Abstract
The level of fetal hemoglobin (HbF) modifies the severity of the common β-globin disorders. Knowledge of the normal mechanisms that repress HbF in the adult stage has remained limited until recently despite nearly 3 decades of molecular investigation, in part because of imperfect model systems. Recent studies have provided new insights into the developmental regulation of globin genes and identified specific transcription factors and epigenetic regulators responsible for physiologic silencing of HbF. Most prominent among these regulators is BCL11A, a transcriptional repressor that inhibits adult-stage HbF expression. KLF1 and c-Myb are additional critical HbF-regulating erythroid transcription factors more broadly involved in erythroid gene expression programs. Chromatin modifiers, including histone deacetylases and DNA methyltransferases, also play key roles in orchestrating appropriate globin gene expression. Taken together, these discoveries present novel therapeutic targets for further consideration. Although substantial hurdles remain, opportunities are now rich for the rational design of HbF inducers.
Introduction
Hemoglobinopathies are among the most common inherited recessive diseases.1 Globin gene mutations occurred numerous times during human history and have been selected to high frequencies in malarial endemic regions. Although many of these globin mutations in the heterozygous state afford modest protection against malaria, the coinheritance of 2 mutant β-globin alleles (in homozygous or compound heterozygous combination) produces the common β-globin disorders. Sickle cell disease (SCD) and the β-thalassemias are chronic diseases with considerable morbidity and mortality. In low-income countries, most affected individuals die in early childhood. Nearly 300 000 infants are estimated born with SCD each year (the majority in Africa), and another 40 000 severely affected with β-thalassemia.2 In the United States alone, the annual medical costs for the approximately 75 000 individuals with SCD are estimated at more than $1 billion.3 Although genetic screening and prenatal diagnosis have reduced the incidence of β-thalassemia in selected countries, such as Sardinia and Cyprus, β-thalassemia remains common in many areas of Asia and the Middle East with limited resources for treatment. Children with β-globin disorders are at particular risk of life-threatening infections. As childhood infectious diseases are brought under better control in the developing world, the β-globin disorders will undoubtedly take on intensified public health significance. Given anticipated population growth, the worldwide prevalence of these diseases is expected to rise dramatically over the next century.4
Since the initial hematologic descriptions of SCD and β-thalassemia by Herrick and Cooley, respectively, in the early 20th century, studies of the hemoglobinopathies have been at the forefront of human genetics and molecular biology. Sickle cell disease, distinguished by its unique hemoglobin structure because of the characteristic glutamate-to-valine substitution of βS, was heralded as the first “molecular disease.”5page543 The subsequent demonstration of globin chain imbalance as the pathophysiologic underpinning of the thalassemias presaged the molecular biology era in which various thalassemia mutations were dissected, illuminating fundamental aspects of gene regulation.6
Fetal hemoglobin
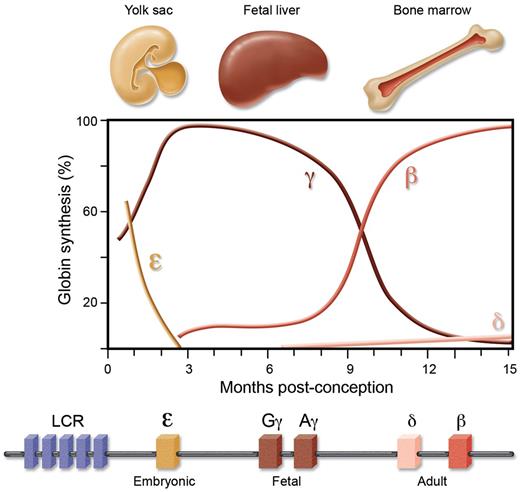
Two gene clusters encode the various globins—the α cluster on chromosome 16 contains the embryonic ζ gene, and adult α1 and α2 genes, and the β cluster on chromosome 11 the embryonic ϵ, the fetal Gγ and Aγ, and adult δ and β genes (Figure 1).7 During early embryonic development, erythropoiesis is yolk-sac–derived, transitioning to the fetal liver midway through the first trimester. Approaching the time of birth, the bone marrow becomes the dominant site of erythropoiesis. Two accompanying switches occur in the expression of genes from the β-globin cluster—a switch from embryonic-to-fetal globins early in gestation, and then from fetal-to-adult globins around the time of birth. Thus HbF (α2γ2) constitutes the major hemoglobin during fetal life, gradually replaced by adult hemoglobin (HbA, α2β2) during infancy. Adults retain low-level expression of HbF (roughly between 0.1% and 1% total hemoglobin), with only a subset of erythrocytes possessing measurable HbF.
The β-globin genes are encoded from a single cluster and under strict developmental control. There are 2 developmental switches in expression from the cluster, from embryonic-to-fetal during the first trimester of conception, and from fetal-to-adult around the time of birth.
The β-globin genes are encoded from a single cluster and under strict developmental control. There are 2 developmental switches in expression from the cluster, from embryonic-to-fetal during the first trimester of conception, and from fetal-to-adult around the time of birth.
A preponderance of genetic, biochemical, and clinical observations suggest a salutary role for HbF in the β-globin disorders. Both SCD and β-thalassemia are genetically heterogeneous conditions. The HbS mutation is carried on 5 independent common haplotypes. These haplotypes have identical βS Glu6Val mutations but vary with regard to the level of γ-globin production from the linked gene cluster. Haplotypes associated with higher levels of HbF are associated with milder clinical courses. β-thalassemia may result from diverse point mutations or deletions affecting the β-globin gene. Deletions with differing breakpoints may be associated with relatively low, intermediate, or high rates of γ-globin production from the linked gene cluster (ie, β0-thalassemia, δβ-thalassemia, or hereditary persistence of fetal hemoglobin [HPFH], respectively). Individuals compound heterozygous for β0 and HPFH have a mild clinical course in contrast to the thalassemia major of those homozygous for β0. Similarly, individuals with 1 βS allele and 1 allele of HPFH are protected from the deleterious consequences of SCD, with HbF remaining in the range of 20%-30%, whereas those with 1 βS allele and 1 β-thalassemia allele (βSβ0) have clinical manifestations comparable with those with HbSS.8
Biochemical studies have demonstrated that the presence of HbF profoundly delays the polymerization and increases the solubility of HbS under deoxygenated conditions. HbF is more potent than HbA in terms of polymer interference.9 The survival advantage of F-cells in SCD has been demonstrated by observing relative enrichment compared with F-reticulocytes.10 γ-globin expression in β-thalassemia mitigates globin chain imbalance, rendering erythropoiesis more effective. In patients with β-hemoglobinopathies who develop mixed chimerism after stem cell transplant, low levels of donor chimerism are sufficient to reverse clinical manifestations of their disease, emphasizing the selective advantage within the bone marrow and peripheral blood of normal erythroid cells.11 Epidemiologic studies have shown that HbF is a major modifier of frequency of painful crises and mortality in SCD.12 Perhaps the most dramatic evidence comes from individual patients—unlike α-thalassemia major which leads to fetal hydrops, β-thalassemia major and SCD only begin to manifest after the first months of life after HbF levels are developmentally silenced.
The sum of this evidence indicates that even modest induction of HbF may be sufficient to ameliorate SCD or β-thalassemia. Importantly, individuals with HPFH as well as their offspring are healthy, suggesting the safety of induction of HbF, even if it were possible to achieve a complete switch from HbA to HbF. Therefore a major goal of hematologists has been to discover targets to reactivate HbF. The supposition has been that the physiologic silencing of HbF during development (known as hemoglobin switching) could be counteracted for therapeutic benefit. In the 1980s, 5-azacytadine, a DNA demethylating agent, was found to increase levels of HbF in baboons and subsequently individuals with β-globin disorders.13,14 Two proposed mechanisms were direct demethylation of the γ-globin promoter and cytotoxicity with induction of HbF inextricably linked to the altered cell-cycle kinetics of stress erythropoiesis.15 Because of concerns related to potential genotoxicity of demethylating agents, studies were conducted with alternative S-phase inhibitors to identify pharmacologics that could induce HbF with more favorable toxicity profiles. This effort led to the discovery of the HbF-inducing potential of hydroxyurea in baboons and then humans.16,17 The mechanism whereby hydroxyurea results in increased HbF remains incompletely understood. Hydroxyurea has been of substantial benefit for many patients with SCD, although it has variable efficacy, requires careful monitoring with dose-limiting myelosuppression, and is of limited utility for β-thalassemia.18,19 A uniformly safe and potent HbF-inducing therapy remains to be discovered. Poor comprehension of the molecular mechanisms operative during the hemoglobin switch has limited the development of novel therapeutics. A recent upsurge of knowledge has reinvigorated the pursuit of rationally designed HbF inducers.
State of the field
Hemoglobin switching, the result of developmental changes in transcriptional output from the β-globin cluster, has been subject to intensive investigation as a model for the control of gene expression.7 The β-like globin genes are linearly arranged 5′ to 3′ in the order expressed during development. A critical distal regulatory element directing expression within the gene cluster is known as the locus control region (LCR).20 Although transgenic mice carrying LCR–γ-globin constructs display partial developmental silencing indicating some gene-autonomous developmental control, larger transgenes with both the β and γ genes linked to the LCR show more appropriate developmental regulation, suggesting that competition between the globin genes for the LCR is critical for proper developmental regulation.21,22 Inherited deletions of the β-globin cluster that result in considerably elevated HbF levels (ie, deletional HPFH) have pointed to the existence of repressive elements prominently located in the γ-δ intergenic region.23,24
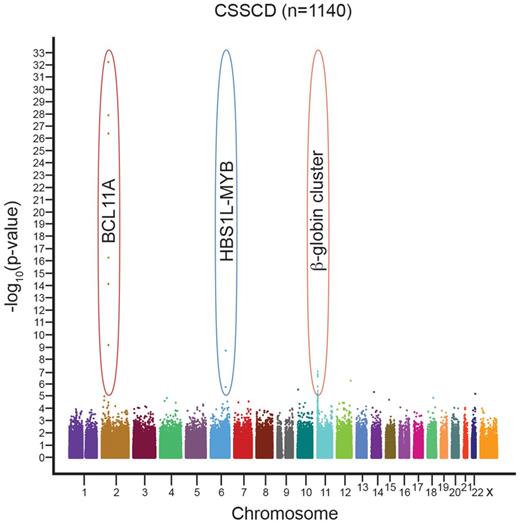
Novel approaches helped break the logjam in the search for γ-globin repressors to serve as therapeutic targets. Genome-wide association studies (GWAS) identified loci beyond the β-globin cluster important in HbF regulation (Figure 2). Because residual adult-stage HbF level is a heritable quantitative trait, it is particularly suited for genotype–phenotype correlation. GWAS of HbF level have included subjects with and without β-hemoglobinopathies and of diverse ethnic ancestry. The findings across numerous studies have been striking and consistent. In addition to cis-acting variants at the β-globin cluster itself, common variation at 2 additional loci, BCL11A on chromosome 2 and the HBS1L-MYB intergenic interval on chromosome 6, accounts for a substantial fraction (estimated at 15%-20% each) of individual variation in HbF level.25-28 Importantly, variants at each of these loci are also associated with the severity of the β-globin disorders, with high-HbF variants associated with milder disease.29,30
Genome-wide association studies have revealed 3 loci consistently associated with HbF level and β-globin disorder severity, across various ethnic backgrounds. These include the β-globin cluster itself on chromosome 11, the HBS1L-MYB intergenic interval on chromosome 6, and BCL11A on chromosome 2. A representative Manhattan plot is shown from the CSSCD cohort. Figure courtesy of Guillaume Lettre (Montreal Heart Institute).
Genome-wide association studies have revealed 3 loci consistently associated with HbF level and β-globin disorder severity, across various ethnic backgrounds. These include the β-globin cluster itself on chromosome 11, the HBS1L-MYB intergenic interval on chromosome 6, and BCL11A on chromosome 2. A representative Manhattan plot is shown from the CSSCD cohort. Figure courtesy of Guillaume Lettre (Montreal Heart Institute).
Knockdown studies demonstrate that BCL11A, a transcriptional repressor, is required to maintain silencing of HbF expression in primary human adult erythroid progenitors.31 Knockout of BCL11A in mice results in impaired developmental silencing of endogenous murine embryonic globin and transgenic human γ-globin genes.32 BCL11A interacts with GATA1, FOG1, and SOX6, erythroid transcription factors, and with the NuRD nucleosome remodeling and deacetylase complex.31,33 BCL11A occupies critical sites within the β-globin gene cluster, including sequences specifically deleted in HPFH, promoting long-range physical interactions between the LCR and the β promoter at the expense of the γ promoter.24,33 These results support a model in which BCL11A coordinates the hemoglobin switch by participating in multiprotein complexes occupying the β-globin gene cluster.
A recent linkage study mapped a novel form of HPFH to mutations in KLF1.34 Interestingly, the Nan (neonatal anemia) mouse mutant also maps to KLF1, with heterozygotes showing derepressed embryonic globin gene expression.35 KLF1 (also known as EKLF) is a critical erythroid transcription factor required for high-level adult-stage β-globin expression.36-38 It interacts with a CACCC motif at the β promoter at higher affinity than similar elements at the γ promoters.39,40 Knockdown of KLF1 in human erythroid progenitor cells leads to increased HbF expression and murine knockout of KLF1 leads to impaired silencing of transgenic human γ-globin.34,41,42 KLF1 protein occupies the BCL11A promoter and activates BCL11A expression.34,41 Therefore, KLF1 appears to influence hemoglobin switching both by directly activating β-globin in the adult stage as well as promoting the expression of the γ-globin silencer BCL11A. Interestingly, KLF1 mutations have been identified in individuals with a variety of erythroid conditions in addition to HPFH, including the In(Lu) blood antigen phenotype, congenital dyserythropoietic anemia, borderline HbA2, and elevated zinc protoporphyrin.43-46 Heterozygous inactivating mutations of KLF1 do not invariably result in elevated HbF, suggesting that genetic modifiers, including variants at BCL11A, may influence HbF level.43 Moreover, KLF1 has been demonstrated to occupy numerous loci within erythroid precursors and influence a broad array of erythroid gene expression programs.47-50
Another locus implicated by GWAS is the HBS1L-MYB intergenic interval. Although a contribution for HBS1L cannot be excluded,26 the bulk of evidence implicates MYB in influencing HbF regulation. c-Myb is a hematopoietic transcription factor essential for definitive hematopoiesis.51 Increased expression of c-Myb inhibits γ-globin expression in the K562 cell line.52 Knockdown of c-Myb within primary human erythroid progenitors results in increased HbF.53 c-Myb can influence KLF1 expression.54 A variety of additional factors have been related to hemoglobin switching, including COUP-TF, FOP (Friend of PRMT1), Ikaros, miR-15 and 16, MBD2, NF-E4, NRF2, and TR2/TR4.53,55-61
The previously mentioned studies implicate specific transcription factors in the developmental control of the globin genes. Each of these factors acts within larger multiprotein complexes with chromatin-modifying activity. The epigenetic state of the globin cluster, including higher-order chromatin structure, histone modifications, and DNA methylation, is correlated with its developmental regulation. Active globin genes are hyperacetylated, whereas inactive globin genes are hypoacetylated.62,63 Moreover, histone deacetylase inhibitors, including short-chain fatty acids, such as butyrate, lead to increased HbF levels.64,65 Specifically, HDAC1 and HDAC2 contribute to HbF repression.66 Chromatin regulators observed to occupy the γ-globin promoter include the arginine methyltransferase PRMT5, lysine methyltransferase SUV4-20h1, serine/threonine kinase CK2α, the NuRD complex, and DNA methyltransferase DNMT3A.67,68 Perhaps the best-characterized epigenetic correlate of HbF level is DNA methylation. Hypermethylation of the β promoter is observed in the embryonic and fetal stages, and hypermethylation of the γ promoter in the adult-stage.69,70
Challenges to clinical translation
Many of the critical discoveries regarding mechanisms of the hemoglobin switch derive from human genetics. In part, this is a credit to generations of astute clinical investigators. However, it also speaks to the fact that cellular and animal models of HbF regulation possess significant limitations to their broad applicability.
Cellular models
K562 cells are an extensively studied human cell line with erythroid and megakaryocytic potential isolated from an individual with BCR-ABL+ chronic myeloid leukemia. When erythroid maturation is induced, modest levels of embryonic and fetal globins ζ, ϵ, and γ are synthesized with minimal production of α or β.71 That is, these cells appear to recapitulate a hybrid of embryonic and fetal but not adult-stage erythropoiesis. Likewise these cells express very low levels of BCL11A.31 Therefore their utility in screening and validating targets that participate in adult-stage silencing of γ-globin appears questionable. A variety of other human cell lines with erythroid potential exist (eg, HEL, KU812, etc) but none of these possess an adult-stage globin gene expression pattern.72
Embryonic stem (ES) and induced pluripotent stem (iPS) cells have been used for erythroid differentiation.73,74 Potential advantages of these systems are the availability from numerous individuals, including those with β-globin disorders. In fact, β-globin disorder patient-derived iPS cells have been generated.75,76 However, current erythroid maturation protocols remain inefficient. Furthermore, erythroid differentiation from pluripotent cells reflects embryonic or fetal stages and does not recapitulate the adult stage.77
Primary human erythroid cultures are widely used. A variety of 2-phase cell culture systems have been described, with expansion of early hematopoietic progenitors before enforced erythroid maturation. These protocols use either rare circulating progenitors present in peripheral blood, or enriched CD34+ hematopoietic stem/progenitor cells from bone marrow aspirates or G-CSF mobilized peripheral blood.78-80 A clear asset of these systems is their capacity to model aspects of human erythropoiesis, including adult-stage pattern globin gene expression and expression of critical regulators including BCL11A, KLF1, and c-Myb. Disadvantages of the primary human cell systems include limited proliferative capacity and asynchronous differentiation manner, especially with regard to the terminal stages of erythroid maturation and enucleation. These cells are particularly subject to maturational switching so careful monitoring of kinetics is mandatory for studies of γ-globin expression. Furthermore these cells typically have a relatively high background level of HbF and appear unusually permissive for HbF induction, thus rendering them sensitive but not necessarily specific indicators of manipulations inducing HbF.81,82 Extrinsic signals, such as fetal calf serum or specific cytokines, such as stem cell factor, may contribute to elevated HbF levels in these cultures.83 Stress erythropoiesis is a final common pathway associated with elevated HbF production; this is seen in patients recovering from myelosuppression (and may underlie some of the HbF induction by hydroxyurea). Therefore exquisite care must be taken to ensure any perturbations of globin gene expression are out of proportion to impacts on cellular growth and proliferation. Agents that increase HbF in primary human erythroid cultures may not necessarily have equivalent behavior in vivo. Additional studies are required to corroborate effects on HbF induction, total globin synthesis, and erythroid differentiation.
Mouse erythroleukemia (MEL) cells demonstrate adult-pattern globin expression, characterized by high-level expression of the adult globins βmajor and βminor and repression of the embryonic globins ϵy and βh1. Knockdown of BCL11A in these cells recapitulates embryonic globin derepression.33 Recently a MEL line was reported in which a human β-globin cluster transgene was engineered to have fluorescent reporter genes in place of the γ and β-globin coding sequences; however, the relatively small shift in ratio of γ/β-globin reporters on BCL11A knockdown suggests limited robustness for small molecule screening.84 Additional cell lines have been generated from mice carrying the entire human β-globin cluster as a transgene.85 All murine cellular systems face the caveat of species-level developmental differences in physiologic hemoglobin switching.
Animal models
A faithful small animal model would offer enormous advantages for screening and validation. The benefits of such a model could include ability to assess impact of modulation of a target: on globin gene expression throughout the developmental stages of erythropoiesis; on aspects of erythropoiesis in addition to globin gene regulation; on other hematopoietic lineages; and beyond the hematopoietic system. A critical drawback of current small animal models is that human HbF regulation lacks a direct analog in rodents. The emergence of fetal stage γ-globin expression occurred approximately 45 million years ago, before the divergence of Old World monkeys, apes, and humans.86 Other vertebrates lack an HbF equivalent although all have at least 1 developmental switch within the β-globin cluster. For example, in contrast to human erythropoiesis consisting of 2 developmental transitions in globin gene expression (from embryonic-to-fetal and from fetal-to-adult) only 1 switch in globin gene expression (from embryonic-to-adult) takes place in the mouse. Therefore, interventions that interfere with mouse globin switching may not directly apply to the human fetal-to-adult transition although certain critical mechanistic aspects do appear conserved.32 Many studies have taken advantage of mice carrying a human β-globin cluster transgene, consisting of all 5 human β-like globin genes as well as the LCR with preserved spatial organization, to circumvent the absence of an endogenous mouse γ-globin gene. These transgenic mice display high-level, lineage-restricted, developmentally regulated expression of the β-like globin genes. However, silencing of human γ-globin occurs alongside that of human ϵ and mouse embryonic β-like globins ϵy and βh1 during the transition from primitive yolk-sac–derived erythropoiesis to the definitive fetal liver stage.32,87,88 The degree of repression is much more profound for embryonic murine globins and transgenic human γ globin (on the order of 1:10 000 relative to adult β-globin expression) in contrast to the relatively more modest degree of repression of γ-globin observed in humans, in whom residual expression of HbF between 0.1% and 1% persists throughout adulthood.89 Therefore interventions seeking to derepress γ globin expression face a greater quantitative hurdle and potentially unique epigenetic mechanisms in the mouse environment compared with the human. BCL11A plays a critical role in the mouse globin switch. However, even in mice deficient for BCL11A within the erythroid lineage, adult γ-globin production remains at ∼ 10%-15% of total human β-like globin production.89 In contrast, in murine Berkeley sickle mice, with basal γ-globin level orders of magnitude higher (∼ 1% of total β-like globin), BCL11A deficiency results in derepression of γ-globin up to ∼ 30%, enough for reversal of the end-organ damage caused by SCD.89 Of note, this SCD model contains a “mini-LCR” globin gene cassette, which does not possess the full complement of regulatory elements at the β-globin cluster so might not completely mirror regulation of the endogenous human genes.90
Recent evidence suggests the regulation of transgenic human γ-globin is more similar to murine embryonic βh1 than to ϵy, with expression in early definitive erythroid precursors of the nascent fetal liver.91,92 Intriguingly, βh1 shares an evolutionary origin with human γ-globin whereas murine ϵy is the paralog of human ϵ.93 Moreover, γ-globin is known to undergo maturational switching during normal human erythropoiesis whereby it is expressed at higher levels in less mature erythroid precursors.94 The mechanistic correlates of embryonic-to-fetal switching as well as maturational switching remain incompletely elucidated. However, loss of BCL11A results in derepression of both ϵy and βh1 to a similar degree during adulthood.
The zebrafish was recently shown to undergo 2 developmental switches in globin gene expression, from embryonic-to-larval and larval-to-adult.95 The globin gene clusters share partial synteny and similar distal regulatory elements with the human genes though it remains to be elucidated whether fish and mammalian hemoglobin switches share trans-acting regulatory mechanisms. Nonetheless, the zebrafish model serves as a complementary system for genetic and chemical screening in numerous aspects of hematopoiesis.96
Large animals have also been used as a model of human hemoglobin switching, as fetal-stage γ-globin expression evolved in a common primate ancestor.86 Studies in baboons have taken advantage of this shared γ-globin regulation to demonstrate that HbF levels increase as a result of stress erythropoiesis or demethylating agents.97 The baboon system has been used to study epigenetic changes at the β-globin cluster.98 Potential limitations with this model include subtle differences in γ-globin regulation, even between various baboon species, and inherent challenges to conducting genetic or even high-throughput chemical studies in such animals.97,99
Provisos and prospects for novel therapeutics
The ideal molecular target for therapeutic induction of HbF for the β-globin disorders would meet several criteria including: direct and significant involvement in HbF silencing; limited effects on total hemoglobin synthesis and on erythroid maturation; absence of critical functions in nonerythroid cells; validation in primary human erythroid cultures as well as in animal models; and feasibility of therapeutic modulation. It should be noted that in other “rational therapy” settings, the criterion of cellular specificity is not always stringently applied. For example, kinase inhibitors that are effective in treating hematopoietic malignancies often also inhibit kinases expressed in noncancerous cells. Until pharmacologic agents are available that directly reactivate HbF expression in adult erythroid cells, it is premature to consider effects in other cell types.
Targets
The genetically implicated transcription factors, BCL11A, KLF1, and c-Myb may all be considered prospective targets for therapy. Assets and deficiencies of each are apparent. The ubiquitous expression of c-Myb in HSCs, and progenitors, and requirement for c-Myb in the maintenance and differentiation of HSCs, raise concerns as to whether an adequate therapeutic window exists whereby c-Myb could be modulated without having negative effects on hematopoiesis. KLF1 has the benefit of exclusive expression within the erythroid lineage. However, pleiotropic erythroid phenotypes observed with KLF1 mutations in both mouse and man indicate that it may be difficult to interfere with this factor without broadly influencing red blood cell production and function. Of note, mice that lack KLF1 die in utero from severe anemia, even if lethal thalassemia is averted by enforced expression of globin genes, demonstrating the critical importance of this factor for erythropoiesis.100 BCL11A has several favorable attributes for therapeutic aim. Its potency in HbF silencing has been demonstrated in a variety of systems from cell lines and primary cell cultures to transgenic mice. For example, in mouse models of sickle cell disease, loss of BCL11A alone produces pancellular HbF induction and reverses the characteristic end-organ damage.89 BCL11A influences the expression of very few genes within the erythroid lineage besides the globins, and its loss is not associated with any discernable erythroid phenotype besides embryonic and fetal globin gene derepression.89 However, there are several potential drawbacks to targeting BCL11A.
One concern relates to essential roles of BCL11A outside the erythroid lineage. BCL11A null mice are perinatal lethal for unclear reasons.101 Within the central nervous system BCL11A is expressed both during embryonic development and after birth.102 BCL11A serves important roles in neuronal differentiation and morphogenesis as well as axonal guidance.103,104 Fortunately, potential untoward CNS effects of BCL11A inhibition might be avoided by an intact blood-brain barrier. Genetic variants at the BCL11A locus have been associated with diabetes mellitus, although a direct role for BCL11A in metabolic homeostasis has not been identified.105,106 Within the hematopoietic compartment, BCL11A is essential for proper development of B-lymphocytes during ontogeny, although its role in postnatal B-cell function remains unclear.101 Given the divergent kinetics of B-cell and erythrocyte production, it seems plausible that intermittent blockade of BCL11A could lead to substantial induction of HbF without excessive interference with B-lymphocyte homeostasis. T-cell neoplasms were identified in recipients of BCL11A-deficient fetal-liver transplants, although the effect appeared to be noncell autonomous, and may have been related to the immunodeficient state of the recipients.101
A more generic concern is that transcription factors have traditionally been considered to be unfavorable drug targets.107 Unlike enzymes, transcription factors lack catalytic domains, and unlike receptors they lack ligands (with the obvious exception of the nuclear hormone receptors). Thus, interference with protein-DNA or protein-protein interactions would appear to be required. By considering the larger chromatin context within which individual transcription factors act, novel targets might materialize. For example, emerging evidence suggests chromatin readers (proteins with recognition modules for specific epigenetic marks) may serve as a susceptible node for small-molecule modulation of transcription. In particular, highly selective small molecules have been designed that interfere with the tandem bromodomain (BET) motif for acetyl-lysine recognition.108,109 These compounds have varied and potent biologic activity in mouse models, preventing LPS-mediated changes in gene expression and subsequent fatal sepsis as well as reversing malignant progression in diverse BET-reliant cancers.108-110 Although the precise contextual determinants of specificity for the chromatin readers remain to be elucidated, the therapeutic potential is striking.
In addition to chromatin “reading,” chromatin “writing” and “erasing” could be important targets in hemoglobin switching. It may be instructive that demethylating agents interfering with DNA methylation were the first compounds to demonstrate HbF induction in patients with β-globin disorders. Next-generation demethylating agents with improved toxicity profiles, such as decitabine, may offer benefit to patients with β-globin disorders.111,112 Although short-chain fatty acid derivatives, many of which appear to exert their action through HDAC inhibition, have shown activity in patients with β-globin disorders, cytotoxicity has limited their clinical applicability.64 Perhaps more specific epigenetic therapies, such as HDAC1 or HDAC2 selective inhibitors, would have an improved therapeutic index.
Purely “agnostic” approaches to genetic or chemical screening may yet yield novel targets for HbF inhibition, although these must tackle the aforementioned challenges of screening platforms and robust validation before moving toward clinical translation. An example of unexpected small molecule modulators is provided by the so-called immunomodulatory compounds lenalidomide and pomalidomide (thalidomide derivatives). These novel agents that have been developed for hematologic malignancies appear to have modest effects in terms of HbF induction.113,114 In this case, the biologic importance of the class of small molecules preceded the identification of the molecular target—although recent studies suggest cereblon, an E3 ubiqutin ligase, may mediate some of its biologic effects.115
Therapeutic modalities
Even after identification of a suitable target, determination of an appropriate modality to address the target remains a formidable challenge (Figure 3). For enzymes, active-site inhibitors would be an obvious strategy. The recognition modules of chromatin readers are another conceptually straightforward approach for small molecule modulation. For multiprotein complexes, critical interfaces between partner protein subunits may be targeted, for example by stabilized peptides.116 Allosteric inhibitors are plausible for a wide spectrum of proteins. Contemporary strategies of compound discovery, such as small molecule microarrays as well as expanding structural knowledge raise hope that transcription factors may not be as “undruggable” as initially supposed.107
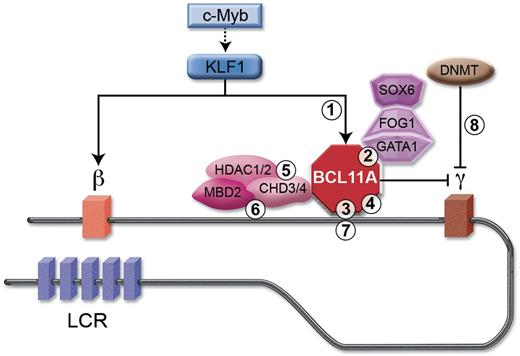
The BCL11A network as a schematic target for various potential therapeutic modalities. BCL11A is shown occupying sequences within the β-globin cluster distal from the γ-globin genes themselves. It is subject to transcriptional activation by KLF1, which itself may be a target of c-Myb. BCL11A interacts with the NuRD nucleosome remodeling and deacetylase complex, which includes the ATPases CHD3/4 and histone deacetylases HDAC1/2 as well as MBD2. BCL11A also interacts with erythroid transcription factors including GATA1, FOG1, and SOX6. BCL11A could conceptually be therapeutically targeted by various strategies including: (1) decreasing its steady-state level, such as by preventing its activation by KLF1, or by RNA interference; (2) interfering with protein–protein interactions such as between BCL11A and GATA1; (3) interfering directly with BCL11A's protein–DNA interactions; (4) allosteric inhibitors of BCL11A itself or various partners; (5) active-site inhibitors of partner proteins with enzymatic activity such as HDAC1/2 and CHD3/4; (6) blocking associated chromatin reader modules, such as PHD fingers and chromodomains on CHD3/4 and MBD domain on MBD2; (7) direct genome editing, such as of critical BCL11A binding regulatory elements; and (8) as part of combination therapy with additional targets such as low-dose demethylase therapy.
The BCL11A network as a schematic target for various potential therapeutic modalities. BCL11A is shown occupying sequences within the β-globin cluster distal from the γ-globin genes themselves. It is subject to transcriptional activation by KLF1, which itself may be a target of c-Myb. BCL11A interacts with the NuRD nucleosome remodeling and deacetylase complex, which includes the ATPases CHD3/4 and histone deacetylases HDAC1/2 as well as MBD2. BCL11A also interacts with erythroid transcription factors including GATA1, FOG1, and SOX6. BCL11A could conceptually be therapeutically targeted by various strategies including: (1) decreasing its steady-state level, such as by preventing its activation by KLF1, or by RNA interference; (2) interfering with protein–protein interactions such as between BCL11A and GATA1; (3) interfering directly with BCL11A's protein–DNA interactions; (4) allosteric inhibitors of BCL11A itself or various partners; (5) active-site inhibitors of partner proteins with enzymatic activity such as HDAC1/2 and CHD3/4; (6) blocking associated chromatin reader modules, such as PHD fingers and chromodomains on CHD3/4 and MBD domain on MBD2; (7) direct genome editing, such as of critical BCL11A binding regulatory elements; and (8) as part of combination therapy with additional targets such as low-dose demethylase therapy.
Interfering with the synthesis of the target is another potential strategy. A direct approach is systemic administration of a compound capable of RNA inhibition, such as modified inhibitory RNA.117 In this approach, effects should be reversible, and potentially titrated to effect. Systemic inhibitory RNA technologies are limited by efficiency of cellular uptake and tissue distribution in vivo. A second approach might involve stable expression of an shRNA for a given target, such as BCL11A, using gene therapy in HSCs ex vivo before transfer back to the patient.118 Expression would need to be persistent for efficacy. Even a limited fraction of transduced HSCs could be of benefit given in vivo selection for HbF-containing erythroid cells. In fact, residual nontransduced HSCs might obviate some possible extraerythroid toxicities. With this strategy, effects of attacking the target outside the hematopoietic lineages would not be relevant. The standard concerns regarding genotoxicity secondary to gene therapy need to be considered. Compared with other gene therapy approaches for SCD, interfering with a target such as BCL11A would be associated with down-regulation of HbS in a reciprocal manner as up-regulation of HbF—an advantage over mere addition of a nonsickling β or γ chain.
Direct genome editing is another possible therapeutic modality.119 Sequence-specific nucleases (such as zinc-finger nucleases or transcription activator-like effector nucleases) are tools that can introduce targeted double-stranded breaks in the genome to produce mutations, such as frameshifts or deletions, or to stimulate homologous recombination. For therapeutic purposes, genome editing would require exquisite specificity to prevent off-target mutagenic events.
Some of these strategies appear more feasible for the relatively short-term horizon; for example, nucleic acid based therapeutics could apply existing technical knowledge to a novel target (eg, BCL11A), and might be suitable for resource-rich environments. Other approaches might require a longer timeframe to materialize as they require the development of novel techniques, the acquisition of structural knowledge, or the identification of novel targets. However, these longer-term approaches might ultimately yield a small-molecule that could be more easily scaled to the developing world where the majority of β-globin disorder patients reside.
Any pharmacologic intervention would be unlikely to achieve complete blockade of its target. For a dose-dependent regulator of HbF, such as BCL11A, incomplete targeting is desirable as it permits a therapeutic window in which adequate HbF induction might occur with minimal toxicity. In addition, combination therapy could allow for various unique strategies to achieve additive or synergistic efficacy. For example, combining genetic BCL11A deficiency with chemical histone deacetylation or DNA demethylation leads to synergistic derepression of transgenic human γ-globin in mice.89 One could envision otherwise modestly effective or toxic therapies playing a valuable role in combination rather than as single agents; for example, low-dose demethylating agents.
After target identification and therapeutic modality design and validation, clinical trials are required to demonstrate safety and efficacy in humans. A challenge for all the model systems is that it is difficult to directly extrapolate the percentage HbF induction expected in humans based on experiments in cells or animals. Fortunately, HbF is an ideal biomarker for clinical studies, inextricably linked to the mechanism of action and compellingly associated with meaningful clinical outcomes in both SCD and β-thalassemia. It may be feasible to define safety and efficacy in healthy individuals with intrapatient dose-escalation before study in patients with β-globin disorders. A greater requirement of γ-globin induction for therapeutic benefit is anticipated for β-thalassemia patients in whom the goal would be to mitigate globin chain imbalance compared with SCD patients in whom the goal is prevention of HbS polymerization.
Conclusion
Targets that directly influence HbF silencing have been identified. Translating this knowledge to the clinics faces hurdles of imperfect model systems and targets. The empiric era of discovery of HbF inducers should now yield to a period of mechanism-based therapeutic design. Exquisitely devised and performed clinical studies will be required to ensure that patients will benefit from safe and efficacious new therapies. It will be necessary to engage interdisciplinary efforts of clinicians, molecular biologists, chemists, and pharmacologists. In addition, as both “orphan” genetic diseases and an emerging public health problem, the β-hemoglobinopathies could benefit enormously from involvement of disease advocates.
Acknowledgments
The authors apologize to the many authors whose work they were unable to cite because of space constraints.
Authorship
Contribution: D.E.B. and S.C.K. reviewed the literature; and D.E.B., S.C.K., and S.H.O. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart H. Orkin, Department of Pediatric Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: orkin@bloodgroup.tch.harvard.edu.
References
Author notes
D.E.B. and S.C.K. contributed equally to this work.