Abstract
VWF is required for platelet adhesion to sites of vessel injury, a process vital for both hemostasis and thrombosis. Enhanced VWF secretion and oxidative stress are both hallmarks of inflammation. We recently showed that the neutrophil oxidant hypochlorous acid (HOCl) inhibits VWF proteolysis by ADAMTS13 by oxidizing VWF methionine 1606 (M1606) in the A2 domain. M1606 was readily oxidized in a substrate peptide, but required urea in multimeric plasma VWF. In the present study, we examined whether shear stress enhances VWF oxidation. With an HOCl-generating system containing myeloperoxidase (MPO) and H2O2, we found that shear stress accelerated M1606 oxidation, with 56% becoming oxidized within 1 hour. Seven other methionine residues in the VWF A1A2A3 region (containing the sites for platelet and collagen binding and ADAMTS13 cleavage) were variably oxidized, one completely. Oxidized methionines accumulated preferentially in the largest VWF multimers. HOCl-oxidized VWF was hyperfunctional, agglutinating platelets at ristocetin concentrations that induced minimal agglutination using unoxidized VWF and binding more of the nanobody AU/VWFa-11, which detects a gain-of-function conformation of the A1 domain. These findings suggest that neutrophil oxidants will both render newly secreted VWF uncleavable and alter the largest plasma VWF forms such that they become hyperfunctional and resistant to proteolysis by ADAMTS13.
Introduction
VWF is an enormous plasma glycoprotein synthesized in endothelial cells and megakaryocytes, the primary function of which is to attach platelets to sites of blood vessel injury.1 VWF also chaperones coagulation factor VIII in plasma, protecting it from degradation and delivering it to the platelet surface, where it participates in blood coagulation.2
VWF comprises homopolymers of a 2050–amino acid polypeptide synthesized through N-terminal disulfide bonding of C-terminal disulfide–bonded dimers.3 Each monomer has many domains, including 3 tandem A domains (A1A2A3) containing, respectively, the platelet glycoprotein (GP) Ib–binding site, the cleavage site for ADAMTS13, and a collagen-binding site.3 The polymers can reach a mass of > 20 million Da4 and are either constitutively secreted or stored in granules, the Weibel-Palade bodies of endothelial cells, or the α-granules of platelets, from which they are released upon stimulation.5 Newly released VWF multimers (termed ultra-large VWF or ULVWF) are hyperadhesive for platelets compared with the VWF normally found in plasma,6 but are rapidly converted to smaller, less reactive plasma forms by cleavage by the metalloprotease ADAMTS13.1
In addition to its hemostatic roles, VWF is also involved in thrombosis. The most obvious example is when ADAMTS13 fails to process ULVWF, resulting in the sometimes fatal microvascular clotting syndrome, thrombotic thrombocytopenic purpura.7 VWF has also been implicated in thrombosis associated with disorders such as HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), antiphospholipid syndrome, and sepsis.8 In addition, VWF may play important roles in other clinical syndromes with connections to microvascular thrombosis that are not as clear. For example, extremely elevated VWF concentrations have been described in patients with acute respiratory distress syndrome, with those having VWF levels > 450% of normal being at markedly increased risk of death.9 Similarly, recent reports implicate VWF in the attachment of malaria-infected erythrocytes to small vessels of the brain in cerebral malaria.10 A common feature of these clinical syndromes is their association with inflammation. During inflammation, cytokines and oxidants body the secretion of Weibel-Palade body contents, presenting both ULVWF and P-selectin on the endothelial surface and providing an adhesive surface for platelets and leukocytes. Leukocytes, particularly neutrophils, are activated by adhesion, cytokines, and/or bacterial products. Activated neutrophils produce potent oxidants, including hypochlorous acid (HOCl) through the action of myeloperoxidase (MPO) on H2O2, itself a product of the respiratory burst.
We recently showed that HOCl oxidizes the ADAMTS13 cleavage site on VWF, converting M1606 in the A2 domain to methionine sulfoxide and rendering the site highly resistant to cleavage.11 This methionine residue was readily oxidized in a substrate peptide, but in plasma VWF required denaturing conditions to be oxidized, similar to the requirements for ADAMTS13 cleavage. In the present study, we examined whether VWF oxidation increased when it was exposed to oxidants in the presence of fluid shear stress, which is known to expose the platelet-binding site in the A1 domain and the ADAMTS13 cleavage site in the A2 domain. We report that shear stress sequentially exposes methionine residues for oxidation in the VWF A1A2A3 region, including M1606 at the ADAMTS13 cleavage site. Further, methionine residues in this region were preferentially oxidized in large VWF multimers and oxidation enhanced the ability of VWF to agglutinate platelets.
Methods
Reagents
The following reagents (with their sources indicated in parentheses) were used in this study: sodium hypochlorite (Fisher Scientific), sequencing-grade trypsin (Promega), acetonitrile (J.T. Baker), DTT and iodoacetamide (Bio-Rad), trifluoroacetic acid and formic acid (EMD; Merck), MPO (Athens Research Technologies), ristocetin (American Biochemical and Pharmaceutical), and lyophilized human platelets (Helena Laboratories). All other reagents were obtained from Sigma-Aldrich.
Partial purification of VWF
Cryoprecipitate was obtained from the Puget Sound Blood Center. The cryoprecipitate was thawed rapidly in a 37°C water bath and suspended in citrate buffer to a final concentration of 4mM citrate, pH 7.4. This suspension was then adjusted to 2M glycine, 300mM NaCl, and 25mM Tris at pH 6.8 and incubated for 30 minutes at 37°C. The mixture was centrifuged at 9000g for 20 minutes to remove fibrinogen. VWF in the supernatant was then precipitated by adding NaCl (90.6 g/L) and mixing for at least 2 hours. The suspension was centrifuged at 15 000g for 30 minutes and the supernatant discarded. The pellet was resuspended in 3 mL of 10mM HEPES, pH 7.4. The concentration of VWF was 650 μg/mL, as measured by ELISA, and the total protein concentration was 19 mg/mL. We used this preparation, in which VWF made up ∼ 3.4% of the total protein, for all experiments reported here.
Oxidation of VWF in stasis or under shear stress
VWF was incubated with 50μM H2O2, or the concentration indicated in the particular experiment, in the absence or presence of 25nM MPO in PBS, pH 7.4, at 37°C for 1 hour in the absence or presence of shear stress. Protein oxidation was stopped by quenching with excess free methionine. Continuous fluid shear stress was applied by perfusing the reaction mixture through a closed loop made up of silicone tubing (Tygon) with an internal diameter of 794 μm using a peristaltic pump at a flow rate of 160 μL/min. VWF in this circulatory system experienced tensile stretch from 2 sources: from the wall shear stress in most of the tubing and from elongational flow and higher wall shear stress in the region where the rollers of the peristaltic pump pinch the tube to induce flow. In most of the system, the calculated wall shear stress was approximately 0.5 dynes/cm2, assuming a viscosity of the protein-containing solution of 1 cP. The descriptions of flow patterns and shear stresses in the region of the peristaltic pump are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The maximum shear stress calculated in the region of the pump rollers was between 68 and 540 dynes/cm2, depending on the extent to which the pump rollers constrict the silicone tubing (see supplemental Methods).
Protein proteolysis
Before analysis for oxidation by mass spectrometry (MS), the reaction mixture was treated for 20 minutes at 70-80°C with 5mM DTT in a buffer containing 50mM ammonium bicarbonate, 5mM L-methionine, and 5% acetonitrile to reduce protein disulfide bonds. The resultant thiols were then alkylated with 12.5mM iodoacetamide for 15 minutes at room temperature. The reduced and alkylated proteins were digested overnight at 37°C with trypsin (trypsin:protein, 1:20, wt/wt). The digestion was stopped with trifluoroacetic acid, pH 2-3. The tryptic peptides were concentrated and desalted using a C18 column (Empore High Performance C18HD Extraction Disk Cartridges; 3M), dried under vacuum, and then resuspended in a solution of 0.1% formic acid, 5mM L-methionine, and 5% acetonitrile before being analyzed by MS.
Analysis of VWF methionine oxidation by MS
Tryptic peptides from plasma VWF were analyzed using liquid chromatography (LC)-electrospray ionization-tandem MS (MS/MS) in the positive ion mode with a Thermo Scientific LTQ Orbitrap Velos mass spectrometer coupled to a Waters nanoACQUITY Ultra Performance LC system. Peptides were separated at a flow rate of 300 nL/min on a C18 column (100 × 0.075 mm, 1.7μm; Waters) using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in CH3CN). Peptides were eluted using a linear gradient of 5%-35% solvent B over 90 minutes. The spray voltage was 2.0 kV, and the temperature of the heated capillary was 200°C. The collision energy for MS/MS was 35%. Methionine oxidation was determined by peak area using selected reaction monitoring.
Effect of VWF oxidation on ristocetin-induced platelet agglutination
VWF (10 μg/mL) was oxidized with 25μM H2O2 and 25nM MPO in PBS at 37°C for 30 minutes under either shear or static conditions. The control was treated identically but without H2O2 or MPO. The oxidation reaction was stopped by adding free methionine, and the reaction mixture was used as the source of VWF to agglutinate fixed platelets in the presence of 0.25 mg/mL of ristocetin. Agglutination was assayed in a platelet aggregometer by monitoring light transmission through the platelet suspension.
Nanobody binding
The effect of oxidation on VWF conformation and function was also assessed by examination of the binding of VWF nanobody (AU/VWFa-11) as described previously.12 This nanobody detects an epitope in the VWF A1 domain exposed when VWF is in a conformation capable of binding platelet GPIbα.12 Briefly, the nanobody was immobilized on an ELISA plate as a capture Ab, and oxidized or nonoxidized VWF in serial dilutions was then added to the coated wells. Bound VWF was detected by an HRP-conjugated polyclonal VWF Ab. Captured VWF was plotted against the concentration of the VWF added to the wells, and the slopes of these plots from different samples were compared.
Fractionation of oxidized VWF multimers by gel filtration
VWF (5 μg/mL) was oxidized by 25μM H2O2 with 25nM MPO in PBS at 37°C for 60 minutes under shear. One milliliter of the oxidized protein mixture was applied to an HR Sephacryl S-500 column (10.2 cm × 1.5 cm) and eluted by gravity (flow rate ∼ 0.3 mL/min) using PBS, pH 7.4, containing 5mM L-methionine to prevent oxidation during processing. Twenty-one milliliter-fractions were collected starting immediately after sample application. The VWF concentration in the collected fractions was determined by ELISA. Fractions 6, 8, and 10, containing large, intermediate, and small multimers, respectively, were subjected to agarose gel electrophoresis and Western blotting for VWF13 and analyzed for methionine oxidation by LC-MS.
Results
Methionines in the VWF A1A2A3 region
Methionine is the amino acid residue most easily oxidized by HOCl. The A1A2A3 region of human VWF contains 14 methionine residues scattered throughout the 3 domains, including M1606 at the ADAMTS13 cleavage site in the A2 domain (Figure 1). Of these, we were able to identify 8 within peptides derived from tryptic digestion of full-length VWF from cryoprecipitate. In the crystal structures of the individual domains, 6 of these 8 methionines, M1303, M1304, M1385, M1521, M1606, and M1761, appeared to be buried in hydrophobic pockets and inaccessible to the surface. Their accessible surface areas (ASAs)14-16 are listed in Figure 4. By contrast, M1860 is partially exposed (ASA 40.3 Å2) and M1495 is almost completely exposed (ASA 112.4 Å2). This analysis suggests that the methionine residues in the A1 domain and M1606 at the ADAMTS13 cleavage site might not be easily oxidized under static conditions.
Methionine residues in the A1A2A3 region of VWF. The A1A2A3 region of VWF contains 14 methionine residues, 4 each in the A1 and A3 domains and 6 in the A2 domain, including M1606 at the ADAMTS13 cleavage site. Methionine residues are the amino acids most susceptible to oxidation by HOCl.
Methionine residues in the A1A2A3 region of VWF. The A1A2A3 region of VWF contains 14 methionine residues, 4 each in the A1 and A3 domains and 6 in the A2 domain, including M1606 at the ADAMTS13 cleavage site. Methionine residues are the amino acids most susceptible to oxidation by HOCl.
Shear stress enhances oxidation of M1606 at the ADAMTS13 cleavage site
Although both Y1605 and M1606 at the ADAMTS13 cleavage site are hypothetically susceptible to oxidation, we previously found that in a 78–amino acid peptide based on the sequence surrounding the cleavage site, only the methionine was oxidized, being converted stoichiometrically to the sulfoxide under conditions that yielded only miniscule quantities of chlorotyrosine.11 However, in multimeric plasma VWF, M1606 was only oxidized in significant quantities under static conditions in the presence of urea, presumably because this chaotropic agent was required to make M1606 accessible to the oxidant. Therefore, we evaluated whether exposure of VWF to shear stress would increase the rate of M1606 oxidation by an MPO-H2O2-Cl− system that generates HOCl. We first exposed VWF to buffer (control), H2O2, or H2O2 and 25nM MPO either under static conditions or under shear stress. After terminating the reaction with excess L-methionine, the reaction mixtures were digested with trypsin and the resulting peptides were then analyzed by LC MS/MS (supplemental Figure 1). The percentage oxidation of M1606 was determined with selected reaction monitoring, as illustrated in Figure 2A. This technique is able to selectively monitor the tryptic peptide containing M1606 and measures the extent of M1606 oxidation. In the absence of MPO, H2O2 oxidized only a minute molar fraction of M1606, and the quantity increased only slightly in the presence of shear stress (Figure 2A-B). In contrast, the presence of MPO greatly increased oxidation of M1606, but only when the VWF was exposed to shear stress. As we reported previously,11 oxidation of M1606 was accompanied by a marked reduction in the ability of ADAMTS13 to cleave the Tyr1605–M1606 bond. These data indicate that solvent exposure of M1606 is very sensitive to shear stress, suggesting that under the right conditions, the ADAMTS13 cleavage site of VWF could be easily oxidized in vivo.
Shear stress greatly enhances oxidation of M1606 at the ADAMTS13 cleavage site in plasma VWF. Plasma VWF (0.75 μg/mL) was incubated with buffer alone, 50μM H2O2, or 50μM H2O2 and 25nM MPO at 37°C for 1 hour under either shear or static conditions. Reactions were quenched by adding excess L-methionine. The treated proteins were digested with trypsin, and the resultant tryptic peptides were analyzed by LC-MS/MS. (A) Selected reaction monitoring of the M1606-containing peptide (EQAPNLVYM1606VTGNPASDEIK), MS2 of m/z 1088.5 → 1424.6 (left) and the corresponding oxidized peptide (EQAPNLVYM1606(O)VTGNPASDEIK), MS2 of m/z 1096.5 → 1440.6 (right). (B) Oxidation of M1606 after treatment under the indicated conditions. The percentage of methionine converted to the sulfoxide was determined by dividing the area of the Met(O) peak by the sum of the areas of the peaks for Met(O) and unoxidized Met. The results represent the means ± SD of 3 experiments.
Shear stress greatly enhances oxidation of M1606 at the ADAMTS13 cleavage site in plasma VWF. Plasma VWF (0.75 μg/mL) was incubated with buffer alone, 50μM H2O2, or 50μM H2O2 and 25nM MPO at 37°C for 1 hour under either shear or static conditions. Reactions were quenched by adding excess L-methionine. The treated proteins were digested with trypsin, and the resultant tryptic peptides were analyzed by LC-MS/MS. (A) Selected reaction monitoring of the M1606-containing peptide (EQAPNLVYM1606VTGNPASDEIK), MS2 of m/z 1088.5 → 1424.6 (left) and the corresponding oxidized peptide (EQAPNLVYM1606(O)VTGNPASDEIK), MS2 of m/z 1096.5 → 1440.6 (right). (B) Oxidation of M1606 after treatment under the indicated conditions. The percentage of methionine converted to the sulfoxide was determined by dividing the area of the Met(O) peak by the sum of the areas of the peaks for Met(O) and unoxidized Met. The results represent the means ± SD of 3 experiments.
Under shear stress, M1606 is preferentially oxidized in larger VWF multimers
Several studies have demonstrated that the ability of shear stress to unfold VWF is related to the size of the VWF polymer.17-21 Therefore, we reasoned that shear-induced oxidation of VWF might selectively favor the largest multimers. After applying the shear/oxidation regimen, we separated VWF on the basis of size using gel filtration (Figure 3A). We analyzed oxidation of M1606 in fractions 6, 8, and 10, which were enriched in large, intermediate, and small VWF multimers, respectively. M1606 was preferentially oxidized in the larger multimers (Figure 3B).
HOCl preferentially oxidizes M1606 in larger VWF multimers. Plasma VWF (5 μg/mL) was oxidized under shear stress as in Figure 2. Oxidized VWF in the reaction mixture was separated on a size-exclusion column into fractions containing predominantly high- (6), medium- (8), and low- (10) molecular-weight VWF multimers. (A) VWF multimer gel of the 3 fractions. (B) Percent oxidation of M1606 in the different fractions. Results represent the means ± SD of 2 experiments, with duplicate LC–MS/MS analyses.
HOCl preferentially oxidizes M1606 in larger VWF multimers. Plasma VWF (5 μg/mL) was oxidized under shear stress as in Figure 2. Oxidized VWF in the reaction mixture was separated on a size-exclusion column into fractions containing predominantly high- (6), medium- (8), and low- (10) molecular-weight VWF multimers. (A) VWF multimer gel of the 3 fractions. (B) Percent oxidation of M1606 in the different fractions. Results represent the means ± SD of 2 experiments, with duplicate LC–MS/MS analyses.
Differential oxidation of other methionine residues in the VWF A1A2A3 region
We also evaluated the effect of shear stress on the oxidation of 7 other methionine residues in the A1A2A3 region: M1303, M1304, and M1385 in the A1 domain; M1495 and M1521 in the A2 domain; and M1721 and M1860 in the A3 domain. As expected, only those methionine residues with the highest accessible surface area (M1860 and M1495) were oxidized to a substantial extent under static conditions, with M1495 fully oxidized (Figure 4). In contrast, application of shear stress greatly increased oxidation of the other 6 residues.
Shear stress facilitates oxidation of methionine residues buried in the VWF A domains. Plasma VWF was incubated with 50μM H2O2 and 25nM MPO under either shear or static conditions. The percentage of methionine converted to the sulfoxide Met(O) was analyzed by LC-MS/MS. The results represent the means ± SD of at least 3 experiments. ASA of the methionine side chain was analyzed on 3D structures of each domain. In the ribbon structures of the A domains, methionine side chains are depicted in ball-and-stick form. In the absence of shear stress, little oxidation was detected at the completely buried methionine residues (A1: M1303, M1304, and M1385; A2: M1521 and M1606; A3: M1761). Their oxidation was greatly increased under shear, indicating that the 3 VWF A domains change their conformations under shear, exposing the buried methionines and facilitating their oxidation.
Shear stress facilitates oxidation of methionine residues buried in the VWF A domains. Plasma VWF was incubated with 50μM H2O2 and 25nM MPO under either shear or static conditions. The percentage of methionine converted to the sulfoxide Met(O) was analyzed by LC-MS/MS. The results represent the means ± SD of at least 3 experiments. ASA of the methionine side chain was analyzed on 3D structures of each domain. In the ribbon structures of the A domains, methionine side chains are depicted in ball-and-stick form. In the absence of shear stress, little oxidation was detected at the completely buried methionine residues (A1: M1303, M1304, and M1385; A2: M1521 and M1606; A3: M1761). Their oxidation was greatly increased under shear, indicating that the 3 VWF A domains change their conformations under shear, exposing the buried methionines and facilitating their oxidation.
Time course and H2O2 concentration dependence of methionine oxidation in the VWF A1A2A3 region
We evaluated the time course of methionine oxidation in the presence of shear stress, sampling the oxidation reactions at 0, 10, 20, 30, 60, and 90 minutes after the addition of H2O2/MPO (Figure 5 left panel). Except for M1495, which was almost completely oxidized by 10 minutes, the extent of oxidation of the other methionine residues increased progressively with time. Of the 6 buried methionine residues, M1521 and M1606 in the A2 domain were the most sensitive to shear stress–induced oxidation at early time points.
Time course and H2O2 concentration dependence of methionine oxidation under shear stress. Left panel: Time course of methionine oxidation in the A1, A2, and A3 domains. Plasma VWF was incubated with 50μM H2O2 and 25nM MPO under shear for the indicated times. Right panel: H2O2 concentration dependence of methionine oxidation in the A1, A2, and A3 domains. Plasma VWF was incubated with the indicated H2O2 concentration and 25nM MPO under shear conditions for 1 hour. Methionine oxidation was analyzed as in Figure 2. The results represent the means ± SD from 2 experiments, with duplicate LC–MS/MS analyses.
Time course and H2O2 concentration dependence of methionine oxidation under shear stress. Left panel: Time course of methionine oxidation in the A1, A2, and A3 domains. Plasma VWF was incubated with 50μM H2O2 and 25nM MPO under shear for the indicated times. Right panel: H2O2 concentration dependence of methionine oxidation in the A1, A2, and A3 domains. Plasma VWF was incubated with the indicated H2O2 concentration and 25nM MPO under shear conditions for 1 hour. Methionine oxidation was analyzed as in Figure 2. The results represent the means ± SD from 2 experiments, with duplicate LC–MS/MS analyses.
We next evaluated whether the oxidation of VWF would accelerate if the MPO (25nM) were provided with increased concentrations of H2O2. At this concentration of the enzyme, methionine oxidation increased progressively with increasing H2O2 concentration (Figure 5 right panel), except for M1495, which was already completely oxidized at 25μM H2O2.
Oxidation of methionine residues is correlate with their accessible surface area
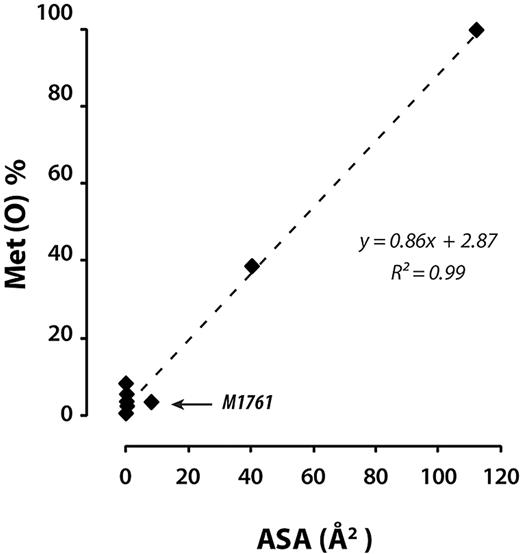
The solvent-accessible surface area assigned to a given amino acid residue in a protein is based on the static X-ray crystal structure of the particular domain in which it resides. The crystal structures of the 3 A domains were each obtained from the isolated domains.22-24 Therefore, accessibility of any residue in the crystal structure of any of the domains could be further restricted by the neighboring domains, such as those in the whole A1A2A3 region or full-length VWF. Therefore, unusual susceptibility of any of the residues to shear-enhanced oxidation may provide some clues as to regions of the molecule that may be shielded by interdomain interactions that are sensitive to the application of shear stress. The accessible surface areas, shear-enhanced oxidation profiles, and position of the methionine residues in the crystal structure are shown in Figure 4. Under stasis, the oxidation of methionine residues were very well correlated with accessible surface area (R2 = 0.99; Figure 6), with one exception. M1761 (A3 domain), with an accessible surface area of 8.1 Å2, deviated the most from the regression curve, suggesting that other domains in globular plasma VWF were shielding it. The other minimally oxidized residues under stasis have very small or no accessible surface. The application of shear stress markedly increased oxidation of these buried methionine residues (Figure 4).
Extent of oxidation of methionine residues in the VWF A1A2A3 region as a function of accessible surface area. Linear regression analysis of the extent of methionine oxidation after 1 hour under static conditions (conditions as in Figure 2) versus the accessible surface area.
Extent of oxidation of methionine residues in the VWF A1A2A3 region as a function of accessible surface area. Linear regression analysis of the extent of methionine oxidation after 1 hour under static conditions (conditions as in Figure 2) versus the accessible surface area.
VWF oxidation by HOCl enhances its platelet-binding functions
The finding that oxidation of the ADAMTS13 cleavage site by neutrophil oxidants renders that site resistant to cleavage suggests that this modification has a (patho)physiologic function, because it would allow the more adhesive population of VWF multimers to persist in the circulation or on the endothelial surface. It also seemed possible that the oxidation itself could alter the conformation of VWF to make it more adhesive, so we tested this possibility. We exposed plasma VWF to the H2O2/MPO system as described in “Methods” and, after quenching the reaction with excess L-methionine, evaluated the ability of the VWF to agglutinate formalin-fixed human platelets. At a ristocetin concentration (0.25 mg/mL) that caused minimal platelet agglutination in the suspension containing unoxidized VWF, the oxidized VWF caused substantial agglutination (Figure 7A). Treatment of the VWF under static conditions also increased its ability to agglutinate platelets, but to a lesser extent than when it was oxidized under shear stress (Figure 7B). Therefore, oxidized VWF is not only resistant to ADAMTS13 cleavage, but is also hyperfunctional with respect to platelet binding.
VWF oxidation enhances its platelet-binding function. (A) Ristocetin-induced platelet agglutination using unoxidized VWF or VWF oxidized in the presence of shear stress. The VWF was mixed with lyophilized platelets in the presence of ristocetin (0.25 mg/mL), and platelet agglutination was monitored by light transmission in a platelet aggregometer. (B) Comparison of VWF oxidized under static or shear conditions for their ability to agglutinate platelets. (C) Binding of nanobody AU/VWFa-11, which detects a gain-of-function conformation in the VWF A1 domain, as detected by ELISA. The results represent the means ± SD of 3 experiments.
VWF oxidation enhances its platelet-binding function. (A) Ristocetin-induced platelet agglutination using unoxidized VWF or VWF oxidized in the presence of shear stress. The VWF was mixed with lyophilized platelets in the presence of ristocetin (0.25 mg/mL), and platelet agglutination was monitored by light transmission in a platelet aggregometer. (B) Comparison of VWF oxidized under static or shear conditions for their ability to agglutinate platelets. (C) Binding of nanobody AU/VWFa-11, which detects a gain-of-function conformation in the VWF A1 domain, as detected by ELISA. The results represent the means ± SD of 3 experiments.
As an independent means of assessing the effect of oxidation on VWF conformation and function, we examined the binding of the conformation-specific nanobody AU/VWFa-11. This reagent detects an epitope within the A1 domain that is exposed when the GPIbα-binding site is exposed; elevated nanobody binding is correlated with increased VWF reactivity with platelets.12 AU/VWFa-11 binding of VWF increased with oxidation (Figure 7C).
Discussion
In this study, we extended our previous work demonstrating that neutrophil oxidants are capable of oxidizing VWF such that it is resistant to proteolysis by ADAMTS13. In particular, we previously showed that HOCl, a product of the reaction of H2O2 with MPO and chloride ion, converts M1606 at the ADAMTS13 cleavage site to methionine sulfoxide, rendering the site resistant to cleavage. In vivo, the effect would be to prolong the lifespan of the largest, most adhesive multimers in the circulation. We found that oxidation of M1606 in vitro mirrored the requirements for ADAMTS13 cleavage at this site: as with proteolysis, M1606 was efficiently oxidized when part of a small ADAMTS13 peptide substrate, but was very inefficiently oxidized in multimeric plasma VWF under static conditions. However, in the presence of the denaturant urea, oxidation of M1606 proceeded rapidly, similar to the requirement for ADAMTS13 cleavage.
In the circulation, the ADAMTS13 cleavage site in VWF is unmasked when VWF multimers are exposed to shear stress, which applies a tensile force to the polymers that unfold one or more A2 domains.21,25,26 We reasoned that shear stress would also promote the oxidation of VWF under the appropriate conditions, a hypothesis we originally attempted to test by applying a constant uniform shear stress to the VWF solution using a cone-and-plate viscometer. This approach was limited by the relatively small volumes we could apply to the plate, by evaporative loss during the experiment, and by adsorptive loss of the VWF onto the surfaces of the cone and plate. We solved these problems by perfusing the VWF-containing solution through a closed circuit of silicone tubing of fixed internal diameter. The tubing adsorbed a relatively small amount of the protein, and we did not have to contend with evaporative fluid loss. We were initially perplexed that we could get such efficient oxidation, especially of M1606, at such a low shear stress (0.5 dynes/cm2), which was much lower than previously published shear stresses required for A2 domain unfolding. For example, Schneider et al,20 using a planar microfluidic device, reported that VWF experienced an abrupt conformational transformation from a compact globule to an elongated strand at a fluid shear rate of 5000/s, which at a viscosity of 1 cP would correspond to a shear stress of 50 dynes/cm2, 2 orders of magnitude above the wall shear stress we calculated for our device. Similarly, Zhang et al21 estimated the most likely unfolding force for a single A2 domain within a VWF polymer to be in the range of 12 pN at a loading rate of 25 pN/s. At a shear stress of 100 dynes/cm2, this tensile force would only be achieved in the central A2 domains of a VWF polymer of 200 monomers; the tensile force on similar A2 domains in a 100mer would barely exceed 2 dynes/cm2. Because neither 100mer nor 200mer VWF molecules have ever been reported and are unlikely to exist in vivo, we postulated that either the VWF was experiencing a higher shear force than we assumed based on our calculations of wall shear stress in the tubing or that other factors were enhancing cleavage. Closer inspection of the shearing apparatus revealed that it was in fact generating local fluid shear stresses much greater than the average wall shear stress in most of the tubing.
The elevated shear stresses produced in parts of the tubing are a consequence of the characteristics of the pump/tubing system. The reaction mixture is passed through a closed loop of silicone tubing of constant internal diameter everywhere except where the fluid is being moved by a peristaltic pump. At the pump site, the tubing is constricted to a very narrow diameter by a roller. As the fluid enters this narrowed region, it experiences a sudden acceleration as is required to maintain a constant flow through a smaller cross-sectional area. Because globular VWF molecules are ellipsoidal, not spherical, one end of the molecule will accelerate suddenly before the rest of the molecule, thus applying a considerable tensile force across the length of the molecule as it enters the region with a velocity gradient. This concept of elongational flow and its effect on VWF structural transitions has been elegantly described in 2 recent papers.21,27 In addition to the elongational flow and its associated shear stress, the wall shear stress at the site where the pump roller compresses the tubing is also markedly higher than in most of the tubing because of the greatly reduced cross-sectional area of the tubing at that point (supplemental Figure 2). Our calculations indicate that the shear stresses reached should be sufficient to unfold VWF, even if only transiently (see the supplemental materials). Consistent with this explanation, we found that the extent of oxidation decreased with increasing length of tubing, which decreases the time the solution traverses the pump rollers (supplemental Figure 3).
It is also possible that initial oxidation of surface-accessible methionines alters the conformation of VWF enough to lower the force required for shear-induced A2 domain unfolding. In this scenario, unfolding could be facilitated by oxidation, and oxidation of individual methionine residues could occur sequentially. Favoring this hypothesis is the fact that the oxidation of methionine to the sulfoxide converts a very hydrophobic amino acid side chain with a preference to be buried to a hydrophilic side chain with a preference for water. Therefore, each methionine oxidation event is likely to produce a rather large conformational alteration of the affected domain and could also affect interdomain interactions, possibly easing the transition between the globular and elongated states and exposing other residues for oxidation.
Whatever mechanism is responsible for making the methionine residues accessible when VWF is sheared, this approach yields interesting insight into shear-induced conformational changes in the A1A2A3 region. In this case, methionine oxidation serves as a probe of these changes. The rate of oxidation of the buried methionines was greatest for the A2 domain (Figure 5), as would be expected given that the domain does not reside within a disulfide loop. In contrast, the unraveling of the A1 and A3 domains by tensile stress is constrained by the disulfide bonds that encompass them. Despite this, in both domains, shear stress promoted the oxidation of methionine residues that are deeply buried in the static structures, indicating that shear stress also alters the structure of each domain within the disulfide bond. A less likely explanation is that shear stress promotes rupture of the disulfide bonds, but the strong oxidative conditions used make this possibility less likely.
Our findings indicate that VWF is much easier to oxidize than was thought previously, especially by the MPO product HOCl. MPO is released from neutrophils and, to a lesser extent, from monocytes, upon their activation. The MPO concentration in plasma has been reported to be as high as 1 μg/mL in sepsis (severe sepsis also manifesting markedly elevated VWF antigen levels),28 and is likely to be even more highly concentrated on the endothelial surface because of its affinity for membrane glycosaminoglycans.29 Likewise, superoxide (the rapidly converted precursor to the MPO substrate H2O2) is produced ubiquitously in the blood, primarily by neutrophils through activation of NADPH oxidases, but also by other blood cells and the endothelium.30 Superoxide is also a byproduct of purine metabolism, one molecule being produced during the conversion of xanthine to hypoxanthine, and another in the conversion of hypoxanthine to urea. Both reactions are catalyzed by xanthine oxidase, which, like MPO, is concentrated on the endothelial surface by virtue of its binding to glycosaminoglycans.31
In preliminary studies of plasma from a patient with sickle cell disease, we have found that both M1606 and M1385 became oxidized (2.8% and 4.6%, respectively, vs 0.2% and 0.5% in normal plasma VWF). We are in the process of examining the oxidation of other methionine residues in the A1A2A3 region and extending the study to more patients so that we can determine whether the extent of oxidation is correlated with multimer patterns, VWF reactivity, and clinical parameters. It appears likely that oxidation of M1606 and M1385, neither of which is solvent exposed in the static crystal structures, will reflect greater oxidation of solvent-exposed residues, as we have observed in the in vitro studies. Oxidation of one of these solvent-accessible residues is very likely to account for the gain of platelet-binding function that we observed, because even VWF oxidation under static conditions (which resulted in minimal oxidation of M1606) significantly increased the ability of VWF to agglutinate platelets (Figure 7).
VWF is often described as “a marker of endothelial activation.”32-34 In many cases, this is undoubtedly true, but is only part of the story, especially given the effect of oxidation on preventing the cleavage and enhancing the function of this important adhesive molecule. In sickle cell disease, for example, we recently demonstrated that patients not only have elevated concentrations of VWF antigen in their plasma,35 but also elevated concentrations of ULVWF, with enhanced activity as detected by increased binding of the llama nanobody AU/VWFa-11, which detects a gain-of-function conformation of the platelet-binding A1 domain.12 Increased total active VWF, defined as the product of the VWF antigen concentration and the VWF activation factor (an index of VWF adhesive activity) was positively correlated with hemolysis, as measured by the plasma lactate dehydrogenase concentration. Therefore, rather than elevated VWF concentrations representing an epiphenomenon only indicative of endothelial activation, it is likely that the VWF itself contributes to the pathology of the inflammatory illness, particularly in the setting of increased oxidative stress. In this case, not only does endothelial activation induce the release of the largest and most adhesive native forms of VWF, the accompanying oxidative stress modifies these multimers, rendering them resistant to cleavage by ADAMTS13. The same oxidants also convert the largest preexisting plasma VWF multimers, which were previously rendered quiescent by ADAMTS13, into hyperfunctional and uncleavable forms. All of these mechanisms converge to generate a highly prothrombotic state, with potentially catastrophic consequences for those experiencing inflammation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Philip de Groot for providing the VWF nanobody and Ms Jennie Le for technical assistance.
This work was supported by National Institutes of Health grants (RO1HL091153 to J.A.L. and R01HL075381 to X.F.) and by institutional funds from Puget Sound Blood Center.
National Institutes of Health
Authorship
Contribution: X.F. designed the experiments, analyzed the data, and wrote the manuscript; J.C. designed and performed the experiments, analyzed the data, and edited the manuscript; R.G. designed and performed the oxidation experiments and edited the manuscript; Y.Z. interpreted the data and edited the manuscript; D.W.C. designed the experiments, interpreted the data, and edited the manuscript; and J.A.L. directed the project, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoyun Fu, PhD, Research Division, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104; e-mail: xiaoyunf@psbcresearch.org or José A. López, MD, Research Division, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104; e-mail: josel@psbcresearch.org.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal