Abstract
Dense, dehydrated red blood cells (DRBCs) are a characteristic feature of sickle-cell disease (SCD). DRBCs play a role in the pathophysiology of SCD acute and chronic organ damage because of heightened tendency to undergo polymerization and sickling because of their higher hemoglobin S concentration. Relations between red cell density (assessed with phthalate density-distribution profile method) and several hematologic, biochemical, genetic parameters, and clinical manifestations were studied in a large cohort of homozygous patients. The percentage of DRBCs was significantly higher in patients who experienced skin ulcers, priapism, or renal dysfunction. Presence of α-thalassemia deletions was associated with fewer DRBCs. A multivariable analysis model showed DRBCs to be positively associated with hemolytic parameters such as lactate dehydrogenase and bilirubin and negatively with fetal hemoglobin. The percentage of DRBCs decreased by 34% at 6 months of hydroxycarbamide (xydroxyurea) therapy. Thus, DRBCs are associated with specific clinical manifestations and biologic markers and may be a useful addition to the biologic and clinical evaluation of patients with SCD, because they can easily be measured in a hematocrit tube.
Introduction
All homozygous patients with sickle-cell disease (SCD) carry the same genetic defect in the β-globin genes. However, the clinical presentation and overall severity of their disease vary greatly, from milder forms that can go undetected for decades to extremely severe forms with multiorgan damage and early mortality. Identification of risk factor(s) and laboratory parameters that might be predictive of SCD severity or complications has important implications not only for understanding the pathophysiology of the disease but also for clinical management.
A distinguishing characteristic of SCD is erythrocyte dehydration, because of K+ efflux from the red blood cell (RBC) and consequently decreased intracellular water content and increased mean corpuscular hemoglobin concentration.1,2 Eaton and Hofrichter reported that the rate of the initial polymerization phase depends on the 20th-40th power of hemoglobin S (HbS) concentration.3 HbS polymerization and sickling rates can be substantially reduced with relatively small diminutions of the intracellular HbS concentration.3-5 Notably, in dense RBCs (DRBCs), defined as having a density exceeding 1.120, the intracellular total Hb concentration is increased from the normal (∼ 33 g/dL) to 40-50 g/dL.6 DRBCs exhibit increased rigidity and decreased stability and include a variable fraction of irreversibly sickled cells.7,8
The DRBC fraction varies within most patients with SCD with wide interpatient variations.6,9 However, in earlier reports, no correlations could be established between DRBCs and clinical SCD severity. Most of those published studies were underpowered to detect this kind of interaction, which requires large numbers of well-characterized patients, and yielded contradictory findings.10,11 Moreover DRBCs have never been studied for their potential association with chronic organ damage, which is increasingly being observed today because of the recent improvements in life expectancy.
Similarly, the lack of precise clinical correlates for the percentage of DRBCs (% DRBCs) has not allowed assessing the potential additional indications for hydroxycarbamide (HC; hydroxyurea), which is currently indicated for the prevention of vaso-occlusive crises (VOCs) or acute chest syndrome (ACS).
Herein, we compared data on RBC density with clinical and hematologic, biochemical, and genetic parameters in a large cohort of homozygous adult patients with SCD with the goal of identifying association with phenotypes and parameters indicative of severity. This population is unique for representing African immigrants with little white admixture and little admixture of haplotypes, contrary to what is seen in American patients.
We also studied the effect of hydroxycarbamide on the % DRBCs.
Methods
Patients
All patients with SS SCD regularly followed in our Center for Sickle Cell Disease at the Hopital Henri Mondor (Creteil, France), for whom a RBC density measurement was available before hydrocarbamide treatment or blood transfusional exchange therapy, were included in this cohort study of the center's clinical and laboratory database. For each patient, sex, age, weight, height, body mass index, and geographic origin were recorded. All clinical data were considered when prospectively collected. Renal dysfunction was defined as proteinuria > 0.3 g/L or estimated glomerular filtration rates (< 80 mL/mn. Estimated glomerular filtration rates were calculated with the 4-point Modification of Diet in Renal Disease formula. Pulmonary hypertension was defined as a mean pulmonary arterial pressure of at least 25 mm Hg confirmed by right heart catheterization, but we did not consider for this analysis whether the elevated pulmonary artery pressures were because of left-sided heart disease (after capillary) or true pulmonary arterial hypertension (before capillary).
Clinical data were collected in 448 patients. Clinical data were available in patients for the absence/presence of skin ulcers (n = 142), pulmonary hypertension (n = 234), priapism (n = 75 men), renal dysfunction (n = 182), stroke (n = 330), osteonecrosis (n = 278), retinal complication (n = 344), and cholecystectomy (n = 186). One hundred patients did not have leg ulcers, pulmonary hypertension, priapism, renal dysfunction, or stroke. ACS and VOCs occurring in the past year (1-year period) were noted in 272 patients.
Countries of origin were grouped as follows: North Africa (Algeria, Morocco, Tunisia), Central Africa (Central African Republic, Gabon, Congo, Democratic Republic of Congo, Angola), West Africa (Benin, Burkina Faso, Ghana, Guinea, Ivory Coast, Mali, Niger, Nigeria, Senegal, Togo), West Indies (Guadaloupe, French Guiana, Martinique), Indian Ocean (Madagascar, Mauritius, Reunion, Comores Islands), and others.
This study was approved by the local Institutional Review Board (CPP–Creteil). All patients gave their signed informed consent for the genetic studies in accordance with the Declaration of Helsinki. All data were rendered anonymous to protect patients' privacy and confidentiality. Patients who received chronic transfusions were excluded.
Laboratory methods
Laboratory data were collected during routine outpatient visits. Hematologic data were the means of 2 or 3 separate steady state determinations. Steady state was defined as a visit ≥ 1 month after an acute clinical event (VOC, infection, ACS, or any other clinical event that resulted in hospitalization and/or blood transfusion) and ≥ 3 months after blood transfusion. All patients were receiving folic acid regularly, and biologic data were not taken into account when iron deficiency was present because it has a clear influence on the density curve and other hematologic parameters. All laboratory analyses were performed on site in the Clinical Laboratories of the Hôpital Henri Mondor, Creteil.
Hematologic studies.
Complete blood cell (CBC) and reticulocyte counts were measured with a Coulter LH 750 counter (Beckman Coulter). Measured parameters included mean corpuscular cell volume (MCV), red cell distribution width (RDW), mean corpuscular hemoglobin content (MCH), as well as Hb, hematocrit (Hct), and RBC count. Reticulocytes were expressed as either absolute reticulocyte count (cells × 109/L) or the reticulocyte percentage.
Values for HbS, HbF, and HbA2 were determined by cation-exchange high-performance liquid chromatography with the use of the Variant Hb analyzer (Variant Hemoglobin Testing System; Bio-Rad).
RBC density measurements.
Density was assessed with the phthalate density-distribution technique.12 Phthalate oil mixtures of precise density were prepared by mixing 2 phthalate esters, n-butyl phthalate and dimethyl-phthalate, to achieve the following densities: 1.060, 1.064, 1.068, 1.076, 1.080, 1.084, 1.088, 1.092, 1.096, 1.100, 1.104, 1.108, 1.112, 1.116, 1.120, 1.124, 1.128, 1.132 and 1.136 g/mL at 20°C.
RBCs were washed 3 times at 4°C with isotonic saline (osmolarity, 290-300 mOsm). For each wash, tubes were centrifuged for 5 minutes at 3000g. After re-suspending the RBC pellet, a 50% suspension was prepared in isotonic saline, which had previously been kept at room temperature, and the cells were left to equilibrate at room temperature for 15 minutes. Then, 0.5-1 cm of each phthalate oil mixture was placed in a separate glass capillary hematocrit tubes. With the use of a 1-mL syringe with 26G needle, each tube was filled with the patient-washed RBC suspension (approximately 2 cm). Particular care was taken to avoid trapping air bubbles in the tubes. Each hematocrit tube was sealed at one end and spun in a temperature-controlled centrifuge (20°C) at 10 000g for 10 minutes. The height of the RBC column below the oil was measured in each hematocrit tube, using either graph paper or a ruler under a magnifying lens. The percentage of DRBC (below the phthalate oil layer) was calculated as follows: cells below/(cells below + cell above). The results were plotted against density to generate a RBC density distribution curve for each patient. For each of these curves, the DRBC percentage was determined as the percentage of RBCs with density > 1.120.13 The D50 was defined as the density for which cells below/(cells below + cell above) was equal to 0.5.
Biochemical studies.
Serum levels of total bilirubin and lactate dehydrogenase (LDH) were determined with a routine chemistry analyzer (Advia 1650; Siemens Medical Solutions Diagnostics).
Genotype analysis.
DNA was isolated from peripheral blood leukocytes by phenol-chloroform extraction. Three forms of deletional α-thalassemia (α−3.7, α−20.5, and Mediterranean type) were ascertained with a PCR. PCR-restriction fragment-length polymorphism was used to determine the β-globin gene cluster haplotypes.14 The polymorphic restriction endonuclease sites studied were HincII 58 to the ϵ- and 38 to the yβ-globin genes, XmnI (−158) 58 to the Gγ-globin gene, HindIII in the IVS-2 of Gγ- and Aγ-globin genes, HinfI 58 and 38 to the β-globin gene, and RsaI 58 to the β-globin gene.15
Haplotypes associated with the β-globin mutation were designed as Bantu (CAR), Benin, Cameroon, Senegal, and others for atypical haplotypes.
Statistical analysis
Results are expressed as means ± SD, numbers, or percentages, as appropriate. Quantitative parameters were compared between groups by use of a Student t test or a Mann-Whitney U nonparametric test when the number of patients was < 30. F test was used to compare the equality of 2 variances. Multivariable linear regression models were used to compare MCV, HbF, D50, and DRBC levels among haplotypes as well as α-thal status. Tukey adjustment for multiple comparisons was applied to both analyses for the pairwise testing. Chi-square test was used to compare the numbers of patients with a α-thalassemia deletion among haplotypes. Simple linear regressions were performed for each biologic parameter to search for potential relation with DRBCs. Multiple linear regressions were used to explore models that better predicted RBC density. All the variables (except D50) related to the hematologic and biochemical profiles that were significantly correlated with DRBCs with an univariate threshold (P < .05) considered in each model. Models were built with a forward stepwise approach. The final models included the variables that remained significantly associated with RBC density after adjustment for the other variables in the models. R-squares (r2) were used as measures of variance explained by the models. Statistical significance was defined as P value < .05. Correlations were established with Pearson correlation coefficient. Statistical analysis was conducted with SPSS 17.0 (SPSS Inc), Statview 5.0 (SAS Institute), and Prism 4 (GraphPad Inc) software.
Results
Demographic data are summarized in Table 1. The West Indies, Central Africa, and West Africa approximately accounted for each a quarter of the patients. The average patient follow-up was 17.7 years.
Sociodemographic characteristics
| . | Value . |
|---|---|
| Sex, no. (%) | |
| Female | 342 (58) |
| Male | 246 (42) |
| Country, no. (%) | |
| West Africa | 226 (38) |
| West Indies | 175 (30) |
| Central Africa | 160 (27) |
| North Africa | 11 (2) |
| Others | 16 (3) |
| Age, y, mean ± SD (range) | 41 ± 9 (19-71) |
| BMI, mean ± SD (range) | 21 ± 3 (15-32) |
| . | Value . |
|---|---|
| Sex, no. (%) | |
| Female | 342 (58) |
| Male | 246 (42) |
| Country, no. (%) | |
| West Africa | 226 (38) |
| West Indies | 175 (30) |
| Central Africa | 160 (27) |
| North Africa | 11 (2) |
| Others | 16 (3) |
| Age, y, mean ± SD (range) | 41 ± 9 (19-71) |
| BMI, mean ± SD (range) | 21 ± 3 (15-32) |
BMI indicates body mass index.
The mean ± SD % DRBCs was 12.8 ± 7.8. No correlation was observed between the patient age and the % DRBCs (P = .27). There was a trend toward higher % DRBCs in males compared with females, respectively, 13.4 ± 7.9 and 12.2 ± 7.6 (P = .055). The DRBC and D50 data were reported in Table 2. The CAR haplotype was associated with the highest percentage of patients with α-thalassemia deletion, which could explain their having the smallest MCV and lowest D50. As previously described,16 the Senegal haplotype was associated with the highest percentage of HbF (% HbF) and the CAR haplotype with the lowest one.
The effects of haplotypes on MCV, HbF, D50 and % DRBCs
| Patient haplotype . | No. . | MCV, mean ± SD . | % HbF, mean ± SD . | α−Thal del, % . | D50, mean ± SD . | % DRBCs, mean ± SD . |
|---|---|---|---|---|---|---|
| Benin/Benin | 200 | 88.7 ± 9.6* | 7.4 ± 5.2* | 49*† | 1.096 ± 0.003* | 13.4 ± 7.7 |
| Benin/CAR | 33 | 88.1 ± 6.7 | 7.0 ± 5.5† | 42‡ | 1.096 ± 0.003 | 14.8 ± 9.6 |
| Benin/Cameroon | 24 | 83.8 ± 10.2 | 5.9 ± 3.7‡ | 42 | 1.095 ± 0.003 | 9.8 ± 7.5 |
| Benin/Senegal | 31 | 88.6 ± 9.6 | 9.6 ± 6.2§ | 35§ | 1.096 ± 0.003 | 13.3 ± 7.8 |
| CAR/CAR | 134 | 83.2 ± 9.9*†‡ | 5.7 ± 4.4*§‖ | 64*‡§ | 1.095 ± 0.003*† | 11.3 ± 7.4 |
| Senegal/Senegal | 38 | 91.5 ± 11.1† | 11.7 ± 5.9*†‡‖¶ | 29*† | 1.097 ± 0.002† | 13.0 ± 7.2 |
| Other | 40 | 89.2 ± 8.6‡ | 7.2 ± 4.7¶ | 50 | 1.096 ± 0.003 | 13.2 ± 7.8 |
| Patient haplotype . | No. . | MCV, mean ± SD . | % HbF, mean ± SD . | α−Thal del, % . | D50, mean ± SD . | % DRBCs, mean ± SD . |
|---|---|---|---|---|---|---|
| Benin/Benin | 200 | 88.7 ± 9.6* | 7.4 ± 5.2* | 49*† | 1.096 ± 0.003* | 13.4 ± 7.7 |
| Benin/CAR | 33 | 88.1 ± 6.7 | 7.0 ± 5.5† | 42‡ | 1.096 ± 0.003 | 14.8 ± 9.6 |
| Benin/Cameroon | 24 | 83.8 ± 10.2 | 5.9 ± 3.7‡ | 42 | 1.095 ± 0.003 | 9.8 ± 7.5 |
| Benin/Senegal | 31 | 88.6 ± 9.6 | 9.6 ± 6.2§ | 35§ | 1.096 ± 0.003 | 13.3 ± 7.8 |
| CAR/CAR | 134 | 83.2 ± 9.9*†‡ | 5.7 ± 4.4*§‖ | 64*‡§ | 1.095 ± 0.003*† | 11.3 ± 7.4 |
| Senegal/Senegal | 38 | 91.5 ± 11.1† | 11.7 ± 5.9*†‡‖¶ | 29*† | 1.097 ± 0.002† | 13.0 ± 7.2 |
| Other | 40 | 89.2 ± 8.6‡ | 7.2 ± 4.7¶ | 50 | 1.096 ± 0.003 | 13.2 ± 7.8 |
The symbols indicate significant differences between the means sharing the same symbol. For example, the mean of MCV for SS patients with Benin/Benin haplotype is significantly different from the mean of patients with CAR/CAR haplotype. The statistical significance, defined as P < .05, was obtained with ANOVA test and Tukey adjustment for multiple comparisons, and χ2 test for α status when authorized.
α-Thal status data are presented in Table 3. The presence of α-thalassemia, as either a 1- or 2-gene deletion, was associated with progressive decreases of MCV, HbF, D50, and % DRBCs. Differences between groups for % DRBCs were all significant (P < .01).
MCV, HbF, D50, and %DRBCs by α-thalassemia status
| α-thalassemia status . | No. . | MCV, mean ± SD . | HbF, mean ± SD . | D50, mean ± SD . | %DRBCs, mean ± SD . |
|---|---|---|---|---|---|
| Normal | 216 | 92.6 ± 8.6*† | 8.4 ± 5.7* | 1.096 ± 0.003* | 14.6 ± 7.7* |
| 1-gene deletion | 183 | 84.6 ± 6.8*‡ | 6.8 ± 4.7* | 1.095 ± 0.003* | 12.5 ± 7.3* |
| 2-gene deletions | 46 | 71.1 ± 4.4†‡ | 4.4 ± 3.7* | 1.092 ± 0.003*† | 4.5 ± 4.3*† |
| Gene triplication | 7 | 92.4 ± 7.9‡ | 7.8 ± 4.8 | 1.098 ± 0.002† | 16.9 ± 4.8† |
| α-thalassemia status . | No. . | MCV, mean ± SD . | HbF, mean ± SD . | D50, mean ± SD . | %DRBCs, mean ± SD . |
|---|---|---|---|---|---|
| Normal | 216 | 92.6 ± 8.6*† | 8.4 ± 5.7* | 1.096 ± 0.003* | 14.6 ± 7.7* |
| 1-gene deletion | 183 | 84.6 ± 6.8*‡ | 6.8 ± 4.7* | 1.095 ± 0.003* | 12.5 ± 7.3* |
| 2-gene deletions | 46 | 71.1 ± 4.4†‡ | 4.4 ± 3.7* | 1.092 ± 0.003*† | 4.5 ± 4.3*† |
| Gene triplication | 7 | 92.4 ± 7.9‡ | 7.8 ± 4.8 | 1.098 ± 0.002† | 16.9 ± 4.8† |
The symbols indicate significant differences between the means sharing the same symbol. For example, the mean MCV for SS patients with normal α-thalassemia status differed significantly from that of patients with 1- or 2-gene deletions. The statistical significance, defined as P < .05, was pairwise comparisons using the Tukey adjustment.
Variables significantly correlated with % DRBCs according to our univariate analysis are reported in Table 4. Hemolytic biologic parameters (LDH and bilirubin) and white blood cell (WBC) and platelet counts were positively correlated with DRBCs, whereas Hb, Hct, RBCs, and % HbF were negatively correlated. Multivariable analysis showed significantly positive associations with MCH, RDW, bilirubin, and LDH and negative association with % HbF and Hct. This statistical model accounts for ∼ 40% of variability of DRBCs in SS patients.
Biologic parameters associated with % DRBCs on 588 patients
| Parameter . | Univariate analysis Pearson r* . | Multivariable analysis β (SE)† . |
|---|---|---|
| D50 | 0.53 | |
| Hb | −0.31 | |
| Hct | −0.40 | −2.0 (0.4) |
| RBC count | −0.42 | |
| MCV | 0.18 | |
| MCH | 0.30 | 0.76 (0.92) |
| RDW | 0.37 | 0.46 (0.10) |
| % Reticulocyte | 0.37 | |
| WBC count | 0.19 | |
| Platelet count | 0.15 | |
| LDH | 0.43 | 0.014 (0.002) |
| Bilirubin | 0.30 | 0.021 (0.01) |
| % HbF | −0.22 | −0.37 (0.07) |
| Parameter . | Univariate analysis Pearson r* . | Multivariable analysis β (SE)† . |
|---|---|---|
| D50 | 0.53 | |
| Hb | −0.31 | |
| Hct | −0.40 | −2.0 (0.4) |
| RBC count | −0.42 | |
| MCV | 0.18 | |
| MCH | 0.30 | 0.76 (0.92) |
| RDW | 0.37 | 0.46 (0.10) |
| % Reticulocyte | 0.37 | |
| WBC count | 0.19 | |
| Platelet count | 0.15 | |
| LDH | 0.43 | 0.014 (0.002) |
| Bilirubin | 0.30 | 0.021 (0.01) |
| % HbF | −0.22 | −0.37 (0.07) |
P < .001 for all parameters.
SE of the coefficients; β is regression coefficient of the multivariable models, indicating the average increase on % DRBCs per 1 unit increase in the covariate (r2 = 0.38; P < .001).
Table 5 shows clinical and biologic associations. % DRBCs were significantly higher in patients with skin ulcers, priapism, or renal dysfunction than either the entire dataset or in a subgroup of patients with none of these complications. Those associations were not explained by differences in % HbF.
Biologic characteristics of clinical complications
| Clinical manifestations . | No. . | Hb, mean ± SD . | % HbF, mean ± SD . | LDH, mean ± SD . | D50, mean ± SD . | % DRBCs, mean ± SD . |
|---|---|---|---|---|---|---|
| Total | 500 | 8.8 ± 1.2 | 7.3 ± 5.3 | 375 ± 133 | 1.096 ± 0.003 | 12.7 ± 7.8 |
| Skin ulcers | 34 | 8.2 ± 0.9*† | 6.4 ± 5.9 | 414 ± 163 | 1.096 ± 0.003 | 16.4 ± 8.5†‡ |
| Pulmonary hypertension | 11 | 7.9 ± 1.7‡§ | 8.3 ± 4.7 | 490 ± 182‡§ | 1.096 ± 0.003 | 14.3 ± 7 |
| Priapism | 33 | 9.2 ± 1.4 | 6.8 ± 6.7 | 382 ± 144 | 1.096 ± 0.003 | 15.7 ± 6.9‡§ |
| Renal dysfunction | 49 | 7.9 ± 2.9*† | 7.5 ± 6 | 440 ± 161*§ | 1.096 ± 0.003 | 16.3 ± 6.9*† |
| Stroke | 22 | 8.3 ± 1§ | 6.7 ± 6.2 | 446 ± 147§ | 1.095 ± 0.003 | 12 ± 6.4 |
| None of the above | 100 | 8.9 ± 1.1 | 7 ± 4.5 | 375 ± 141 | 1.095 ± 0.004 | 11.3 ± 8.6 |
| Osteonecrosis | 81 | 9 ± 1.3 | 7.9 ± 5.3 | 367 ± 126 | 1.095 ± 0.003 | 12.3 ± 7.6 |
| Retinal complication | 151 | 8.7 ± 2.4 | 6.8 ± 4.9 | 383 ± 128 | 1.095 ± 0.003 | 13.3 ± 7.8 |
| Cholecystectomy | 113 | 8.6 ± 1.2§ | 6.6 ± 4.8 | 411 ± 160‡ | 1.095 ± 0.003 | 14.3 ± 8.2§ |
| ACS during 1 y | 34 | 8.2 ± 3.2§ | 8 ± 4.4 | 423 ± 132 | 1.095 ± 0.002 | 13.3 ± 6.6§ |
| VOC during 1 y | 81 | 8.9 ± 1.4 | 8.3 ± 5.4 | 378 ± 124 | 1.095 ± 0.003 | 12.3 ± 7.6 |
| Clinical manifestations . | No. . | Hb, mean ± SD . | % HbF, mean ± SD . | LDH, mean ± SD . | D50, mean ± SD . | % DRBCs, mean ± SD . |
|---|---|---|---|---|---|---|
| Total | 500 | 8.8 ± 1.2 | 7.3 ± 5.3 | 375 ± 133 | 1.096 ± 0.003 | 12.7 ± 7.8 |
| Skin ulcers | 34 | 8.2 ± 0.9*† | 6.4 ± 5.9 | 414 ± 163 | 1.096 ± 0.003 | 16.4 ± 8.5†‡ |
| Pulmonary hypertension | 11 | 7.9 ± 1.7‡§ | 8.3 ± 4.7 | 490 ± 182‡§ | 1.096 ± 0.003 | 14.3 ± 7 |
| Priapism | 33 | 9.2 ± 1.4 | 6.8 ± 6.7 | 382 ± 144 | 1.096 ± 0.003 | 15.7 ± 6.9‡§ |
| Renal dysfunction | 49 | 7.9 ± 2.9*† | 7.5 ± 6 | 440 ± 161*§ | 1.096 ± 0.003 | 16.3 ± 6.9*† |
| Stroke | 22 | 8.3 ± 1§ | 6.7 ± 6.2 | 446 ± 147§ | 1.095 ± 0.003 | 12 ± 6.4 |
| None of the above | 100 | 8.9 ± 1.1 | 7 ± 4.5 | 375 ± 141 | 1.095 ± 0.004 | 11.3 ± 8.6 |
| Osteonecrosis | 81 | 9 ± 1.3 | 7.9 ± 5.3 | 367 ± 126 | 1.095 ± 0.003 | 12.3 ± 7.6 |
| Retinal complication | 151 | 8.7 ± 2.4 | 6.8 ± 4.9 | 383 ± 128 | 1.095 ± 0.003 | 13.3 ± 7.8 |
| Cholecystectomy | 113 | 8.6 ± 1.2§ | 6.6 ± 4.8 | 411 ± 160‡ | 1.095 ± 0.003 | 14.3 ± 8.2§ |
| ACS during 1 y | 34 | 8.2 ± 3.2§ | 8 ± 4.4 | 423 ± 132 | 1.095 ± 0.002 | 13.3 ± 6.6§ |
| VOC during 1 y | 81 | 8.9 ± 1.4 | 8.3 ± 5.4 | 378 ± 124 | 1.095 ± 0.003 | 12.3 ± 7.6 |
Significant differences between the means sharing the same character compared with total, P < .005.
Significant differences between the means sharing the same character compared with none of the above, P < .005.
Significant differences between the means sharing the same character compared with total, P < .05.
Significant differences between the means sharing the same character compared with none of the above, P < .05.
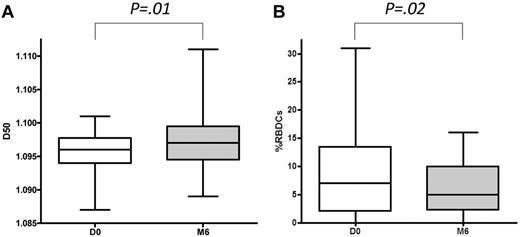
The mean % DRBCs decreased by 34% (n = 33; P = .02) after 6 months of hydroxycarbamide treatment, despite an increase in D50 (Figure 1). The % DRBC distribution was more homogenous under HC than before treatment, as proved by variances difference (P = .004). Interestingly, the change in % DRBCs was not correlated with the increase level in % HbF (P = .58)
Effects of hydroxycarbamide therapy. Effects on D50 (A) and DRBCs (B) after 6 months of hydroxycarbamide therapy. Values for D50 and % RBDCs (± SD) for 33 patients with Hb SS disease are plotted at baseline and after 6 months of hydroxycarbamide therapy.
Effects of hydroxycarbamide therapy. Effects on D50 (A) and DRBCs (B) after 6 months of hydroxycarbamide therapy. Values for D50 and % RBDCs (± SD) for 33 patients with Hb SS disease are plotted at baseline and after 6 months of hydroxycarbamide therapy.
Discussion
Prior studies on the clinical correlates of dense cells were hampered by the small number of patients and the lack of rigorous laboratory assessments. In the present study, all laboratory analyses were conducted under homogenous conditions, using standardized procedures on patients who were all followed in our SCD Referral Center for > 1 year. RBC density was directly measured in each patient at steady state with the phthalate-density method. The validity and reproducibility of this technique in SCD have been previously confirmed17 and does not depend on patient age.
Our results show for the first time in a large cohort of patients that the % DRBCs is specifically associated with defined clinical complications of the disease and with HbS polymerization contributing factors (Tables 4–5). The percentage of DRBCs, which is the important measure, is easy to perform and needs only 1 hematocrit tube. However, a temperature-controlled centrifuge must be used, which could be a limiting factor for wider applicability of this assay. The reproducibility is excellent with no difference between 2 analyses with a mean interval of 3 years (n = 26 patients; P = .79); the mean % DRBCs of the first and the second analysis were, respectively, 15.5 ± 8.4 and 15.8 ± 8.1. Linear regression analysis of duplicate measures yielded an r2 value of 0.6468 (P < .001). We have confirmed in a large cohort that DRBCs are correlated with biologic parameters of hemolysis: positively with LDH, bilirubin, and percentage of reticulocytes and negatively with Hct. Enhanced hemolysis might be related to mechanical destruction because of rheologic anomalies because of HbS polymerization8 and/or because of immune-mediated destruction because of defective control of membrane attack complex against DRBCs,18 membrane abnormalities,19 and increased DRBC opsonization by autologous immunoglobulin.20
The % DRBCs is significantly associated with hematologic parameters such as MCH, RDW, and % HbF (Table 4). Cell hemoglobin concentration is known to directly influence the rate of HbS polymerization,3,4 particularly under deoxygenated conditions.21,22 DRBC formation is not completely understood, but some studies argue strongly for a direct relation between HbS polymerization and DRBC formation,22-24 mediated in part, by erythrocyte dehydration because of K+ loss.2,25,26 Other observations also support the hypothesis that DRBCs are not the result of aging per se, because young cells, including reticulocytes, could be transformed into DRBCs.27 DRBCs are in a dynamic equilibrium between the processes leading to their formation and those responsible for their removal. Erythrocyte lifespan is shortest in patients with the highest numbers of DRBCs,17 with survival half-time of 40 hours for DRBCs with the lowest HbF content.28
Studies on small patient cohorts have shown reduced numbers of DRBCs in patients with HbS/α-thalassemia and patients with elevated HbF levels.9,29-32 HbF directly inhibits HbS polymerization33 and also decreases RBC dehydration.3,34 Concomitant α-thalassemia and SCD has been described to be associated with fewer complications related to hemolytic phenotype.29,35-37 Our data indicated that α-thalassemia had a significant biologic effect by reducing the % DRBCs in SS disease (Table 3). Despite those correlations, it is intriguing that the haplotypes with the highest HbF percentage (Senegal/Senegal) and the highest percentage of patients with α-thalassemia deletion (CAR/CAR) were not those with the lowest mean % DRBCs. These differences might be explained by the Senegal haplotype being associated with a lower percentage of α-thalassemia deletions or the CAR haplotype being associated with a lower % HbF. Interestingly, in the Bantou/Bantou haplotype the % HbF was not associated with the % DRBCs, whereas the relation with α-thalassemia deletions was strong (P < .001). However, we cannot exclude the possibility of a false-positive finding because we performed multiple hypothesis tests that may increase the probability of type I errors.
We also reported here a relation between the % DRBCs and WBC count in the univariate but not in the multivariable analysis, meaning that % DRBCs had no independent predictive utility when considering MCH, RDW, bilirubin, LDH, % HbF, and Hct simultaneously. WBC count has been shown to be a strong, independent predictor of ACS,38 stroke,39,40 and overall SCD severity in children.41
We demonstrated here for the first time that % DRBCs are associated with skin ulcers, priapism, and renal dysfunction. In our dataset enhanced hemolysis, as shown by higher LDH levels, was present only in patients with pulmonary hypertension and renal dysfunction but not in patients with priapism or skin ulcers, whereas these clinical manifestations have been described to be associated with hemolysis biologic parameters.42,43 The strong association of % DRBCs with skin ulcers and priapism in the absence of increased LDH could suggest that intrinsic cellular characteristics of DRBCs may be involved in the pathophysiology of these complications. Kaul et al reported that increased viscosity was correlated with increased cell density and that deoxygenation dramatically increased the DRBC fractions and, subsequently, peripheral vascular resistance.44 Although less adherent to vasculature,45,46 DRBCs caused persistent blockage of small postcapillary venules in an ex vivo model45,47 and were more prone to being trapped, in vivo, with acute effects, resulting in perfusion deficits followed by metabolic as shown by 99mTc imaging and magnetic resonance spectroscopy.48 More recently, the extent of mechanical deformation of the RBC membrane was found to control shear-induced ATP release and to regulate blood pressure by releasing ATP as a vasodilatory signaling molecule; therefore, increased RBC rigidity could be responsible for impaired ATP delivery and could favor vasoconstriction.49
Hydrocarbamide, the only drug with clinical proven benefit in SCD, significantly decreases the % DRBCs after 6 months of therapy without a correlation with HbF % increase (Figure 1). Because % DRBCs could be involved in the pathophysiology of skin ulcers, priapism, and renal dysfunction, HC use with the aim of reaching a sharp decrease in % DRBCs should be prospectively evaluated in clinical trials for patients with these specific complications. Drugs that specifically inhibit sickle cell dehydration, resulting in significantly lower % DRBCs and concomitantly less hemolysis and anemia, should also be reconsidered for patients with high % DRBCs and skin ulcers, priapism, or renal dysfunction, perhaps in conjunction with phlebotomy to prevent excessive increases in Hb levels.50
Our study highlights the notion that simple determination of the % DRBCs can be a meaningful and useful addition to the hematologic characterization of patients with sickle cell syndromes. New studies to establish the clinical utility of this test in guiding therapeutic approaches are needed.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jugurtha Berkenou and Christine Fauroux for the data management and Orah Platt, Janet Jacobson, Gil Tchernia, Marie Cambot, and Henri Wajcman for helpful feedback and discussions.
Authorship
Contribution: P.B. analyzed, interpreted data, performed statistical analysis, and wrote the manuscript; C.B. analyzed and interpreted data and edited manuscript; A.T.-P. performed the statistical analysis; S.P., K.M., and H.J. conducted laboratory tests; and F.G. designed the study and collected and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlo Brugnara, Children's Hospital Boston, Department of Laboratory Medicine, 300 Longwood Avenue, BA 760, Boston, MA 02115; e-mail: carlo.brugnara@childrens.harvard.edu.