (D) patient 351.
Evolution of ristocetin cofactor activity (VWF:RCo) and Willebrand factor antigen (VWF:Ag) and serum monoclonal immunoglobulin M (IgM) concentration over time in 4 patients with acquired von Willebrand syndrome. The solid line indicates the evolution of serum IgM concentration, the dashed line indicates VWF:Ag, and the line of alternating dashes and dots indicates the VWF:RCo. Arrows indicate that plasma exchanges have been performed. (A) Patient 296, (B) patient 314, (C) patient 333, and (D) patient 351.
Clinical presentation and outcome of patients according to VWF:Ag levels
Recursive partitioning analysis on overall survival of the whole cohort of 72 patients identified 2 cutoff values, 110 and 250 U/dL, for VWF:Ag level. Forty-three patients (59%) presented with VWF:Ag level higher than 110 U/dL (median, 234 U/dL; range, 111-804 U/dL), including 13 patients with very high level (higher than 250 U/dL). Twenty-nine patients (41%) had VWF:Ag less than 110 U/dL (including the 10 patients with AVWS). These cutoff values were used to define 3 subgroups of patients, based on their VWF:Ag levels (VWF:Ag less than 110, 110-250, and higher than 250 U/dL). No significant difference in the distribution of both clinical and inflammatory characteristics (fibrinogen and C-reactive protein) was observed among these 3 subgroups (supplemental Table 1). However, these subgroups retained significantly different overall survival (Figure 4A), survival after first-line therapy (Figure 4B) and survival after VWF assessment (P < .0001 for each analysis). Conversely, the presence of AVWS had no influence on overall survival (P = .09) and survival after treatment initiation (P = .18). Bivariate Cox models of survival after treatment initiation and overall survival showed that AVWS retained no independent prognostic value beside VWF:Ag (supplemental Table 2; P = .31, respectively). Cox model of survival after the initiation of therapy, taking intermediate or high ISSWM and VWF:Ag level above 250 U/dL as adverse covariates, selected both characteristics as independent prognostic factors (P < .001). Similar results were found in a model that included VWF:Ag level with a cutoff value set at 110 U/dL instead of 250 U/dL (P = .015). VWF:Ag was also an independent prognostic factor in bivariate Cox models with each characteristic of the ISSWM (including age; supplemental Table 2). Functional form analysis of the Martingale residuals distribution confirmed the prognostic value associated with nearly normal level of VWF:Ag level (supplemental Figure 3).
Prognostic significance of von Willebrand factor (VWF) level in patients with Waldenström macroglobulinemia (WM). (A) Overall survival in 72 patients with WM and (B) survival after first-line therapy in 55 patients with WM, according to VWF antigen (VWF:Ag) level. The line of alternating dashes and dots indicates VWF:Ag < 110 U/dL, the dotted line indicates VWF:Ag level between 110 and 250 U/dL, and the solid line indicates VWF:Ag more than 250 U/dL.
Prognostic significance of von Willebrand factor (VWF) level in patients with Waldenström macroglobulinemia (WM). (A) Overall survival in 72 patients with WM and (B) survival after first-line therapy in 55 patients with WM, according to VWF antigen (VWF:Ag) level. The line of alternating dashes and dots indicates VWF:Ag < 110 U/dL, the dotted line indicates VWF:Ag level between 110 and 250 U/dL, and the solid line indicates VWF:Ag more than 250 U/dL.
The VWF:Ag concentration had been regularly measured for 1-5 years in 17 patients with normal or elevated baseline VWF:Ag level. No variation was observed in the 6 untreated patients or in the 11 patients who received therapy during this period. Late increase (more than a doubling with absolute value higher than 250%) was recorded in 2 patients, 2 and 4 years after first sampling and treatment initiation.
The VWFpp was available in 14 patients without AVWS. Patients with VWF:Ag lower than 110 U/dL, between 110 and 250 U/dL, and above 250 U/dL had a similar VWFpp/VWF:Ag ratio close to 1 (Kruskal-Wallis test: P = .39; Figure 2). This distribution in VWFpp/VWF:Ag ratio might rather suggest the presence of a possible chronic endothelial activation in patients with high VWF:Ag levels but required confirmation on a larger number of cases.
Plasma concentration of VEGF was above the upper value of normal range in 75% of patients (median, 190 pg/mL; range, 19-1134 pg/mL; 30 patients). We failed to identify any correlation between VWF and plasma concentration of VEGF (P = .17). Bone marrow immunostaining for VWF identified a markedly increased MVD (grade 3) in 6 of the 22 tested patients (Table 2; Figure 1A-B) in agreement with a previous report.25 VEGF was detected on LPCs (grade 1-3) in 13 patients (of 22; Table 2; Figure 1C-F) and on mast cells (grade + to ++) in 8 patients (of 23; Table 2; Figure 1G-H). Patients with markedly increased MVD and patients expressing VEGF on mast cells had higher levels of VWF:Ag than the others (Mann-Whitney test: P = .06 and .003, respectively). Markedly increased MVD was more frequently observed in patients with VWF:Ag higher than 250 U/dL (2-sided Fisher test: bootstrapped P = .02). In addition, patients with markedly increased MVD (grade 3) expressed VEGF on mast cells more frequently (bootstrapped P = .05) than patients with slightly to moderately increased MVD (grade 1 or 2).
Discussion
The present study showed that 10 of 72 patients with WM (13%) fulfilled criteria of AVWS, which is, so far, one of the largest reports of this association. Patients with AVWS had higher serum IgM concentration, higher serum viscosity, and more frequent cryoglobulinemia than unaffected patients. Systematic screening of AVWS in patients with WM may have important clinical implications. Plasma exchanges by reducing hyperviscosity allow a quick resolution of the VWF defect, and some patients may also benefit from desmopressin or replacement therapy to cover invasive procedures. We sought to explain the mechanism of AVWS. The high VWFpp/VWF:Ag ratio observed in most tested patients suggested an increase in VWF clearance. Antibody-mediated inhibition of VWF activity has been reported in patients with WM,16,17 albeit infrequently.6 The dramatic increase in VWF:RCo level immediately after plasma exchanges differs from the pattern of response described in Ig-mediated disorders such as mixed cryoglobulinemia or IgM-related neuropathies,26 and we did not identify anti-VWF activity in all tested patients. Although we cannot rule out an autoimmune mechanism, the negative relations between VWF levels and both mIgM concentration and serum viscosity are more consistent with a specific (and nonimmune) interaction between VWF and mIgM, as previously described.18 The increased serum viscosity and the low VWF:RCo/VWF:Ag ratio observed in some patients with AVWS may suggest an increased cleavage and clearance of VWF multimers, although the demonstration could not be provided by the multimeric pattern analysis. Indeed, shear force applied to VWF or cells is directly linked to viscosity, and 2- to 3-fold increase in shear stress has been associated with VWF proteolysis in small vessels or capillaries. Thus, high viscosity may accelerate the proteolysis of the high molecular weight multimers and may explain a disproportionate decrease in VWF:RCo.12 Finally, imunohistochemical studies failed to identify VWF adsorption on LPCs; therefore, the drop in VWF levels in patients with high mIgM appears to be unrelated to bone marrow LPC infiltration.
Surprisingly, 59% of patients with WM had high levels of VWF:Ag (median, 234 IU/dL). In addition, high VWF:Ag levels significantly affected both overall survival and survival after first-line treatment. Despite the large sample size and the prolonged follow-up, external validation would be useful to confirm these data. Functional form analysis of the distribution of hazard confirmed the prognostic value associated with nearly normal value of VWF:Ag level, whereas AVWS was not associated with a particular prognostic role. Similar distribution of hazard has already been observed in other malignancies, for example, with LDH level around the upper limit of normal in the mantle cell international prognostic index study.27 Moreover, VWF:Ag level and ISSWM (or each parameter of this scoring system) retained independent prognostic values. Because ISSWM was built as a combination of age and covariates mainly related to tumor burden, we suggested that VWF:Ag may provide additional prognostic information related to the microenvironment pattern.
In patients without AVWS (ie, with normal or high levels of VWF:Ag) the VWFpp/VWF:Ag ratio was close to 1, suggesting a possible endothelial activation, especially in patients with VWF levels greater than 250 U/dL. Endothelial cell activation occurs in response to inflammation and cytokine secretion, including VEGF28 and interleukin-6 (IL-6).29 IL-6 is a proinflammatory cytokine with an important role in normal and malignant B-cell biology; IL-6 up-regulates IgM secretion in WM cells via the JAK/STAT pathway.30
In addition, VEGF expression on bone marrow mast cells was associated with high VWF concentration. Besides growth and survival signals provided to WM LPCs,31,32 mast cells regulate endothelial cell proliferation and function by means of mediators such as heparin, histamine, and angiogenic factors, especially VEGF.33–37 Histamine and VEGF have been reported to be involved in VWF exocytosis also.8–10
Finally, inflammatory parameters were slightly albeit nonsignificantly increased in patients with high VWF:Ag (whereas those parameters had no significant independent prognostic value for subsequent outcome; data not shown). An adverse prognostic effect of very high VWF levels has been reported in inflammatory condition, similarly to the present study.38 Enhanced VWF secretion and oxidative stress are both hallmarks of inflammation. VWF may amplify the consequences of the inflammatory process because oxidative stress induces structural changes that render multimers more resistant to cleavage by ADAMTS13.39
Although we found increased plasma levels of VEGF in 75% of patients with WM as described previously,40 no correlation was observed between plasma VEGF and VWF. Beside a possible role of VWF itself on angiogenesis41 and the aforementioned potential mechanisms, plasma concentration of VEGF may only reflect a limited role in VWF production and clearance.
In conclusion, our results suggest that VWF should be systematically assessed in patients with WM. Few patients will present with decreased VWF activity (AVWS) that probably results, at least partly, from hyperviscosity that requires specific management, including plasma exchanges. Conversely, a large proportion of patients presents with high or very high VWF levels along with higher bone marrow microvessel density, higher VEGF expression on BM mast cells, and impaired prognosis, suggesting that VWF could drive or be associated with a microenvironment pattern favorable to LPC growth and survival. Put together, these findings raise new issues on the underlying cellular interactions among LPCs, mast cells, and endothelial cells in WM.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Valerie Tournilhac, MD, for her assistance in manuscript preparation.
Authorship
Contribution: C. Caron, J.G., and P.M. designed the study; B.H. and P.M. collected patients clinical data; S.P, C. Charpy, and O.T. performed the immunohistochemical studies; C. Caron, C.F.-S., and J.G performed the hemostasis studies; C.Z. performed plasma VEGF study; S.L. and M.R. collected biochemistry data (electrophoresis, viscosity); A.D. and P.M. performed statistical analysis; B.H, C. Caron, J.G., O.T., and P.M. wrote the manuscript; C. Charpy, C.Z., S.P., and S.S. wrote some parts of the manuscript; O.T. and A.D. commented on the manuscript; and all authors approved the manuscript..
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Morel, Service d'Hematologie Clinique, Centre hospitalier Schaffner, 99 rute de La Bassee, 62300 Lens; e-mail: pmorel@ch-lens.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal