There is no generally established prognostic index for patients with mantle cell lymphoma (MCL), because the International Prognostic Index (IPI) and Follicular Lymphoma International Prognostic Index (FLIPI) have been developed for diffuse large cell and follicular lymphoma patients, respectively. Using data of 455 advanced stage MCL patients treated within 3 clinical trials, we examined the prognostic relevance of IPI and FLIPI and derived a new prognostic index (MCL international prognostic index, MIPI) of overall survival (OS). Statistical methods included Kaplan-Meier estimates and the log-rank test for evaluating IPI and FLIPI and multiple Cox regression for developing the MIPI. IPI and FLIPI showed poor separation of survival curves. According to the MIPI, patients were classified into low risk (44% of patients, median OS not reached), intermediate risk (35%, 51 months), and high risk groups (21%, 29 months), based on the 4 independent prognostic factors: age, performance status, lactate dehydrogenase (LDH), and leukocyte count. Cell proliferation (Ki-67) was exploratively analyzed as an important biologic marker and showed strong additional prognostic relevance. The MIPI is the first prognostic index particularly suited for MCL patients and may serve as an important tool to facilitate risk-adapted treatment decisions in patients with advanced stage MCL.
Introduction
Mantle cell lymphoma (MCL) is a relatively rare lymphoma entity accounting for approximately 3% to 6% of all non-Hodgkin lymphoma (NHL) cases.1,–3 It has a poor prognosis with reported median overall survival (OS) of only 30 to 43 months.1,2,4 In the late 1980s, it was controversially debated whether centrocytic lymphoma, as defined by the Kiel classification,5 represented a separate lymphoma entity and whether it corresponded to the intermediate lymphocytic lymphoma or lymphocytic lymphoma of intermediate differentiation of the Working Formulation.6 After detection of the characteristic translocation t(11;14) associated with those lymphoma subtypes and based on further morphologic analyses, Banks et al7 proposed in 1992 the term “mantle cell lymphoma” for this now widely accepted entity of malignant lymphomas.
Since the international acceptance of MCL in the revised European-American lymphoma (REAL) classification8 in 1994 many reports on clinicopathologic features, treatment, clinical course, and prognostic factors for patients with MCL have been published.1,2,4,9,,,,,,,–17 Treatment results have overall been unsatisfactory, although a substantial variation in outcome was noted among individual cases with a small fraction of patients even achieving long lasting remissions. Unlike the International Prognostic Index18 (IPI) for diffuse large B-cell lymphomas or the Follicular Lymphoma International Prognostic Index19 (FLIPI) for follicular lymphomas, no broadly accepted prognostic index has been defined for MCL so far. Previous reports revealed serious limitations applying the IPI and FLIPI to MCL patients,1,2,4,9,,,,–14,16,17,20 and the development of a prognostic index specific to MCL patients has been postulated.12,20 In addition, controversial results have been published concerning the prognostic value of several candidate prognostic factors, but results were mostly based on limited samples of fewer than 130 patients.
We analyzed the pooled data of the most recent randomized trials of the German Low Grade Lymphoma Study Group (GLSG) and the European MCL Network to validate the IPI, FLIPI, and other prognostic factors, and to develop, if needed, a new prognostic index that is particularly suited for patients with advanced stage MCL.
Methods
Patients
Data from 3 consecutive randomized trials, GLSG1996,21 GLSG2000,22 and European MCL Trial 1,23 which were performed between 1996 and 2004, were included in this analysis. Local ethics committees of the participating centers approved the study protocol, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Patient entry criteria of all trials were identical with the exception of age, which was limited up to 65 years in the European MCL trial. Otherwise all 3 trials recruited treatment-naive patients with advanced Ann Arbor stage III or IV MCL. Central pathology review was mandatory to ensure a high quality of histologic diagnosis. Exclusion criteria comprised serious concomitant diseases, bad performance status, or significant impairment of organ function.21,–23 The initial diagnostic work-up comprised a bone marrow biopsy, ultrasound examination of the abdomen, and computed tomography (CT) scans of chest and abdomen. Normal organ function was assured by the respective laboratory tests, as well as by echocardiograms and electrocardiograms.
Treatment protocols
The GLSG1996 trial21 compared the response rates of initial chemotherapy with CHOP (cyclophosphamide, hydroxy-daunorubicin, vincristine and prednisone) and MCP (mitoxantrone, chlorambucil, prednisone). In 2000, the GLSG2000 trial22 was initiated to assess the additional efficacy of incorporating the monoclonal CD20-antibody rituximab to primary CHOP in terms of time-to-treatment failure. In both GLSG trials, patients older than 65 years and responding to initial chemotherapy received interferon-α (IFNα) maintenance therapy. Younger patients were randomized between consolidating myeloablative radiochemotherapy followed by autologous stem cell transplantation (ASCT) and IFNα maintenance within the European MCL Trial 1.23 Detailed study design and results of the respective trials have been published previously.21,–23
Candidate prognostic factors and outcome parameters
All baseline variables were considered as candidate prognostic factors, namely age, sex, Eastern Cooperative Oncology Group (ECOG) performance status,24 Ann Arbor stage, presence of B-symptoms, bone marrow involvement, spleen involvement, number of involved nodal areas, number of extranodal sites, size of largest involved lymph node, and laboratory parameters such as white blood cell (WBC), lymphocyte, granulocyte, monocyte, and platelet counts, hemoglobin (Hb), lactate dehydrogenase (LDH), albumin, and serum β2-microglobulin levels. Because upper limits of normal (ULN) differed considerably between laboratories for LDH, albumin, and β2-microglobulin, ratios to ULN were calculated. In addition, the known prognostic indices established in other lymphomas, IPI,18 and FLIPI19 were assessed. The outcome parameter was OS calculated from the day of recruitment to death from any cause or to the latest follow-up date.
In line with IPI and FLIPI, our aim was to establish a clinical prognostic index (mantle-cell lymphoma international prognostic index, MIPI) easily available in clinical practice. Besides the clinical parameters, the proliferation markers Ki-67 and number of mitoses, possibly the most important prognostic markers in MCL,1,2,4,11,14,,–17 have been assessed centrally and in a standardized way. Thus, we analyzed the additional prognostic relevance of cell proliferation and exploratively developed a combined biologic index (MIPIb) incorporating the effect of cell proliferation.
Statistical methods
To validate IPI and FLIPI, Kaplan-Meier OS curves stratified for risk group were calculated and compared by the log rank test. In addition, the individual risk factors constituting IPI and FLIPI, respectively, were checked for independent prognostic relevance by multiple Cox regression analyses.
The prognostic relevance of the candidate prognostic factors was evaluated using univariate Cox regression for OS. Subsequently, multiple Cox regression with backward variable selection was performed to identify independent prognostic factors. Size of largest involved lymph node, spleen involvement, lymphocyte, granulocyte, and monocyte counts, albumin, and serum β2-microglobulin levels were not included in multiple regression because of a high number of multivariately missing values. Bone marrow involvement was excluded because of high congruence with stage IV. Continuous parameters were not categorized a priori because this would have negatively affected the power of the analysis.25 ECOG performance status was classified into 3 groups: asymptomatic (ECOG 0), symptomatic but ambulatory and able to carry out light work (ECOG 1), and unable to work or bedridden (ECOG 2-4). WBC count, LDH, and β2-microglobulin were analyzed on the log-scale because of highly skewed distributions. The proportional hazards assumption and the linearity assumption for the final model were checked using scaled Schoenfeld residuals26,27 and martingale residuals,28 respectively.
Prognostic groups were defined by categorizing the prognostic score (linear predictor) of the final Cox regression model. 2 optimal cutpoints were found maximizing the log rank statistic according to the “minimal P value approach,”29 and P values for the log rank statistic were adjusted for multiple testing by the Bonferroni method. For practical reasons referring to clinical application of the prognostic index, the goal was to define 3 risk groups, none of which comprised more than 50% of the patients. An explorative simplification of the new classification rule was developed categorizing the prognostic factors according to standard cutpoints. The simplification with the best concordance to the original index measured by Cohen weighted kappa30 was chosen.
Internal validation was performed applying the refined bootstrap described by Efron.31 Random data splitting in training and validation sample was not performed because this internal validation procedure reduces the power for both model development and validation and is known to be inferior to bootstrap validation.31,32 Bootstrap validation used the likelihood ratio or log rank test statistic, the calibration slope (regression coefficient of the prognostic score), Harrell concordance index c33 for censored outcome variables, and the measure of separation SEP34 for survival curves.
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and R version 2.2.1 (www.r-project.org). Significance level was 5% for all analyses.
Results
Patient characteristics, treatment, and outcome
Between May 1996 and October 2004, 455 patients with advanced stage MCL entered the trials. The median age was 60 years (34-86 years) and 76% of the patients were male. Eighty-four percent of the patients were in Ann Arbor stage IV, 79% had bone marrow involvement. Forty-three percent presented with B-symptoms and 9% were unable to work or bedridden (ECOC 2-4). Further patient characteristics are depicted in Table 1.
Baseline patient characteristics
| Parameter . | Quantity . | n . |
|---|---|---|
| Median age, y (range) | 60 (34-86) | 455 |
| Sex, no. male (%) | 344 (76) | 455 |
| ECOG 0, no. (%) | 147 (33) | 452 |
| ECOG 1, no. (%) | 263 (58) | 452 |
| ECOG 2-4, no. (%) | 42 (9) | 452 |
| Stage IV, no. (%) | 384 (84) | 455 |
| B-symptoms present, no. (%) | 196 (43) | 451 |
| Bone marrow involvement present, no. (%) | 360 (79) | 453 |
| Extranodal sites more than 1, no. (%) | 143 (32) | 445 |
| Median no. involved nodal areas, (range) | 8 (0-11) | 432 |
| Median max. lymph node size, cm (range) | 4 (0.5-24) | 387 |
| Spleen involvement present, no. (%) | 225 (54) | 420 |
| Median WBC count, 109/L (range) | 7.9 (1.0-764) | 451 |
| Median lymphocyte count, 109/L (range) | 2.1 (0.35-625) | 422 |
| Median granulocyte count, 109/L (range) | 4.2 (0.19-26.4) | 413 |
| Median monocyte count, 109/L (range) | 0.5 (0.014-10.9) | 408 |
| Median platelet count, 109/L (range) | 188 (3-1346) | 452 |
| Median LDH/ULN (range) | 0.86 (0.15-5.3) | 443 |
| Median hemoglobin (males),g /L, (range) | 133 (55-175) | 342 |
| Median hemoglobin (females),g /L, (range) | 124 (30-149) | 109 |
| Median albumin/ULN (range) | 0.8 (0.36-1.26) | 268 |
| Median β2-microglobulin/ULN (range) | 1.1 (0.06-8) | 285 |
| Parameter . | Quantity . | n . |
|---|---|---|
| Median age, y (range) | 60 (34-86) | 455 |
| Sex, no. male (%) | 344 (76) | 455 |
| ECOG 0, no. (%) | 147 (33) | 452 |
| ECOG 1, no. (%) | 263 (58) | 452 |
| ECOG 2-4, no. (%) | 42 (9) | 452 |
| Stage IV, no. (%) | 384 (84) | 455 |
| B-symptoms present, no. (%) | 196 (43) | 451 |
| Bone marrow involvement present, no. (%) | 360 (79) | 453 |
| Extranodal sites more than 1, no. (%) | 143 (32) | 445 |
| Median no. involved nodal areas, (range) | 8 (0-11) | 432 |
| Median max. lymph node size, cm (range) | 4 (0.5-24) | 387 |
| Spleen involvement present, no. (%) | 225 (54) | 420 |
| Median WBC count, 109/L (range) | 7.9 (1.0-764) | 451 |
| Median lymphocyte count, 109/L (range) | 2.1 (0.35-625) | 422 |
| Median granulocyte count, 109/L (range) | 4.2 (0.19-26.4) | 413 |
| Median monocyte count, 109/L (range) | 0.5 (0.014-10.9) | 408 |
| Median platelet count, 109/L (range) | 188 (3-1346) | 452 |
| Median LDH/ULN (range) | 0.86 (0.15-5.3) | 443 |
| Median hemoglobin (males),g /L, (range) | 133 (55-175) | 342 |
| Median hemoglobin (females),g /L, (range) | 124 (30-149) | 109 |
| Median albumin/ULN (range) | 0.8 (0.36-1.26) | 268 |
| Median β2-microglobulin/ULN (range) | 1.1 (0.06-8) | 285 |
Data in column 3 (“n”) are number of data points for parameter.
Initial cytoreductive chemotherapy comprised CHOP in 56% of the patients, 31% of the patients received R-CHOP, 11% MCP, and 2% other chemotherapy regimens. Of the 438 patients evaluable for treatment response, 351 (80%) achieved a complete or partial remission, and 80 (18%) a complete remission. Treatment in remission was ASCT in 80 patients, IFNα maintenance in 199 patients, and 72 patients obtained no therapy in remission. Of the 455 patients, 159 had died and the median OS was 57 months with a median follow-up of surviving patients of 32 months.
IPI and FLIPI
According to the IPI, 99 patients (23%) were classified as low risk (LR), 173 patients (40%) as low intermediate risk (LIR), 119 patients (28%) as high intermediate risk (HIR), and 41 patients (9%) as high risk (HR); in 23 patients the IPI could not be evaluated due to missing data. The median OS was not yet reached in the LR group with a 5-year OS rate of 59%, and 61 months in the LIR group, 45 months in the HIR group and 20 months in the HR group (P < .001, Figure 1). LIR and HIR groups comprised together more than two-thirds of the patients and were not well separated. In multiple Cox regression age, ECOG performance status and LDH were prognostic for OS, whereas the number of extranodal sites was not (Table 2).
Overall survival according to the International Prognostic Index risk groups.18 LR indicates low risk; LIR, low intermediate risk; HIR, high intermediate risk; and HR, high risk.
Overall survival according to the International Prognostic Index risk groups.18 LR indicates low risk; LIR, low intermediate risk; HIR, high intermediate risk; and HR, high risk.
Prognostic relevance of the IPI18 (n = 432) risk factors for overall survival determined in a multiple Cox regression
| Prognostic factor . | Comparison . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|
| Age, y | ≥ 60 vs < 60 | 1.7 | 1.2 | 2.3 | .002 |
| ECOG | 2-4 vs 0-1 | 2.8 | 1.8 | 4.5 | <.001 |
| LDH | elevated vs normal | 1.6 | 1.2 | 2.3 | .006 |
| No. of extranodal sites | > 1 vs ≤1 | 1.2 | 0.8 | 1.6 | .33 |
| Prognostic factor . | Comparison . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|
| Age, y | ≥ 60 vs < 60 | 1.7 | 1.2 | 2.3 | .002 |
| ECOG | 2-4 vs 0-1 | 2.8 | 1.8 | 4.5 | <.001 |
| LDH | elevated vs normal | 1.6 | 1.2 | 2.3 | .006 |
| No. of extranodal sites | > 1 vs ≤1 | 1.2 | 0.8 | 1.6 | .33 |
Ann Arbor stage was III or IV in all patients.
RR indicates relative risk; LCL, lower confidence limit; UCL, upper confidence limit; and ECOG, ECOG performance status.
The FLIPI could be determined in 418 patients of whom 26 (6%) were classified as LR, 124 patients (30%) as intermediate risk (IR), and 268 patients (64%) as HR. The median OS was not yet reached for LR and IR patients with a 5-year OS rate of 61% and 57%, respectively, and 48 months in the HR group (P< .001, Figure 2). LR and IR groups were not separated and the large high risk group had relatively good outcome. In the multiple Cox regression, age and LDH were prognostic for OS, whereas neither hemoglobin nor the number of involved nodal areas were of prognostic relevance (Table 3).
Overall survival according to the Follicular Lymphoma International Prognostic Index19 risk groups. LR, low risk; IR, intermediate risk; and HR, high risk.
Overall survival according to the Follicular Lymphoma International Prognostic Index19 risk groups. LR, low risk; IR, intermediate risk; and HR, high risk.
Prognostic relevance of the FLIPI19 (n = 418) risk factors for overall survival determined in a multiple Cox regression
| Prognostic factor . | Comparison . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|
| Age, y | ≥ 60 vs < 60 | 1.8 | 1.3 | 2.5 | .001 |
| LDH | elevated vs normal | 1.8 | 1.3 | 2.5 | .001 |
| Hemoglobin, g/L | <120 vs ≥120 | 1.1 | 0.8 | 1.6 | .50 |
| No. involved nodal areas | > 4 vs ≤ 4 | 1.1 | 0.7 | 1.6 | .80 |
| Prognostic factor . | Comparison . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|
| Age, y | ≥ 60 vs < 60 | 1.8 | 1.3 | 2.5 | .001 |
| LDH | elevated vs normal | 1.8 | 1.3 | 2.5 | .001 |
| Hemoglobin, g/L | <120 vs ≥120 | 1.1 | 0.8 | 1.6 | .50 |
| No. involved nodal areas | > 4 vs ≤ 4 | 1.1 | 0.7 | 1.6 | .80 |
Ann Arbor stage was III or IV in all patients.
RR indicates relative risk; LCL, lower confidence limit; and UCL, upper confidence limit.
Prognostic factors
Neither sex, Ann Arbor stage (III vs IV), bone marrow involvement, number of extranodal sites or number of nodal areas, nor platelet count or albumin showed prognostic relevance for OS in univariate analyses. In contrast, age, ECOG performance status, B-symptoms, spleen involvement, maximal lymph node size, WBC, lymphocyte, granulocyte, and monocyte counts, LDH, Hb, and β2-microglobulin had a significant impact on OS (Table 4).
Prognostic relevance for overall survival according to univariate Cox regression
| Candidate prognostic factor . | Comparison . | n . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|---|
| Age | 10 years older | 455 | 1.38 | 1.15 | 1.65 | <.001 |
| Sex | Male vs female | 455 | 1.12 | 0.77 | 1.63 | .55 |
| ECOG performance status | 1 vs 0 | 452 | 1.61 | 1.10 | 2.35 | .014 |
| ECOG performance status | 2-4 vs 0 | 452 | 4.80 | 2.84 | 8.12 | <.001 |
| ECOG performance status | 2-4 vs 0-1 | 452 | 3.48 | 2.22 | 5.43 | <.001 |
| Ann Arbor stage | IV vs III | 455 | 1.09 | 0.70 | 1.70 | .70 |
| B-symptoms | present vs absent | 451 | 1.60 | 1.17 | 2.19 | .003 |
| Bone marrow | involved vs not | 453 | 1.04 | 0.70 | 1.54 | .85 |
| No. of extranodal sites | 1 vs 0 | 445 | 1.25 | 0.72 | 2.16 | .42 |
| No. of extranodal sites | > 1 vs 0 | 445 | 1.57 | 0.89 | 2.77 | .12 |
| No. of extranodal sites | > 1 vs 0-1 | 445 | 1.30 | 0.94 | 1.80 | .11 |
| No. nodal areas | 1 more | 432 | 1.03 | 0.97 | 1.08 | .34 |
| Maximum tumor size | 1 cm larger | 387 | 1.06 | 1.01 | 1.10 | .016 |
| Spleen | involved vs not | 420 | 1.40 | 1.01 | 1.95 | .044 |
| WBC count | 10-fold* | 451 | 2.25 | 1.59 | 3.18 | <.001 |
| Lymphocyte count | 10-fold* | 422 | 1.97 | 1.40 | 2.77 | <.001 |
| Granulocyte count | 10-fold* | 412 | 4.69 | 2.06 | 10.71 | <.001 |
| Monocyte count | 10-fold* | 408 | 2.61 | 1.65 | 4.12 | <.001 |
| Platelet count | 100 ×109/L more | 452 | 1.01 | 0.88 | 1.16 | .89 |
| LDH | 2-fold* | 443 | 1.72 | 1.32 | 2.24 | <.001 |
| Hemoglobin | 10 g/L more† | 451 | 0.91 | 0.85 | 0.98 | .009 |
| Albumin | +ULN‡ | 268 | 0.23 | 0.05 | 1.08 | .063 |
| β2-microglobulin | 2-fold* | 285 | 1.53 | 1.22 | 1.92 | <.001 |
| Candidate prognostic factor . | Comparison . | n . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|---|
| Age | 10 years older | 455 | 1.38 | 1.15 | 1.65 | <.001 |
| Sex | Male vs female | 455 | 1.12 | 0.77 | 1.63 | .55 |
| ECOG performance status | 1 vs 0 | 452 | 1.61 | 1.10 | 2.35 | .014 |
| ECOG performance status | 2-4 vs 0 | 452 | 4.80 | 2.84 | 8.12 | <.001 |
| ECOG performance status | 2-4 vs 0-1 | 452 | 3.48 | 2.22 | 5.43 | <.001 |
| Ann Arbor stage | IV vs III | 455 | 1.09 | 0.70 | 1.70 | .70 |
| B-symptoms | present vs absent | 451 | 1.60 | 1.17 | 2.19 | .003 |
| Bone marrow | involved vs not | 453 | 1.04 | 0.70 | 1.54 | .85 |
| No. of extranodal sites | 1 vs 0 | 445 | 1.25 | 0.72 | 2.16 | .42 |
| No. of extranodal sites | > 1 vs 0 | 445 | 1.57 | 0.89 | 2.77 | .12 |
| No. of extranodal sites | > 1 vs 0-1 | 445 | 1.30 | 0.94 | 1.80 | .11 |
| No. nodal areas | 1 more | 432 | 1.03 | 0.97 | 1.08 | .34 |
| Maximum tumor size | 1 cm larger | 387 | 1.06 | 1.01 | 1.10 | .016 |
| Spleen | involved vs not | 420 | 1.40 | 1.01 | 1.95 | .044 |
| WBC count | 10-fold* | 451 | 2.25 | 1.59 | 3.18 | <.001 |
| Lymphocyte count | 10-fold* | 422 | 1.97 | 1.40 | 2.77 | <.001 |
| Granulocyte count | 10-fold* | 412 | 4.69 | 2.06 | 10.71 | <.001 |
| Monocyte count | 10-fold* | 408 | 2.61 | 1.65 | 4.12 | <.001 |
| Platelet count | 100 ×109/L more | 452 | 1.01 | 0.88 | 1.16 | .89 |
| LDH | 2-fold* | 443 | 1.72 | 1.32 | 2.24 | <.001 |
| Hemoglobin | 10 g/L more† | 451 | 0.91 | 0.85 | 0.98 | .009 |
| Albumin | +ULN‡ | 268 | 0.23 | 0.05 | 1.08 | .063 |
| β2-microglobulin | 2-fold* | 285 | 1.53 | 1.22 | 1.92 | <.001 |
LCL indicates lower confidence limit; UCL, upper confidence limit; and RR, relative risk.
Log scale
No significant interaction of hemoglobin and sex (P = .43)
Quotient to ULN
Sex, stage, B-symptoms, number of extranodal sites, number of nodal areas, platelet count, and hemoglobin lost their prognostic relevance in multiple Cox regression with backward variable selection on the dataset of 409 complete cases. Age, ECOG performance status, LDH, and WBC counts were identified as the 4 independent prognostic factors for OS. Relative risk (RR) of increased age was 1.04 per year (95% CI 1.02-1.06, P<.001), RR of patients with poor performance (ECOG > 1) was 2.01 (95% CI 1.19-3.39, P = .009), and patients with a tenfold elevated LDH or WBC count had a RR of 3.92 (95% CI 1.48-10.37, P = .006) and 2.56 (95% CI 1.66-3.95, P < .001), respectively (Table 5). Accordingly, the MIPI prognostic score was calculated as
MIPI score = [0.03535 × age (years)]
× age (years)] + 0.6978 (if ECOG > 1)
+ [1.367 × log10(LDH/ULN)]
+ [0.9393 × log10(WBC count)]
Independent prognostic factors for overall survival identified by backward variable selection with multiple Cox regression on significance level 5% for the Wald statistic
| Prognostic factor . | Comparison . | β . | SE . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|---|---|
| Age | 1 year older | 0.03535 | 0.009604 | 1.04 | 1.02 | 1.06 | <.001 |
| ECOG | 2-4 vs 0-1 | 0.6978 | 0.2663 | 2.01 | 1.19 | 3.39 | .009 |
| LDH | 10-fold | 1.367 | 0.4962 | 3.92 | 1.48 | 10.37 | .006 |
| WBC count | 10-fold | 0.9393 | 0.2220 | 2.56 | 1.66 | 3.95 | <.001 |
| Prognostic factor . | Comparison . | β . | SE . | RR . | 95% LCL . | 95% UCL . | P . |
|---|---|---|---|---|---|---|---|
| Age | 1 year older | 0.03535 | 0.009604 | 1.04 | 1.02 | 1.06 | <.001 |
| ECOG | 2-4 vs 0-1 | 0.6978 | 0.2663 | 2.01 | 1.19 | 3.39 | .009 |
| LDH | 10-fold | 1.367 | 0.4962 | 3.92 | 1.48 | 10.37 | .006 |
| WBC count | 10-fold | 0.9393 | 0.2220 | 2.56 | 1.66 | 3.95 | <.001 |
The candidate prognostic factors included were age, sex, ECOG performance status, Ann Arbor stage, B-symptoms, number of extranodal sites, number of nodal areas, WBC count, platelet count, LDH, and hemoglobin. The model was developed using 409 complete cases for the candidate prognostic factors. The same model was identified using forward variable selection or a significance level of 10%.
β indicates the regression coefficient; SE, standard error; RR, relative risk; LCL, lower confidence limit; and UCL, upper confidence limit.
Mantle cell lymphoma international prognostic index (MIPI)
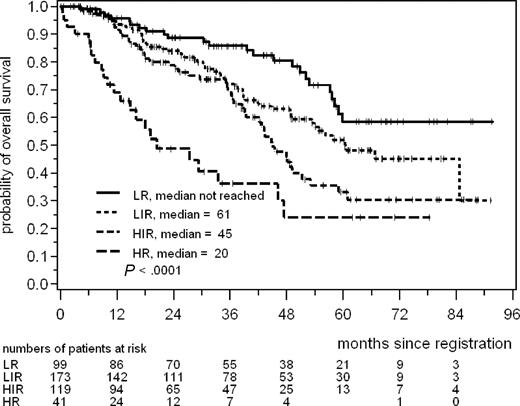
The median value of the prognostic score in 409 patients was 5.78 (4.31-9.18), 10% of the values were below 5.16 and 90% of the values below 6.63. Potential cutpoints between 5.15 and 6.65 in steps of 0.05 were assessed. On this basis, the value 6.2 showed the best discrimination (log rank statistic 53.06, 1 degree of freedom, df) and defined a high-risk group of 84 patients (21%, score ≥ 6.2). For the definition of an intermediate-risk group, the value of 5.7 was identified as best discriminator (log rank statistic 61.11, 2 df). In this way 145 patients were classified as intermediate risk (35%, 5.7 ≤ score < 6.2). The remaining 180 patients belonged to the low-risk group (44%, score < 5.7). After Bonferroni correction for the P values calculated from the log rank statistics, the separation of these risk groups remained statistically significant. Median OS was not reached in the low risk group with a 5-year OS of 60%, and it was 51 months and 29 months in the intermediate-risk group and the high-risk group, respectively (Figure 3).
Overall survival according to the new prognostic index (MIPI). LR indicates low risk, prognostic score less than 5.7; IR, intermediate risk, score 5.7 or more but less than 6.2; and HR, high risk, score 6.2 or more. The prognostic score is calculated as [0.03535 × age (years)] + 0.6978 (if ECOG > 1) + [1.367 × log10(LDH/ULN)] + [0.9393 × log10(WBC count].
Overall survival according to the new prognostic index (MIPI). LR indicates low risk, prognostic score less than 5.7; IR, intermediate risk, score 5.7 or more but less than 6.2; and HR, high risk, score 6.2 or more. The prognostic score is calculated as [0.03535 × age (years)] + 0.6978 (if ECOG > 1) + [1.367 × log10(LDH/ULN)] + [0.9393 × log10(WBC count].
The internal validation procedure correcting for overoptimism by bootstrap showed stability of statistical significance and prognostic separation (Table 6). In addition, the bootstrap corrected performance measures for the new prognostic classification system appeared to be better than the results of the external validation of the IPI and FLIPI.
Bootstrap validation according to Efron et al31
| Model . | Method . | Log rank-χ2 . | df . | c . | SEP . |
|---|---|---|---|---|---|
| Cox model | optimistic | 55.84* | 4 | 0.6867 | 1.00† |
| Cox model | bootstrap | 46.62* | 4 | 0.6791 | 0.91† |
| MIPI | optimistic | 61.11 | 2 | 0.6668 | 1.59 |
| MIPI | bootstrap | 47.64 | 2 | 0.6536 | 1.49 |
| MIPIb | optimistic | 39.78 | 2 | 0.7096 | 1.74 |
| MIPIb | bootstrap | 31.78 | 2 | 0.6962 | 1.58 |
| Risk groups using standard cutpoints | optimistic | 34.77 | 2 | 0.6393 | 1.51 |
| Simplified MIPI | optimistic | 63.15 | 2 | 0.6716 | 1.60 |
| IPI | external | 38.83 | 3 | 0.6359 | 1.41 |
| IPI LR/LIR vs HI vs HR | external | 35.36 | 2 | 0.6149 | 1.40 |
| IPI LR vs LI/HI vs HR | external | 35.10 | 2 | 0.6170 | 1.31 |
| IPI LR vs LI vs HI/HR | external | 24.22 | 2 | 0.6178 | 1.41 |
| FLIPI | external | 14.14 | 2 | 0.5773 | 1.36 |
| Model . | Method . | Log rank-χ2 . | df . | c . | SEP . |
|---|---|---|---|---|---|
| Cox model | optimistic | 55.84* | 4 | 0.6867 | 1.00† |
| Cox model | bootstrap | 46.62* | 4 | 0.6791 | 0.91† |
| MIPI | optimistic | 61.11 | 2 | 0.6668 | 1.59 |
| MIPI | bootstrap | 47.64 | 2 | 0.6536 | 1.49 |
| MIPIb | optimistic | 39.78 | 2 | 0.7096 | 1.74 |
| MIPIb | bootstrap | 31.78 | 2 | 0.6962 | 1.58 |
| Risk groups using standard cutpoints | optimistic | 34.77 | 2 | 0.6393 | 1.51 |
| Simplified MIPI | optimistic | 63.15 | 2 | 0.6716 | 1.60 |
| IPI | external | 38.83 | 3 | 0.6359 | 1.41 |
| IPI LR/LIR vs HI vs HR | external | 35.36 | 2 | 0.6149 | 1.40 |
| IPI LR vs LI/HI vs HR | external | 35.10 | 2 | 0.6170 | 1.31 |
| IPI LR vs LI vs HI/HR | external | 24.22 | 2 | 0.6178 | 1.41 |
| FLIPI | external | 14.14 | 2 | 0.5773 | 1.36 |
df indicates degrees of freedom; c, Harrell measure of concordance34 between expected and observed survival times; SEP, measure of separation35 of survival curves; standard cutpoints: 60 years of age, upper limit of normal for LDH, 10 × 109 leukocytes/L; optimistic, estimation using the data set of model development; bootstrap, estimation corrected for overoptimism; and external, estimation using an independent data set.
Likelihood ratio statistic.
Calibration slope: regression coefficient of the prognostic score.
Simplified MIPI
The classification according to the new prognostic index involves some elementary mathematical operations best performed using an electronic calculator. To make the index most practicable, we exploratively simplified it categorizing the individual prognostic factors maintaining high concordance to the original index. Dichotomizing the prognostic factors, as is commonly done, at standard cutpoints (60 years for age, ULN for LDH, 10 × 109/L for WBC count) yielded low concordance to the MIPI (weighted kappa 0.56), and suboptimal separation of survival curves (Table 6, SEP). Instead, weighting age, ECOG performance status, LDH, and WBC with 0 to 3 points using 3 cutpoints each (Table 7) and classifying patients with a total of at most 3 points as LR, patients with 4 to 5 points as IR and patients with more than 5 points as HR, yielded high concordance (weighted kappa 0.79) and good separation of OS curves (Table 6, SEP).
Simplified prognostic index
| Points . | Age, y . | ECOG . | LDHULN . | WBC, 109/L . |
|---|---|---|---|---|
| 0 | <50 | 0-1 | <0.67 | < 6.700 |
| 1 | 50-59 | — | 0.67-0.99 | 6.700-9.999 |
| 2 | 60-69 | 2-4 | 1.000 -1.49 | 1.000-14.999 |
| 3 | ≥70 | — | ≥1.5000 | ≥15000 |
| Points . | Age, y . | ECOG . | LDHULN . | WBC, 109/L . |
|---|---|---|---|---|
| 0 | <50 | 0-1 | <0.67 | < 6.700 |
| 1 | 50-59 | — | 0.67-0.99 | 6.700-9.999 |
| 2 | 60-69 | 2-4 | 1.000 -1.49 | 1.000-14.999 |
| 3 | ≥70 | — | ≥1.5000 | ≥15000 |
For each prognostic factor, 0 to 3 points were given to each patient and points were summed up to a maximum of 11. Patients with 0 to 3 points in summary were classified as low risk, patients with 4 to 5 points as intermediate risk, and patients with 6 to 11 points as high risk. ECOG performance status was weighted with 2 points if patients were unable to work or bedridden (ECOG 2-4). LDH was weighted according to the ratio to the ULN. Thus, for an ULN of 240 U/L, the cutpoints were 180 U/L, 240 U/L, and 360 U/L, for example.
— indicates not applicable.
Prognostic relevance of cell proliferation
The percentage of Ki-67 positive cells was available in 236 lymph node biopsies of the 455 MCL patients with a median of 14.5% and range of 1.2% to 91.0%. The number of mitoses per square millimeter was counted in 162 cases with a median of 4, range 0 to 72 and one extreme value of 1039, which was excluded from further analysis. Both proliferation parameters showed strong univariate prognostic relevance for OS with an RR of 1.29 (95% CI 1.16-1.44, P < .001) for Ki-67 elevated by 10% and 1.27 (95% CI 1.09-1.48, P = .003) for the number of mitoses elevated by 10/mm2. In bivariable regression including Ki-67 and number of mitoses, only Ki-67 remained independently significant, whereas the number of mitoses lost its prognostic relevance (P = .68, n = 126), and was thus excluded from further analyses. Patients with Ki-67 assessed showed significantly less spleen and bone marrow involvement, better performance status, more involved nodal areas, and larger lymph nodes than patients without available Ki-67. However, there was no significant difference in OS of patients with or without data on Ki-67 (median OS 58 and 57 months, P = .39).
Ki-67 remained independently significant from the MIPI clinical score (regression coefficient 0.02142, P < .001) and inclusion of Ki-67 did not substantially change the regression coefficient of the MIPI score (0.9554, P < .001). Thus, we calculated the combined biologic score by addition of 0.02142 times Ki-67 (%) to the clinical score. Optimal cutpoints were 5.7 and 6.5 for the log rank statistics with respect to OS, and Figure 4 shows OS according to the so defined combined biologic index (MIPIb).
Overall survival according to the combined biologic index (MIPIb) in 220 patients with Ki-67 available. LR indicates low risk, combined biologic score (CBS) less than 5.7; IR, intermediate risk, CBS 5.7 or more but less than 6.5; and HR, high risk, CBS 6.5 or more. The combined biologic score is calculated as 0.03535 times age (years) plus 0.6978 (if ECOG > 1) plus 1.367 times log10(LDH/ULN) plus 0.9393 times log10(WBC count) plus 0.02142 times Ki-67 (%).
Overall survival according to the combined biologic index (MIPIb) in 220 patients with Ki-67 available. LR indicates low risk, combined biologic score (CBS) less than 5.7; IR, intermediate risk, CBS 5.7 or more but less than 6.5; and HR, high risk, CBS 6.5 or more. The combined biologic score is calculated as 0.03535 times age (years) plus 0.6978 (if ECOG > 1) plus 1.367 times log10(LDH/ULN) plus 0.9393 times log10(WBC count) plus 0.02142 times Ki-67 (%).
Discussion
Based on data of 455 patients with advanced stage MCL from 3 randomized trials of GLSG and European MCL Network, a new prognostic index (MIPI, mantle cell lymphoma international prognostic index) has been developed. The MIPI is the first prognostic index specific to MCL patients and, more importantly, allows a clear separation of 3 well-balanced groups of patients with significantly different prognoses. The low-risk group comprised 44% of the patients with a median OS not yet reached after a median follow-up of 32 months, and a 5-year OS rate of 60%. The intermediate risk group consisted of 35% of the patients and revealed a median OS of 51 months, while the high-risk group (21%) had a poor outcome with a median survival of only 29 months. The MIPI is based on the 4 independent prognostic factors age, ECOG performance status, LDH, and WBC counts, which are clinical variables easily and routinely available in clinical practice. A simplification of the MIPI was developed allowing for bedside application reproducing the original index remarkably well.
The application of the IPI18 for diffuse large B-cell lymphoma to our MCL patient cohort revealed a lack of separation of the 2 intermediate-risk groups, which comprised more than two-thirds of the patients. The application of the FLIPI19 for follicular lymphoma to our MCL patients showed even worse results, with no separation of low- and intermediate-risk groups and a large high-risk group with relatively good outcome. In addition, of the individual IPI and FLIPI risk factors, the number of extranodal sites and the number of involved nodal areas showed no prognostic relevance and hemoglobin no independent prognostic relevance in our MCL patient cohort. ECOG performance status, which showed high prognostic relevance in our analyses and many previous reports, was not included in the development of the FLIPI.
Previous attempts to apply the IPI in particular to patients with MCL proved rather unsatisfactory1,12,–14,16,20 (Table 8). The IPI showed either no prognostic relevance for OS,4,10,17 or the original 4 risk groups were combined to 312,14 or 2 risk groups1,11,16 or different risk groups had very similar outcome.2,9,13,20 In addition, of the 5 IPI risk factors, the number of extranodal sites showed mostly no, and Ann Arbor stage inconsistent, prognostic relevance in previous univariate analyses (Table 8).
Previous reports on clinical characteristics, outcome, and prognostic factors of patients with MCL
| First author . | Tiemann . | Räty . | Samaha . | Andersen . | Schrader . | Oinonen . | Møller . | Argatoff . | Weisenburger . | Zucca . | Bosch . | Decaudin . | Velders . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 17 | 15 | 13 | 1 | 16 | 12 | 20 | 4 | 14 | 9 | 11 | 10 | 2 |
| Number | 304 | 127 | 121 | 105* | 95 | 94 | 93* | 80* | 68 | 65 | 59 | 45 | 41* |
| Median age, y | 64 | 65 | 63 | 66 | 62% (> 60) | 66 | 68 | 65 | 64 | 64 | 63 | 59 | 68 |
| Male, % | 76 | 64 | 67 | 75 | 77 | 59 | 71 | 70 | 75 | 67 | 74 | 78 | 61 |
| Stage I/II, % | 8 | 17 | 13 | 16 | 8 | 24 | 17 | 14 | 25 | 22 | 5 | 13 | 20 |
| Median OS, mo | ∼36 | 34 | 37 | 30 | ∼30 | 41 | 37 | 43 | 38 | 42 | 49 | ∼56 | 31.5 |
| Older than 60 y | + | na | +† | +‡ | + | + | na | − | na | +‡ | − | na | na |
| Sex | − | na | na | − | − | − | na | na | na | − | − | na | na |
| ECOG 2-4 vs 0-1 | + | na | + | + | − | +§ | + | + | + | − | + | na | + |
| Stage III/IV vs I/II | + | na | + | + | − | + | na | − | −‖ | − | −¶ | − | + |
| B-symptoms | + | na | – | − | − | + | na | na | + | − | + | na | na |
| Spleen inv. | na | na | + | + | na | − | na | na | na | − | + | – | na |
| Bone marrow inv. | + | na | na | − | − | + | na | na | + | − | na | + | + |
| Peripheral blood inv. | na | na | + | na | na | + | na | + | na | na | − | −‖ | na |
| Extranodal sites, more than 1 | +| | na | + | − | −| | − | − | − | na | na | − | − | + |
| Bulk, larger than 10 cm | na | na | − | na | na | − | na | na | na | na | na | na | na |
| Elevated LDH | + | na | + | + | + | + | na | − | na | + | + | − | − |
| WBC count | na | na | na | + | na | + | na | na | na | na | + | na | na |
| Hemoglobin | na | na | + | + | na | + | + | na | na | na | na | − | na |
| Growth pattern | + | + | − | na | na | na | na | − | + | na | na | na | na |
| Cytology | − | + | − | na | na | na | na | + | + | na | + | −‖ | na |
| Mitotic index | + | + | na | na | na | na | na | + | − | na | + | na | na |
| Ki-67 | + | + | na | − | + | na | na | na | na | na | na | na | + |
| IPI | − | na | +** | + | + | +†† | +‡‡ | − | + | +†† | + | − | +‡‡ |
| IPI risk group, no. | 2 | na | 4 | 2 | 2 | 3 | 4 | 4 | 3 | 4 | 2 | 2 | 4 |
| First author . | Tiemann . | Räty . | Samaha . | Andersen . | Schrader . | Oinonen . | Møller . | Argatoff . | Weisenburger . | Zucca . | Bosch . | Decaudin . | Velders . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 17 | 15 | 13 | 1 | 16 | 12 | 20 | 4 | 14 | 9 | 11 | 10 | 2 |
| Number | 304 | 127 | 121 | 105* | 95 | 94 | 93* | 80* | 68 | 65 | 59 | 45 | 41* |
| Median age, y | 64 | 65 | 63 | 66 | 62% (> 60) | 66 | 68 | 65 | 64 | 64 | 63 | 59 | 68 |
| Male, % | 76 | 64 | 67 | 75 | 77 | 59 | 71 | 70 | 75 | 67 | 74 | 78 | 61 |
| Stage I/II, % | 8 | 17 | 13 | 16 | 8 | 24 | 17 | 14 | 25 | 22 | 5 | 13 | 20 |
| Median OS, mo | ∼36 | 34 | 37 | 30 | ∼30 | 41 | 37 | 43 | 38 | 42 | 49 | ∼56 | 31.5 |
| Older than 60 y | + | na | +† | +‡ | + | + | na | − | na | +‡ | − | na | na |
| Sex | − | na | na | − | − | − | na | na | na | − | − | na | na |
| ECOG 2-4 vs 0-1 | + | na | + | + | − | +§ | + | + | + | − | + | na | + |
| Stage III/IV vs I/II | + | na | + | + | − | + | na | − | −‖ | − | −¶ | − | + |
| B-symptoms | + | na | – | − | − | + | na | na | + | − | + | na | na |
| Spleen inv. | na | na | + | + | na | − | na | na | na | − | + | – | na |
| Bone marrow inv. | + | na | na | − | − | + | na | na | + | − | na | + | + |
| Peripheral blood inv. | na | na | + | na | na | + | na | + | na | na | − | −‖ | na |
| Extranodal sites, more than 1 | +| | na | + | − | −| | − | − | − | na | na | − | − | + |
| Bulk, larger than 10 cm | na | na | − | na | na | − | na | na | na | na | na | na | na |
| Elevated LDH | + | na | + | + | + | + | na | − | na | + | + | − | − |
| WBC count | na | na | na | + | na | + | na | na | na | na | + | na | na |
| Hemoglobin | na | na | + | + | na | + | + | na | na | na | na | − | na |
| Growth pattern | + | + | − | na | na | na | na | − | + | na | na | na | na |
| Cytology | − | + | − | na | na | na | na | + | + | na | + | −‖ | na |
| Mitotic index | + | + | na | na | na | na | na | + | − | na | + | na | na |
| Ki-67 | + | + | na | − | + | na | na | na | na | na | na | na | + |
| IPI | − | na | +** | + | + | +†† | +‡‡ | − | + | +†† | + | − | +‡‡ |
| IPI risk group, no. | 2 | na | 4 | 2 | 2 | 3 | 4 | 4 | 3 | 4 | 2 | 2 | 4 |
Prognostic relevance reported from univariate analyses on significance level .05 for overall survival.
+ indicates prognostic; −, not prognostic; inv, involvement; and na, not analyzed.
Population based data.
Age cutpoint 70 years.
Age cutpoint 65 years.
ECOG performance status 0 vs > 0.
Stage I-III vs. IV.
P < .1.
LR, LIR, HIR not separated.
LIR, HIR not separated.
LIR, HIR, HR not separated.
Extra nodal involvement, yes vs no.
In contrast to our results, a recent report by Møller et al20 stated superiority of the FLIPI over the IPI in terms of size and number of risk groups, separation of survival curves, and prognostic value of individual risk factors. However, the number of patients analyzed, 93, was rather small, low- and intermediate-risk groups were not well separated, and the high-risk group included more than half of the patients. Interpretation of multiple Cox regression results was hampered by inclusion of the interdependent factors IPI, FLIPI and the individual prognostic factors.
Of the independent prognostic factors of the MIPI, consistent prognostic relevance on OS had been shown for age, performance status, and LDH in previous univariate analyses (Table 8). Hematologic parameters were mostly not considered as candidate prognostic factors. Three studies,1,11,12 however, included the WBC count and described a prognostic relevance in univariate analysis. In several studies peripheral blood involvement was included4,10,,–13 and showed significance in 34,12,13 of 5 univariate analyses and in 212,13 multiple regression analyses. The methods for the detection of peripheral blood involvement, however, were mostly not adequately described and sensitivity of different methods may vary substantially. Although peripheral blood involvement may occur without leukocytosis, a high WBC count probably reflects peripheral blood involvement, and our results thus may confirm previous observations.12,13
Despite these encouraging results, it must be emphasized that the current analyses did not include patients with limited stage I or II MCL because for these patients no comparable clinical trial is available. However, the prognostic relevance of stage is not consistently seen in the literature, the proportion of patients with stages I or II is rather low in MCL (Table 8) and they also require a different therapeutic approach. Thus, as recommended by Altman,35 to avoid any treatment bias, we limited our investigation to the advanced-stage MCL patients with standardized treatments in randomized trials. The present data are also limited to patients who can tolerate moderately intensive chemotherapy. This limitation, however, also applies to the IPI18 and FLIPI19 and does not hamper the relevance of the MIPI within these borders.
Spleen involvement, maximal tumor size, serum β2-microglobulin levels, and lymphocyte, granulocyte, and monocyte counts were excluded from multiple Cox regression due to missing data, but had shown univariate prognostic relevance on OS. Nevertheless, further exploratory analyses revealed no additional prognostic relevance of these variables to the 4 MIPI prognostic factors. For β2-microglobulin this stands in contrast to the results of Khouri et al.36 Two MIPI adverse prognostic factors, higher age and worse ECOG performance status, are also contraindications against more aggressive experimental therapies, for which the MIPI could be applied to select candidate patients. However, excluding age and ECOG performance status as candidate prognostic factors, only LDH and WBC count remained independent prognostic factors still allowing for patient stratification. In addition, in the cohort of patients younger than 65 years, the same 4 independent prognostic factors were identified, thus, there was no “age-adjusted” prognostic index as developed for the IPI. Finally, our findings are independent of the choice of initial cytoreductive therapy, because inclusion of treatment variables did not change the relevance of the 4 independent prognostic factors. In particular, the inclusion of primary rituximab treatment as covariate did not change the final prognostic model. In addition, a significant improvement in OS has not been shown for ASCT so far for MCL patients.
In an attempt to develop a combined biologic index as postulated by Räty et al15 and Tiemann et al,17 we included the proliferation marker Ki-67 in our analyses and showed high prognostic relevance independent from the MIPI prognostic score. Including Ki-67 we exploratively defined a combined biologic index (MIPIb) which revealed a low-risk group with relatively good outcome. However, proliferation data were available in only approximately half of the patients, and the selection of patients with Ki-67 appeared to be nonrandom with less spleen and bone marrow involvement and more and larger lymph node involvement. As it is assessed on lymph node biopsies, proliferation is not available in all MCL patients. In addition, standardization and reproducibility of proliferation assessment still requires improvement as essential prerequisite to be used as molecular prognostic marker.37 Hence, a combined biologic prognostic index including Ki-67 is currently not applicable except for research studies.
We performed internal validation by bootstrap, the method that achieves the highest possible power from the available data,31,32 and could confirm a high stability of the developed prognostic model. Nevertheless, an external validation on an independent data set is still warranted to allow a broad application of this prognostic tool.
In conclusion, our new prognostic classification tool, MIPI, might be helpful to allow individualized, risk-adapted treatment decisions in patients with advanced stage MCL, to hopefully optimize treatment and to improve outcomes of this aggressive disease. In addition, our results will allow stratification in clinical trials, interstudy-comparisons of clinical trial results according to patient risk profiles, and provide a basis for establishing future novel biologic prognostic markers.
The online version of this article contains a data supplement.
Presented in part in oral form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 12, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the German Low Grade Lymphoma Study Group and the European MCL Network for participation in this study. Many thanks to Olaf Determann from the Lymph Node Registry Kiel, who also worked intensively in providing the proliferation data.
This work was supported in part by grants from the Deutsche Krebshilfe (T14/96/Hi 1, project No. 70-2208-Hi 2), the Bundesministerium für Bildung und Forschung, Kompetenznetz Maligne Lymphome (no. 01 GI 9994), the European Commission (no. LSHC-CT-2004-503351), and the Lymphoma Research Foundation (no. MCLI-04-016).
Authorship
E.H. analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; MD, W.H., and M.U. designed the trials and revised the manuscript; W.K. provided proliferation data; J.H. provided statistical expertise and revised the manuscript; C.G., A.v.H., H.C.K.-N., M.P., M.R., B.M., H. Einsele, N.P., W.J., B.W., W.-D.L., U.D., H. Eimermacher, and H.W. recruited, treated, and documented patients.
E.H. is a candidate at the Faculty of Medicine of the Ludwig-Maximilians-University Munich for her doctoral degree and part of this work has been developed in partial fulfillment of the requirements for her degree.
A complete list of the members of the German Low Grade Lymphoma Study Group and the European MCL Network who contributed to this analysis appears in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: M.P. is a member of the Advisory Board of Roche, Genentech, and Lilly. M.D. received support for clinical studies and speaker honoraria from Roche. W.H. is member of the Advisory Board of Roche and received support for clinical studies and speaker honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Dr. Michael Unterhalt, Medizinische Klinik III, Klinikum Groβhadern, Marchioninistr. 15, D-81377 München, Germany; e-mail: Michael.Unterhalt@med.uni-muenchen.de.


![Figure 3. Overall survival according to the new prognostic index (MIPI). LR indicates low risk, prognostic score less than 5.7; IR, intermediate risk, score 5.7 or more but less than 6.2; and HR, high risk, score 6.2 or more. The prognostic score is calculated as [0.03535 × age (years)] + 0.6978 (if ECOG > 1) + [1.367 × log10(LDH/ULN)] + [0.9393 × log10(WBC count].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-06-095331/4/m_zh80050813140003.jpeg?Expires=1769098127&Signature=F8L8epdsxfsbUgzwHGAvJizZT7vMErbpyVyRyu0NX3w7O~1Ols9uu0xATzjUavLqo4FTG3hxZjcDHvU9o2VAu1tBu857KS9SCZ5jrJs-dsVja-bzi2foXfHfkVO8swqH0twqQjtp-DLJrHtWnnkxWqQ2aqvvGTgm5PHHup~HEapOmRAYhMPIZOLe0Al~Gda5jpaCIzRUVVR-jpZOa7mzSSjmWVdKNe6t8JiG42lk9JLgr2XODXCODJcw6rqSQgObCSkfsgbCDHBU0epgDSeL-RkPyKztytQ8F36PQtajQN0XANG8~866wB~czpcY3GxE9trIHLDtte0IxNhKP2uiDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal