In high doses with stem-cell transplantation, melphalan is an effective but toxic therapy for patients with systemic light-chain (AL-) amyloidosis, a protein deposition and monoclonal plasma cell disease. Melphalan can eliminate the indolent clonal plasma cells that cause the disease, an achievement called a complete response. Such a response is usually associated with extended survival, while no response (a less than 50% reduction) is not. Gene-expression studies and a stringently supervised analysis identified calreticulin as having significantly higher expression in the pretreatment plasma cells of patients with systemic AL-amyloidosis who then had a complete response to high-dose melphalan. Calreticulin is a pleiotropic calcium-binding protein found in the endoplasmic reticulum and the nucleus whose overexpression is associated with increased sensitivity to apoptotic stimuli. Real-time PCR and immunohistochemical staining also showed that expression of calreticulin was higher in the plasma cells of those with a complete response. Furthermore, wild-type murine embryonic fibroblasts were significantly more sensitive to melphalan than calreticulin knock-out murine embryonic fibroblasts. These data have important implications for understanding the activity of melphalan in plasma-cell diseases and support further investigation of calreticulin and its modulation in patients with systemic AL-amyloidosis receiving high-dose melphalan.
Introduction
Systemic light-chain (AL-) amyloidosis is a rare protein conformation and clonal plasma cell disorder associated with multiorgan failure and early death due to fibrillar tissue-deposits formed by aberrant monoclonal immunoglobulin free light chains (FLC).1 Approximately 3000 new cases are diagnosed annually in the United States. Small numbers of clonal plasma cells in the bone marrow are usually the source of the FLC, and κ disease is less common than λ, with a case ratio of 1:4, unlike multiple myeloma in which the ratio is 3:2.2,–4 A paradox of systemic AL-amyloidosis is how apparently indolent clonal plasma cells tolerate making and secreting FLC toxic to normal tissues. High-dose melphalan with autologous stem-cell transplantation (SCT) is an effective therapy in selected patients but a treatment-related mortality of up to 15% is seen even in centers with SCT experience, and only one-third of patients at diagnosis are eligible for SCT.4
After SCT, about one-third of patients achieve a durable complete or near complete response of the clonal plasma cell disease, while one-third achieve a partial response (> 50% reduction), and one-third have minimal to no response. The achievement of a complete response is associated with subsequent improvement of the amyloid organ disease and extended survival, while no response is associated with progression of organ disease and shortened survival.4,–6 The availability of the serum free light chain assay, a measure of the toxic FLC in almost all cases, has improved management during treatment.7 Reduction in the level of pathologic FLC and normalization of the FLC ratio are associated with achievement of a complete response.8,9 There are no known baseline predictors of the response of the clonal plasma-cell disease to high-dose melphalan in patients with systemic AL-amyloidosis, although patients with κ disease have an increased complete response rate.4
Melphalan (L-phenylalanine mustard) is a bifunctional alkylating agent whose mechanism of cytotoxicity is related to the formation of reactive oxygen species and to DNA adducts and cross-links.10 Melphalan is highly active in systemic AL-amyloidosis and multiple myeloma and is the standard single-agent used in high-dose therapy in both diseases.11 Sensitivity to high-dose melphalan in multiple myeloma has been associated with increased formation and decreased repair of DNA cross-links in the p53 gene in peripheral blood mononuclear cells obtained at treatment.12,13 There have, however, been no studies in systemic AL-amyloidosis attempting to identify a basis for the difference in response to melphalan. Given the risk of SCT in systemic AL-amyloidosis, predictors of response would help to identify patients likely to benefit from SCT and provide a rational basis for patient selection.
We have begun to study this phenomenon, assuming that among the factors contributing to the variable response to melphalan are ones intrinsic to the clonal plasma cells. Using stringent criteria, we have identified 5 genes significantly overexpressed in the purified clonal plasma cells of patients with complete response compared with those with no response. In this report we focus on one of them, calreticulin, a gene that encodes a pleiotropic calcium-binding chaperone protein found predominantly in the endoplasmic reticulum.14,15 Our findings show that higher levels of calreticulin expression are associated with response to high-dose melphalan in systemic AL-amyloidosis and support further investigation of calreticulin in plasma-cell disorders and their treatment.
Methods
Patients and screening
Patients referred for assessment of plasma-cell disorders were evaluated for monoclonal gammopathy, hereditary forms of amyloidosis, and organ-involvement with amyloid as previously described.16,–18 Diagnosis of amyloidosis required tissue biopsy. Patients with systemic AL-amyloidosis undergoing autologous stem-cell transplantation were followed for outcome assessment as previously described.19
All patients were offered the opportunity to contribute research specimens at the time of clinically indicated bone marrow studies. Written informed consent was obtained from patients for studies of bone marrow cells on protocols approved by the Institutional Review Board (IRB) at Memorial Sloan-Kettering Cancer Center and in accordance with the Declaration of Helsinki. The study of marrow biopsies was performed under IRB waiver. The availability and adequacy of specimens determined their use in these studies.
Plasma-cell procurement and purification
Heparinized bone marrow aspirates (6 to 10 mL in volume) were obtained from 31 patients with systemic AL amyloidosis and 3 patients with hereditary (transthyretin-related, ATTR-) amyloidosis. Bone marrow biopsies from 29 of the 31 AL patients and from an additional 19 AL patients (a total of 48 biopsies were available from the 50 AL patients) were obtained at diagnosis, fixed in formalin, and paraffin-embedded for immunohistochemical staining. All AL patients underwent SCT.
Plasma-cell specimens used to obtain RNA for gene-expression profiles were purified from light-density mononuclear cells that had been labeled with phycoerythrin-linked anti-CD138 monoclonal antibody (Miltenyi Biotec, Auburn, CA) and 4′-6-diamidino-2-phenylindole (DAPI). DAPI was used to minimize contamination with dead cells. Greater than 95% pure CD138+/DAPI− plasma cells were then obtained by FACS-sorting, confirmed by confocal immunofluorescence microscopy (IFM; Nikon Diaphot, Melville, NY) using polyclonal goat antihuman antibodies for cytoplasmic kappa and lambda light chains (Southern Biotechnology Associates, Birmingham, AL). Plasma cell specimens used to obtain RNA for real-time PCR were selected using immunomagnetic technique with anti-CD138 linked to microbeads (Miltenyi). We repeated the selections twice for each specimen, using the positively selected cells from the first selection as starting material for the second round of selection (double enrichment).
Gene-expression profiles
cRNA was made and processed, and Affymetrix U133 PLUS 2.0 arrays (Affymetrix, Santa Clara, CA) performed as previously described.20,21 We then performed a supervised analysis comparing the plasma-cell gene- expression profiles of complete responders (CR) with nonresponders (NR). The basic analysis was a t test for differential expression between the groups. We filtered genes at P less than .01. The genes that passed the filter were subject to Expression Analysis Systematic Explorer (EASE; National Institutes of Health, Bethesda, MD) software analysis to find whether there were gene ontology families overrepresented in the gene list. Our final filtering selected those genes with P less than .01, 2-fold difference in expression between complete and no response, average expression of at least 1000, and EASE score less than 0.05.
Real-time polymerase chain reaction
Real-time polymerase chain reaction (qRT-PCR) for calreticulin (CALR) was performed with SYBR-Green Master Mix on an ABI 7300/7500 platform and related software as previously described (Applied Biosystems, Foster City, CA).20 GAPDH was used as an internal control. Primers were as follows: CALR forward 5′-AAATGAGAAGAGCCCCGTTCTTCCT-3′, and reverse 5′-AAGCCACAGGCCTGAGATTTCATCTG-3′ (amplicon 116bp); and GAPDH forward 5′-TTCGACAGTCAGCCGCATCTTCTT-3′, and reverse 5′-GCCCAATACGACCAAATCCGTTGA-3′ (amplicon 105bp). cDNA was synthesized (ThermoScript RT-PCR System, Invitrogen Life Technologies, Carlsbad, CA) from patient specimen whole-cell RNA preparations treated with DNase I (RNeasy Mini Kit; QIAGEN, Valencia, CA). We analyzed all samples in duplicate and performed the assays at least twice on separate days. The comparative Ct method was used and the amount of target was normalized to the GAPDH control (2−ΔΔCt) assuming an efficiency of 2.
Immunohistochemical staining
To optimize immunohistochemical (IHC) staining for calreticulin, SV40-immortalized murine embryonic fibroblasts (MEF) from wild type (K41) and calreticulin knock-out (K42) mice were obtained (gift of Dr Marek Michalak, University of Alberta, Edmonton, AB). MEF cells were grown in DMEM with 4.5g/L glucose and 10% fetal bovine serum with penicillin and streptomycin, passaged when confluent, harvested with 0.1% trypsin and EDTA, and fixed in 4% formalin and pelleted for histologic study. We obtained a polyclonal rabbit anti–human calreticulin antibody (Affinity Bioreagents, Golden, CO) previously shown to work in IHC tissue staining.
After optimizing the staining conditions with the use of MEF controls, adjacent sections of formalin-fixed marrow biopsy specimens were mounted for IHC staining for calreticulin and plasma cells. The rabbit anti–human calreticulin antibody was used at a dilution of 1:20 000 after HIER (heat-induced epitope retrieval) with citrate buffer, pH 6.0, and steam pretreatment for 30 minutes. Secondary biotinylated anti–rabbit IgG (H+L, Vector Labs, Burlingame, CA) was used at a 1:500 dilution, and tertiary streptavidin-horseradish peroxidase (DAKO; Glostrup, Denmark) was used at a 1:500 dilution. A mouse monoclonal anti-CD138 antibody (Serotec; Raleigh, NC) was used to stain plasma cells at a dilution of 1:20 after HIER with citrate buffer, pH 6.0, and steam pretreatment for 30 minutes. Secondary biotinylated antimouse antibody (Vector Labs) was used at a 1:500 dilution, and tertiary strepavidin-horseradish peroxidase (DAKO) was used at a 1:500 dilution.
Images were obtained with an Olympus BX41 microscope and attached DP20 digital camera and software and 1600 × 1200 Ultra eXtended Graphics Array (UXGA) high-definition monitor (Olympus Imaging America, Center Valley, PA), and as noted through a 60× Achromat objective. Calreticulin staining intensity was scored as 1, rare scattered; 2, granular cytoplasmic; 3, pancytoplasmic. The fraction of calreticulin-positive cells was scored over the total CD138-positive plasma cells and scored as 1 (one-third or less), 2 (one-third to two-thirds) and 3 (more than two-thirds). A combined score (intensity × fraction positive) was calculated (range, 1-9).
Melphalan exposure of murine embryonic fibroblast cell lines
Wild-type and calreticulin knock-out MEF cells were grown as described above. Melphalan (L-phenylalanine mustard; Sigma-Aldrich, St Louis, MO) was dissolved in acid alcohol and used at the concentrations specified. Cell viability studies were performed with 105 cells per well in 6-well flat-bottom plates. Cells were seeded and allowed to adhere over 24 hours before the addition of freshly solubilized melphalan. After 24-hour incubation with melphalan, cells were washed, harvested with 0.1% trypsin and EDTA, and assessed by trypan blue staining. Cytotoxicity studies were performed with a lactate dehydrogenase (LDH) Cytotoxicity Detection Kit (Roche Molecular Biochemicals, Mannheim, Germany) with all procedures optimized and performed according to the manufacturer's instructions. The absorbance of the samples at 490 nm (reference wavelength 655 nm) was measured with a Biorad Model 680 microplate reader (Biorad, Hercules, CA).
Statistics
Affymetrix gene-expression data were normalized and a supervised analysis was performed comparing the plasma-cell gene-expression profiles of complete responders with nonresponders as described above. For other statistical analyses we used PRISM (GraphPad, San Diego, CA). The calreticulin real-time PCR data and calreticulin indices of marrow biopsy slides were analyzed using the Mann-Whitney test. The wild-type and calreticulin knock-out MEF cell culture results were analyzed using the paired t test. All analyses were 2-tailed using P value less than .05 for significance.
Results
Bone marrow specimens from patients with amyloidosis and purification of plasma cells from bone marrow aspirates
To perform these studies we used marrow specimens obtained at diagnosis prior to treatment from 50 patients with systemic AL-amyloidosis who then underwent high-dose melphalan treatment with SCT and from 3 patients with transthyretin-associated hereditary amyloidosis (ATTR). Thirty-one marrow aspirate specimens were obtained from AL patients. Seven were used for gene-expression profiles, 22 for calreticulin qRT-PCR, and 2 for both. Marrow biopsy specimens from diagnosis were available from 29 of these 31 AL patients, and from an additional 19 AL patients chosen because of availability of adequate material. The 48 marrow biopsies included 8 of the 9 patients whose aspirates were for gene-expression profiles and all whose aspirates were used for calreticulin qRT-PCR.
We separated light-density mononuclear cells from marrow aspirates and selected CD138+/DAPI− plasma cells either by FACS-sorting at our core facility or by double-enrichment using immunomagnetic beads. Selected plasma-cell specimens contained a median of 2 × 105 cells (range, 0.1 × 105-106). We analyzed the cells by flow cytometry and immunofluorescence microscopy and confirmed that suspensions contained more than 95% plasma cells with light-chain isotype restriction (Figure 1A).
Clonal plasma cells from patients with systemic AL-amyloidosis can be highly enriched after FACS-sorting. (A) Flow cytometric plot of FACS-sorted cells is shown with CD138+/DAPI− cells in the bottom right quadrant. CD138+/DAPI− cells were used for gene-expression analysis in 9 cases. In the insert, an IFM image of enriched clonal plasma cells from the marrow aspirate of an AL-amyloidosis patient is shown. These cells were stained intracellularly for lambda light chains with a FITC-linked monoclonal antibody and were more than 95% pure. (B) Box-and-whiskers plots of the expression levels of lineage-specific genes are depicted for the 9 specimens of purified clonal plasma cells used in gene-expression studies. The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile), and the whiskers extend down to the smallest and up to the largest value. The genes (and probe sets in parentheses) include genes for clonality, namely the λ and κ light-chain constant region genes (Affymetrix probe sets 221651 and 215121). Eight of the 9 specimens were λ and hence the nonclonal gene was κ in those cases. The plot of expression levels for the clonal and nonclonal genes clearly shows that the clonal genes were expressed at higher levels than the nonclonal constant region genes (paired t test, P = .005). The other lineage markers were CD38 (205692), XBP-1 (200670), CD138 (201286), CD4 (200670), CD14 (201743), CD16 (206398), CD19 (206398), CD33 (206120), CD45 (212588) and CD64 (214511). Myeloma cells can aberrantly express some of these markers.70 Comparison of the CR and NR sets showed no significant differences in gene- expression levels by paired t test for clonal and nonclonal IgVL constant region genes, CD38, XBP-1, CD138, or other lineage markers with expression levels more than 100.
Clonal plasma cells from patients with systemic AL-amyloidosis can be highly enriched after FACS-sorting. (A) Flow cytometric plot of FACS-sorted cells is shown with CD138+/DAPI− cells in the bottom right quadrant. CD138+/DAPI− cells were used for gene-expression analysis in 9 cases. In the insert, an IFM image of enriched clonal plasma cells from the marrow aspirate of an AL-amyloidosis patient is shown. These cells were stained intracellularly for lambda light chains with a FITC-linked monoclonal antibody and were more than 95% pure. (B) Box-and-whiskers plots of the expression levels of lineage-specific genes are depicted for the 9 specimens of purified clonal plasma cells used in gene-expression studies. The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile), and the whiskers extend down to the smallest and up to the largest value. The genes (and probe sets in parentheses) include genes for clonality, namely the λ and κ light-chain constant region genes (Affymetrix probe sets 221651 and 215121). Eight of the 9 specimens were λ and hence the nonclonal gene was κ in those cases. The plot of expression levels for the clonal and nonclonal genes clearly shows that the clonal genes were expressed at higher levels than the nonclonal constant region genes (paired t test, P = .005). The other lineage markers were CD38 (205692), XBP-1 (200670), CD138 (201286), CD4 (200670), CD14 (201743), CD16 (206398), CD19 (206398), CD33 (206120), CD45 (212588) and CD64 (214511). Myeloma cells can aberrantly express some of these markers.70 Comparison of the CR and NR sets showed no significant differences in gene- expression levels by paired t test for clonal and nonclonal IgVL constant region genes, CD38, XBP-1, CD138, or other lineage markers with expression levels more than 100.
Calreticulin expression is significantly higher in the plasma cells of patients who respond to high-dose melphalan
Newly diagnosed untreated patients with systemic AL-amyloidosis were enrolled on a clinical trial using high-dose melphalan as initial therapy and after treatment were assessed for response as previously described (see www.ClinicalTrials.gov,NCT00089167).6,19 Pretreatment marrow aspirates from 9 patients provided purified plasma cells for gene-expression studies. Four of these patients achieved a complete response (CR, 3λ, 1κ) and 5 had no response (NR, 5λ). The median percentages of plasma cells in the marrow aspirates of CR and NR patients were 10% (range, 5%-22%) and 13.5% (range, 4-26) respectively (P, nonsignificant), and all samples showed clonal dominance on marrow biopsy by light chain isotype staining.22,23
RNA from purified plasma cells was given to the core Affymetrix facility for quality control, amplification, and hybridization on the Affymetrix U133 PLUS 2.0 chip. Transcript expression was detected (present calls) in over 30% of all probe sets. To assess the fidelity of the expression profiles to the plasma-cell lineage, we compared the expression of plasma cell–specific genes, such as immunoglobulin kappa and lambda light chain (IgVL) constant region genes, CD38, XBP-1, and CD138, to that of genes specific for other hematopoietic lineages, such as T cells (CD4) and myelomonocytic cells (CD14, CD16, CD33, CD64; Figure 1B). The results confirmed that we obtained purified plasma cells and furthermore that we obtained specimens highly enriched in clonal plasma cells. Comparison of the CR and NR sets showed no differences in gene-expression levels for clonal and nonclonal IgVL constant region genes, CD38, XBP-1, CD138, or other lineage markers with expression levels more than 100 by paired t test. The gene-expression data from these 9 cases are being entered into the NCBI Gene Expression Omuibus (http://www.ncbi.nlm.nih.gov/projects/geo/index.cgi.
We performed a supervised analysis comparing the plasma-cell gene-expression profiles of complete responders (CR, n = 4) with nonresponders (N = 5) using the stringent criteria described above. With this approach we identified 5 genes that were overexpressed in the plasma cells of patients with CR (Table 1). Tryptophanyl-tRNA synthetase (WARS) exists in human cells as full-length and truncated proteins and is inducible by serum starvation, tunicamycin,or interferon-gamma as part of the endoplasmic reticulum response to stress.25,26 The truncated form can act as an angiostatic factor.27,28 Isoleucine-tRNA synthetase (IARS), another aminoacyl synthetase, may also have multiple functions.29 Serine hydroxymethyltransferase 2 (SHMT2) is a mitochondrial enzyme that is myc responsive and important for conversion of serine to one-carbon units for metabolism.30 The β4 proteasome subunit (PSMB4) is part of the proteasome but does not display major peptidase or interferon-γ inducible activity.31
Overexpressed genes in patients achieving a complete response
| Gene . | Gene symbol . | Probe set . | CR mean . | NR mean . | Fold-change . | P . | EASE score . |
|---|---|---|---|---|---|---|---|
| Tryptophanyl-tRNA synthetase | WARS | 200629 | 2312.458 | 362.7378 | 6.375012 | .007 | 2.07×10−5 |
| Serine hydroxymethyltransferase 2 | SHMT2 | 214096 | 1271.041 | 276.7321 | 4.59304 | .007 | 0.003 |
| Isoleucine-tRNA synthetase | IARS | 204744 | 2003.228 | 555.4616 | 3.60642 | .006 | 2.07×10−5 |
| Calreticulin | CALR | 212952 | 2522.444 | 884.3507 | 2.852312 | .006 | 0.03 |
| Proteasome subunit, beta type, 4 | PSMB4 | 202244 | 2546.411 | 1152.955 | 2.208595 | .006 | 0.01 |
| Gene . | Gene symbol . | Probe set . | CR mean . | NR mean . | Fold-change . | P . | EASE score . |
|---|---|---|---|---|---|---|---|
| Tryptophanyl-tRNA synthetase | WARS | 200629 | 2312.458 | 362.7378 | 6.375012 | .007 | 2.07×10−5 |
| Serine hydroxymethyltransferase 2 | SHMT2 | 214096 | 1271.041 | 276.7321 | 4.59304 | .007 | 0.003 |
| Isoleucine-tRNA synthetase | IARS | 204744 | 2003.228 | 555.4616 | 3.60642 | .006 | 2.07×10−5 |
| Calreticulin | CALR | 212952 | 2522.444 | 884.3507 | 2.852312 | .006 | 0.03 |
| Proteasome subunit, beta type, 4 | PSMB4 | 202244 | 2546.411 | 1152.955 | 2.208595 | .006 | 0.01 |
Expression of calreticulin (CALR) in the CR set was 2.9-fold increased over the NR set, a difference that was significant by t test and EASE scoring. The quantitative level of expression of calreticulin in the CR set was 2522 versus 884 in the NR set. Calreticulin was of interest because of its links with radiation and drug sensitivity in other cell types and because of its role as a chaperone and in nascent protein N-glycosylation.32 Furthermore, a role for calreticulin in secretory cells such as plasma cells had not been studied but could hypothetically be relevant to the folding and secretion of unstable amyloid-forming FLC.
In comparison of genes overexpressed in the NR set compared with the CR set, no genes met the stringent filter we imposed with respect to expression level of at least 1000. Genes that met all other criteria including EASE score as overexpressed in the NR set are shown in Table 2.
Overexpressed genes in patients with no response
| Gene . | Gene symbol . | Probe set . | CR mean . | NR mean . | Fold change . | P . | EASE score . |
|---|---|---|---|---|---|---|---|
| Adrenomedullin | ADM | 202912 | 83.16284 | 379.3584 | 4.561634 | .009 | 9.89 × 10−5 |
| Suppression of tumorigenicity 14 (colon carcinoma, matriptase, epithin) | ST14 | 202005 | 69.9292 | 213.2634 | 3.049704 | .004 | 0.017 |
| HGFL gene | MGC17330 | 221756 | 97.40954 | 275.9443 | 2.832827 | .009 | 0.017 |
| Adenosine monophosphate deaminase (isoform E) | AMPD3 | 207992 | 67.24567 | 177.7494 | 2.643284 | <.001 | 0.012 |
| Interleukin 2 receptor, beta | IL2RB | 205291 | 43.66505 | 100.9797 | 2.312597 | .002 | 0.017 |
| Gene . | Gene symbol . | Probe set . | CR mean . | NR mean . | Fold change . | P . | EASE score . |
|---|---|---|---|---|---|---|---|
| Adrenomedullin | ADM | 202912 | 83.16284 | 379.3584 | 4.561634 | .009 | 9.89 × 10−5 |
| Suppression of tumorigenicity 14 (colon carcinoma, matriptase, epithin) | ST14 | 202005 | 69.9292 | 213.2634 | 3.049704 | .004 | 0.017 |
| HGFL gene | MGC17330 | 221756 | 97.40954 | 275.9443 | 2.832827 | .009 | 0.017 |
| Adenosine monophosphate deaminase (isoform E) | AMPD3 | 207992 | 67.24567 | 177.7494 | 2.643284 | <.001 | 0.012 |
| Interleukin 2 receptor, beta | IL2RB | 205291 | 43.66505 | 100.9797 | 2.312597 | .002 | 0.017 |
Calreticulin expression varies in purified plasma cells from patients with systemic AL-amyloidosis and correlates with response to high-dose melphalan
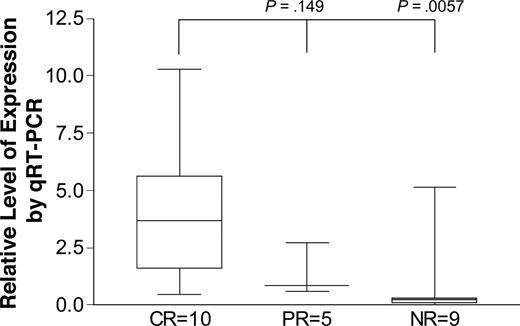
Using 24 specimens of purified plasma cells (20λ, 4κ) from patients before high-dose melphalan with SCT, we assessed the relative expression of calreticulin by real-time PCR. We used purified pooled marrow plasma cells from patients with hereditary amyloidosis (mutant transthyretin, ATTR-amyloidosis) as control. Assays were run in duplicate wells on separate days with the same controls. Results were nearly identical in all paired wells and the values of at least 2 separate runs were averaged for each specimen. The differences between the patients with CR and NR achieved significance (P < .01; Figure 2). There was no association between serum free light chain levels and calreticulin expression by qRT-PCR (data not shown).
Expression levels of calreticulin by real-time (qRT-) PCR correlate with response to high-dose melphalan. Twenty-four specimens of purified plasma cells from patients with systemic AL-amyloidosis were obtained before treatment with high-dose melphalan and assessed for calreticulin expression by qRT-PCR. GAPDH and pooled cDNA from the purified plasma cells of 3 patients with hereditary amyloidosis were used as controls. Patients were assessed for response to high-dose melphalan at 3 months after treatment. Calreticulin expression in responders was significantly higher than in nonresponders (P < .01, Mann-Whitney). The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile), and the whiskers extend down to the smallest and up to the largest value.
Expression levels of calreticulin by real-time (qRT-) PCR correlate with response to high-dose melphalan. Twenty-four specimens of purified plasma cells from patients with systemic AL-amyloidosis were obtained before treatment with high-dose melphalan and assessed for calreticulin expression by qRT-PCR. GAPDH and pooled cDNA from the purified plasma cells of 3 patients with hereditary amyloidosis were used as controls. Patients were assessed for response to high-dose melphalan at 3 months after treatment. Calreticulin expression in responders was significantly higher than in nonresponders (P < .01, Mann-Whitney). The box extends from the 25th percentile to the 75th percentile, with a horizontal line at the median (50th percentile), and the whiskers extend down to the smallest and up to the largest value.
Calreticulin expression is variable in the plasma cells of patients with systemic AL-amyloidosis by immunohistochemical staining of bone marrow biopsies
To evaluate calreticulin expression immunohistochemically in bone marrow biopsy specimens, we obtained wild-type and calreticulin knock-out murine embryonic fibroblasts and a polyclonal rabbit anti-human calreticulin antibody. Immunohistochemical staining conditions were optimized for formalin-fixed paraffin-embedded tissue, and we then stained 48 marrow biopsies obtained at diagnosis from patients with systemic AL-amyloidosis treated with high-dose melphalan (λ = 40, κ = 8). Sections from each biopsy were also stained for CD138+ to identify plasma cells, and the CD138 and calreticulin stained slides were scored blindly by a hematopathologist. In all cases, the marrow biopsies at diagnosis had been routinely stained for light chain isotype and clonal predominance demonstrated as previously described.22
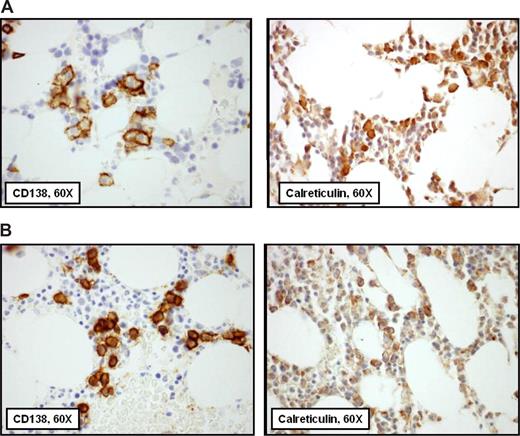
Two patterns of calreticulin distribution in clonal plasma cells were observed, pancytoplasmic staining sparing the nucleus and rare scattered cytoplasmic staining. A calreticulin immunohistochemical staining index was developed with scoring of the intensity of calreticulin staining and the fraction of plasma cells expressing calreticulin (over total CD138+ cells), both scored 1, 2, or 3, with the 2 scores multiplied to compute the index (range 1-9). Examples of high- and low-scoring specimens are shown in Figure 3.
Calreticulin expression is variable in the plasma cells of patients with systemic AL-amyloidosis by immunohistochemical staining of bone marrow biopsies. In these images from adjacent sections of bone marrow biopsies from 2 patients, CD138+ plasma cells stained immunohistochemically are shown on the left and calreticulin on the right. A pathologist who did not know the clinical status of the patients scored all cases blindly for intensity and distribution of calreticulin to compute the calreticulin index. In panel A, the calreticulin index of 9 is a function of the intensity of staining, indicating that calreticulin was found in a pancytoplasmic distribution. In panel B, the calreticulin index of 3 is a function of an intensity score of 1, indicating that the distribution of calreticulin was rare and scattered.
Calreticulin expression is variable in the plasma cells of patients with systemic AL-amyloidosis by immunohistochemical staining of bone marrow biopsies. In these images from adjacent sections of bone marrow biopsies from 2 patients, CD138+ plasma cells stained immunohistochemically are shown on the left and calreticulin on the right. A pathologist who did not know the clinical status of the patients scored all cases blindly for intensity and distribution of calreticulin to compute the calreticulin index. In panel A, the calreticulin index of 9 is a function of the intensity of staining, indicating that calreticulin was found in a pancytoplasmic distribution. In panel B, the calreticulin index of 3 is a function of an intensity score of 1, indicating that the distribution of calreticulin was rare and scattered.
The calreticulin indices for 3 of the 4 patients in the CR set assessed by gene-expression profiling were 9, 4, and 3, while the indices for the 5 patients in the NR set were 6, 6, 2, 2, and 1. Calreticulin indices and qRT-PCR levels were available for 1 CR and 1 NR patient in the Affymetrix sets and were 0.440 and 4 in the CR case and 0.070 and 2 in the NR case.
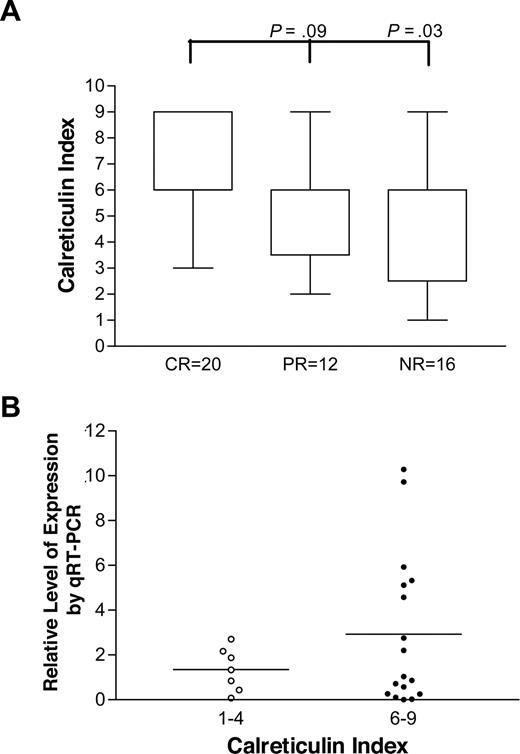
Overall, for all 48 biopsies stained, patients with CR had significantly higher indices than those who had NR, as shown in Figure 4A (P = .03, Mann-Whitney) and the difference between those with CR and PR approached significance (P = .09). In addition, 8 of the 20 patients achieving CR had an index of 9 compared with 3 of 28 patients not achieving CR (P = .034, Fisher exact). Of note, the 8κ plasma cell clones had significantly higher indices than the 40λ clones, with medians of 9 (range, 6-9) and 6 (range, 1-9) respectively (P = .002, Mann-Whitney).
The distribution of calreticulin indices and response to high-dose melphalan. Immunohistochemical staining of baseline marrow biopsies was performed as described. (A) Distribution of calreticulin indices for patients with CR (n = 20), PR (n = 12), and NR (n = 16) shown. The difference between CR and NR groups is significant and there is a trend toward significance in the comparison of CR and PR groups. (B) qRT-PCR levels of patients with indices of 1 to 4 (n = 7) are compared with those of patients with indices 6 to 9 (n = 17; P = .09, unpaired t test). Horizontal bars are mean values.
The distribution of calreticulin indices and response to high-dose melphalan. Immunohistochemical staining of baseline marrow biopsies was performed as described. (A) Distribution of calreticulin indices for patients with CR (n = 20), PR (n = 12), and NR (n = 16) shown. The difference between CR and NR groups is significant and there is a trend toward significance in the comparison of CR and PR groups. (B) qRT-PCR levels of patients with indices of 1 to 4 (n = 7) are compared with those of patients with indices 6 to 9 (n = 17; P = .09, unpaired t test). Horizontal bars are mean values.
All 24 patients whose marrow aspirates were used for calreticulin qRT-PCR also had marrow biopsies stained for calreticulin and indices scored. The qRT-PCR results of patients with calreticulin indices of 1 to 4 were compared with those with indices of 6 to 9, as shown in Figure 4B, and the differences approached significance (P = .09, t test).
Calreticulin knock-out murine embryonic fibroblasts resist melphalan-induced cell death
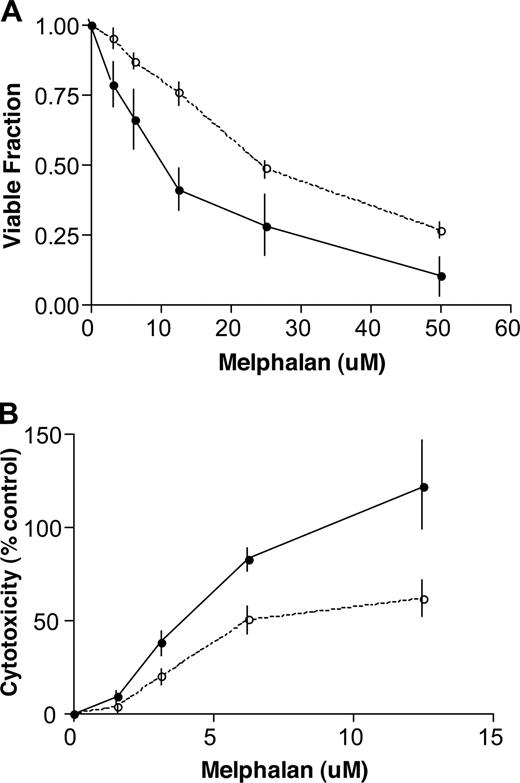
To further study calreticulin and the cellular response to melphalan we used wild-type and calreticulin knock-out murine embryonic fibroblasts (MEF). We exposed these cells for 24 hours to increasing doses of melphalan and assessed viability by trypan blue staining and cytotoxicity by an in situ lactate dehydrogenase cytotoxicity detection assay. At all dose levels in both assays, wild-type MEF cells were significantly more sensitive to melphalan than calreticulin knock-out MEF cells (Figure 5).
The response of wild-type and calreticulin knock-out murine embryonic fibroblasts (MEF) to melphalan exposure. (A) Wild-type and calreticulin knock-out MEF cells were seeded onto flat-bottom 2 mL wells at 105 cells per well in standard medium overnight and were then exposed to increasing doses of melphalan for 24 hours. Cells were then harvested with 0.1% trypsin and counted for viability with trypan blue staining. The results of 3 experiments are shown. The wild-type MEF cells (—●—) are significantly more sensitive to all doses of melphalan than the calreticulin knock-out cells (—○—; mean ± SD; P= .04, 2-tailed paired t test). (B) Lactate dehydrogenase release was directly measured to assess the cytotoxic effects of increasing doses of melphalan on wild type and calreticulin knock-out MEF cells. Triton-X 100–treated wells provided positive controls. The results of 3 experiments are shown. The wild-type MEF cells (—●—) are significantly more sensitive to all doses of melphalan than the calreticulin knock-out cells (—○—; mean ± SD; P = .006, 2-tailed paired t test).
The response of wild-type and calreticulin knock-out murine embryonic fibroblasts (MEF) to melphalan exposure. (A) Wild-type and calreticulin knock-out MEF cells were seeded onto flat-bottom 2 mL wells at 105 cells per well in standard medium overnight and were then exposed to increasing doses of melphalan for 24 hours. Cells were then harvested with 0.1% trypsin and counted for viability with trypan blue staining. The results of 3 experiments are shown. The wild-type MEF cells (—●—) are significantly more sensitive to all doses of melphalan than the calreticulin knock-out cells (—○—; mean ± SD; P= .04, 2-tailed paired t test). (B) Lactate dehydrogenase release was directly measured to assess the cytotoxic effects of increasing doses of melphalan on wild type and calreticulin knock-out MEF cells. Triton-X 100–treated wells provided positive controls. The results of 3 experiments are shown. The wild-type MEF cells (—●—) are significantly more sensitive to all doses of melphalan than the calreticulin knock-out cells (—○—; mean ± SD; P = .006, 2-tailed paired t test).
Discussion
In this study we sought to understand why the plasma-cell clones of some patients with systemic AL-amyloidosis are more sensitive to high-dose melphalan than others. We include evidence from hybridization, amplification, and immunohistochemical studies of patient specimens. Using the pretreatment purified plasma cells of newly diagnosed untreated patients, we compared the gene-expression profiles of patients achieving CR to the profiles of patients who had NR to high-dose melphalan. Then, using stringent criteria, we identified 5 genes with a significant difference in expression between these sets. With additional purified specimens from patients with CR and NR, we then performed qRT-PCR testing for 2 of these genes, tryptophanyl-tRNA synthetase (WARS) and calreticulin. WARS expression in CR and NR sets did not achieve statistical significance (data not shown) but calreticulin expression did. The qRT-PCR results for calreticulin were concordant with the supervised Affymetrix gene-expression analysis. Patients with CR had levels of calreticulin message that were significantly higher than those with NR (Figure 2).
We extended these preliminary observations with marrow biopsy specimens obtained at diagnosis assessed for calreticulin by immunohistochemical (IHC) staining using wildtype and calreticulin knock-out murine embryonic fibroblasts as controls to establish the technique. In the plasma cells of these biopsies, which were clonally restricted by light-chain isotype staining, we saw 2 patterns of calreticulin distribution: punctate and peripheral in the cytoplasm with faint Golgi staining and dense throughout the cytoplasm sparing the nucleus. A calreticulin index was devised based on these observed differences. With blinded scoring, patients with CR were found to have higher calreticulin indices than those with NR (Figure 4A).
In this series of marrow biopsies stained for calreticulin, κ clones had significantly higher calreticulin indices than λ clones. Calreticulin is involved in N-linked glycosylation of proteins.33,34 Whether light- or heavy-chain N-linked glycosylation played a role in the variability we observed in calreticulin staining is not known. Amyloid-forming κ1 light chains are frequently characterized by the acquisition of an N-linked glycosylation site due to somatic mutation and, as previously noted, patients with κ clones have a higher rate of CR to high-dose melphalan than those with λ plasma cells.4,35 The results with IHC staining and the scoring of calreticulin indices provide further support for a role for plasma-cell calreticulin in the response to high-dose melphalan in patients with systemic AL-amyloidosis. Because the IHC is cumbersome and operator-dependent, however, we are currently investigating the use of flow cytometry to provide more reliable quantitative measures of intracellular calreticulin.
We do not know how calreticulin transcription, mRNA stability, or protein turnover are regulated in clonal plasma cells secreting amyloid-forming light chains. The 5 genes we have identified as overexpressed in the patients achieving CR are associated with protein translation and the endoplasmic reticulum, and conceivably the level of calreticulin mRNA may be related to intracellular management of amyloid-forming light chains. The suggestion then would be that such plasma cells are poised to succumb to high-dose melphalan. Whether calreticulin plays a direct role, or is a marker of cell stress or vulnerability, is not known. However, to assess further the link between calreticulin and sensitivity to melphalan, we tested wild-type and calreticulin knock-out murine embryonic fibroblasts using different assays for viability and cytotoxicity. The wild type cells were significantly more sensitive than the calreticulin knock-out cells at all doses of melphalan tested in both assays, consistent with our observations in plasma cells from patients with systemic AL-amyloidosis. This finding suggests that calreticulin may play a direct role in sensitivity to melphalan but does not indicate whether its cytoplasmic, endoplasmic, or nuclear localization might matter.
Melphalan is a phenylalanine derivative of nitrogen mustard and a bifunctional alkylating agent used in pulse oral and high-dose regimens.36,,,,,,–43 Its cytotoxic activity is thought to be related to the generation of reactive oxygen species (ROS) and inactivation of ROS by glutathione on the one hand, and DNA damage and the response of damage repair systems on the other.42,43 Both possibilities have been explored in attempts to potentiate its activity. Buthionine sulfoximine, an inhibitor of γ-glutamylcysteine synthetase, and O6-benzylguanine, a pseudosubstrate for the important DNA repair enzyme alkylguanine transferase, have been evaluated in clinical trials without significant success despite supportive preclinical data.44,–46 A number of mechanisms have been associated with resistance to melphalan in vitro, including deficiencies in drug transport causing decreased uptake, increased glutathione stores resulting in decreased intracellular oxidative stress, and more efficient repair of DNA cross-links.47,–49 Cells deficient in fanc-c in the Fanconi anemia DNA repair pathway displayed marked sensitivity to melphalan.50 Similarly, in studies using human myeloma cell lines cultured to grow in melphalan, resistance was shown to be associated with increased expression of genes in the Fanconi anemia complementation groups.51
In the administration of high-dose melphalan to patients with systemic AL-amyloidosis, the exposure to melphalan is brief but intense. This may reduce pharmacokinetic variability between patients, increasing the likelihood that differences in response are due to factors intrinsic to the clonal plasma cells.52 In addition, the majority of patients with systemic AL-amyloidosis who receive high-dose melphalan as initial therapy are not previously treated; therefore, differences in response are not likely to be related to prior drug exposure or selection of resistance genes in plasma cells. Moreover, the plasma cells in systemic AL-amyloidosis are less proliferative than myeloma cells, raising the question of melphalan activity in indolent cells.53 These observations are consistent with our claim that some of the variability in response may be linked to factors specific to clonal plasma cells.
When the gene-expression profile data became available, we focused on calreticulin because it is a major component of the endoplasmic reticulum, perhaps its most pleotropic protein.14 Among its functions, calreticulin has been shown in various cell types to be a chaperone involved in N-linked glycosylation, a regulator of calcium homeostasis, a ligand for integrins, a modulator of steroid hormone nuclear receptors, an inhibitor of angiogenesis (vasostatin), a self-antigen, a vehicle for antigen delivery, and a component of the phagocytic synapse.54,,,,–59 Calreticulin with its links to endoplasmic calcium stores has also been studied in relationship to apoptosis.58,60,61 HeLa cells inducible for calreticulin are more sensitive to drug-dependent apoptosis than wild type, and the sensitivity of glioblastoma cells to radiation directly correlates with the calreticulin level.58,59 Furthermore, investigators studying the melphalan-resistant and susceptible variants of the human MCF-7 breast cancer cell line with a combination of 2-dimensional gel electrophoresis and mass spectrometry identified calreticulin as one of the 3 downregulated proteins in resistant cells.62
Recently calreticulin was identified as a component of a histone-associating protein complex recruited immediately after radiation injury, suggesting that the nuclear presence of calreticulin could have stoichiometric implications with respect to mediating chromatin structure.63,64 Given its pleotropic character, there may be multiple mechanisms by which calreticulin expression is linked to increased sensitivity to drug and radiation injury. Moreover, calreticulin surface expression on apoptotic cells is an “eat me” signal and plays a key role in anthracycline-related tumor-cell immunogenicity, enabling a T cell–mediated immune response to tumors in mice.65,66 Antibodies to calreticulin can block this response. Of particular interest in that regard is the presence of calreticulin autoantibodies in patients with cancer, raising the possibility that humoral responses to tumor-associated antigens may help cancer cells resist treatment.54
Calreticulin in clonal plasma cells has not been previously studied except for the observation that its expression is significantly increased in RPMI 8226 myeloma cells after exposure to the proteasome inhibitor bortezomib.67 This is of interest because bortezomib and melphalan are synergistic in the treatment of multiple myeloma.68,69 It is also of interest because it suggests that calreticulin expression in plasma cells is variable, as we report, and subject to modulation. Both the significance of calreticulin variability in the clonal plasma cells of patients with systemic AL-amyloidosis and the pharmacologic modulation of its expression to enhance melphalan activity are matters for further study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the many patients who contributed their cells to research and Stephen D. Nimer for his continued support and encouragement. We also thank Joanne Santorsa, RN, for her assistance and care in obtaining clinical and research marrow specimens. We also appreciate greatly the care provided to our patients by the nurses and clinical fellows in the outpatient areas and on the Memorial 8 Bone Marrow Transplant and Memorial 12 Telemetry floors.
This work was supported by Food and Drug Administration grant R03-002174, the Amyloidosis Research Fund, and the Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation.
Authorship
Contribution: P.Z. designed research, conducted experiments, analyzed data, and wrote the paper; J.T-.F. and P.L. conducted experiments, analyzed data and wrote the paper; M.F. designed research and conducted experiments; A.O. performed statistical analyses of the data; R.L.C. conceived and designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond L. Comenzo, MD, Howard 802, 1275 York Avenue, Memorial Sloan-Kettering Cancer Center, New York, NY 10021; e-mail: comenzor@mskcc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal