Abstract
The clinical value of serial minimal residual disease (MRD) monitoring in core binding factor (CBF) acute myeloid leukemia (AML) by quantitative RT-PCR was prospectively assessed in 278 patients [163 with t(8;21) and 115 with inv(16)] entered in the United Kingdom MRC AML 15 trial. CBF transcripts were normalized to 105ABL copies. At remission, after course 1 induction chemotherapy, a > 3 log reduction in RUNX1-RUNX1T1 transcripts in BM in t(8;21) patients and a > 10 CBFB-MYH11 copy number in peripheral blood (PB) in inv(16) patients were the most useful prognostic variables for relapse risk on multivariate analysis. MRD levels after consolidation (course 3) were also informative. During follow-up, cut-off MRD thresholds in BM and PB associated with a 100% relapse rate were identified: for t(8;21) patients BM > 500 copies, PB > 100 copies; for inv(16) patients, BM > 50 copies and PB > 10 copies. Rising MRD levels on serial monitoring accurately predicted hematologic relapse. During follow-up, PB sampling was equally informative as BM for MRD detection. We conclude that MRD monitoring by quantitative RT-PCR at specific time points in CBF AML allows identification of patients at high risk of relapse and could now be incorporated in clinical trials to evaluate the role of risk directed/preemptive therapy.
Introduction
Despite improved survival rates, relapse remains the main cause of treatment failure in acute myeloid leukemia (AML). The core binding factor (CBF)–positive leukemias comprise AML with t(8;21) (q22;q22) and inv(16) (p13q22)/t(16,16) (p13;q22), which are characterized by the presence of RUNX1-RUNX1T1 (AML 1-ETO) and CBFB-MYH11 fusion transcripts, respectively.1 Although CBF AML belongs to the favorable cytogenetic subgroup, with a cure rate of > 65% achievable with chemotherapy alone, in particular with the use of high-dose cytarabine-based consolidation regimens, disease relapse remains the most important single cause of treatment failure, occurring in up to 35% of patients.2-7 At present, little is known about the kinetics of relapse or the development of resistance mechanisms; and furthermore, post-remission therapy in AML takes no account of the level of residual leukemia. However, the development of real-time quantitative RT-PCR techniques has enabled the sensitive detection and quantification of leukemia-associated genes as a measure of residual leukemia, thus allowing the tracking of reemergent leukemic clones. Fusion genes, such as RUNX1-RUNX1T1 and CBFB-MYH11 in CBF AML, PML-RARA in acute promyelocytic leukemia (APL), DEK-CAN, and mutations in the nucleophosmin (NPM1) gene and the Wilms' Tumor (WT1) gene, can now be used as molecular targets for monitoring residual leukemia.8-14
Monitoring of minimal residual disease (MRD) using quantitative RT-PCR has become an important diagnostic tool that permits the assessment of response to therapy and for detecting early relapse during remission and to guide therapeutic decisions. Although MRD monitoring is now an integral part in the management of acute lymphoblastic leukemia15,16 (generally done by multiparameter flow cytometry) and chronic myeloid leukemia,17 its clinical utility in AML is currently restricted to the APL subtype, albeit where the relapse rate is now low, in which preemptive treatment at the time of molecular relapse has been shown to be of clinical benefit.11 There is now cumulative evidence that monitoring of MRD can predict relapse in other subtypes of AML. In 2 recent studies from the German-Austrian AML Study Group, involving AML patients with inv(16)/t(16;16)18 and NPM1 gene mutations,19 the authors reported clinically relevant MRD checkpoints that allowed the identification of patients who were at high risk of relapse. Early reports in AML with t(8;21) suggested that quantitative monitoring of MRD was useful in distinguishing patients at high risk of relapse from those in durable remission.20,21 This observation was confirmed in subsequent studies in CBF AML using quantitative RT-PCR methodology.22-28 However, in CBF AML, especially in the t(8;21) subtype, there has been, to date, a paucity of large prospective studies to assess the clinical utility of MRD monitoring, and in particular limited data on serial MRD assessment in BM and/or peripheral blood (PB) samples during and after chemotherapy.
Within the United Kingdom Medical Research Council AML-15 trial, we prospectively assessed the clinical value of serial MRD monitoring in CBF AML, and we herein report out findings.
Methods
Patients and samples
The MRC AML-15 trial29 compared 3 induction regimens (DA vs ADE vs FLAG-Ida), followed by randomization in consolidation (courses 3 and 4) to either MACE and MidAC or 2 doses of Ara-C (3 g/m2 or 1.5 g/m2) or to stop or have a fifth course (Ara-C 1.5 g/m2). Patients were also randomized to receive gemtuzumab ozogamicin (GO; 3 mg/m2) at induction and/or consolidation in a 2 × 2 design. Allografting in the first complete remission (CR) was not recommended for CBF AML patients. At diagnosis, all trial patients had routine cytogenetic investigations and were also screened for the presence of RUNX1-RUNX1T1 and CBFB-MYH11 transcripts. The diagnosis of CBF AML was based on a positive cytogenetic and/or molecular result. Between July 2002 and January 2009, 361 CBF AML patients were recruited, of whom 278 [163 with t(8;21) and 115 inv(16)/t(16;16)], were included in the MRD study. BM and PB samples were requested at diagnosis, after each course of chemotherapy and serially every 3 months in the first year of follow up, every 4 months in the second year, 6 months in the third year, and at relapse. Informed consent was obtained from patients according to standard procedures, in accordance with the Declaration of Helsinki, in each center, and samples were collected as part of the treatment protocol, which was approved by the Wales Research Ethics Committee. Results of MRD monitoring were not disclosed to participating clinicians.
Nucleic acid isolation, cDNA synthesis, quantitative RT-PCR
Methodologies for nucleic acid isolation and cDNA synthesis are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Real-time quantitative RT-PCR protocols for RUNX1-RUNX1T1, CBFB-MYH11, and ABL transcripts were performed on the ABI 7900HD platform, in line with recommendations of the EAC program.9,30 For patients with inv(16), including t(16;16), only screening for the 3 major fusion subtypes A, D, and E comprising ∼ 98% of all breakpoints was carried out. The absolute copy numbers of fusion gene transcripts was normalized to ABL, expressed per 105 copies of ABL. Median sensitivity of the quantitative RT-PCR assay for RUNX1-RUNX1T1 and CBFB-MYH11 transcripts was 10−5 and was calculated as described.31 PCR negativity and positivity were defined, according to EAC criteria.30 Diagnostic and follow-up samples with < 102 and < 103 copies of ABL, respectively, were excluded from analysis.
Clinical endpoints and statistical analysis
The outcome definitions (eg, RFS and OS) follow IWG guidelines.32 CR was defined as < 5% blasts in a normocellular marrow, with peripheral neutrophil recovery to 1 × 109/L, and platelet count to 100 × 109/L, without evidence of extramedullary disease. Cumulative incidence of relapse (CIR) is measured from the date of achievement of remission (CR or CRi [without complete peripheral blood recovery]) until relapse with death as a competing risk. Survival from remission is measured from CR/CRi until death or date last seen.
Statistical methods
Surviving patients were censored at January 1, 2009. Correlations were calculated using Spearman rank correlation coefficient, or Pearson correlation for log copy number as these showed approximate normality. Time-to-event outcomes are presented using Kaplan-Meier curves, with univariate analyses by the log-rank method. For multivariable analysis, Cox proportional hazards regression was used. To evaluate the effect of MRD positivity (as defined by transcript copy numbers) during sequential monitoring, Mantel-Byar analysis33 was used to adjust for zero time-shift bias in that patients who relapsed after becoming MRD-positive will have been in remission while MRD-negative. In Mantel-Byar analysis, patients start in the MRD-negative group and transfer to the MRD-positive group when they become MRD-positive at the specified level. All outcomes are given at 5 years except where otherwise specified.
Results
Patients' clinical characteristics are shown in Table 1; 163 patients with t(8;21) and 115 with inv(16) were included in the MRD study. Patients were excluded because of lack of or insufficient diagnostic and/or follow-up samples for molecular analysis; 8 patients were transplanted and were therefore censored from the study. All t(8;21) patients were positive for RUNX1-RUNX1T1, whereas 5 patients who were cytogenetically negative for inv(16)/t(16;16) were positive for the CBFB-MYH11 transcript. The total number of samples analyzed in the study were as follows: 822 from BM and 855 from PB in t(8;21) patients and 681 from BM and 661 from PB in inv(16) patients. CBF transcript levels at diagnosis are shown in supplemental Results. Pretreatment RUNX1-RUNX1T1 and CBFB-MYH11 copy numbers in BM and PB did not correlate with age, WBC, performance status, secondary disease, or sex. Furthermore, pretreatment transcript levels in BM and PB for both t(8;21) and inv(16) patients, expressed as a log-transformed continuous variable, did not impact on CR, CIR, and OS in both unadjusted and adjusted analyses.
Patient demographics and clinical characteristics
| . | Total no. . | t(8;21) . | inv(16) . |
|---|---|---|---|
| Patients | 278 | 163 | 115 |
| Age, y | |||
| 15-29 | 67 | 33 | 34 |
| 30-39 | 58 | 33 | 25 |
| 40-49 | 72 | 40 | 32 |
| 50-59 | 60 | 44 | 16 |
| 60+ | 21 | 13 | 8 |
| Median (range) | 42 (15-70) | 45 (15-70) | 38 (16-64) |
| Sex | |||
| Female | 118 | 69 | 49 |
| Male | 160 | 94 | 66 |
| Diagnosis | |||
| De novo | 270 | 158 | 112 |
| Secondary | 8 | 5 | 3 |
| Performance Status (WHO) | |||
| 0 | 198 | 120 | 78 |
| 1 | 67 | 39 | 28 |
| 2 | 6 | 2 | 4 |
| 3 | 5 | 2 | 3 |
| 4 | 5 | 0 | 2 |
| WBC, × 109/L | |||
| < 10 | 99 | 76 | 23 |
| 10-19.9 | 49 | 39 | 10 |
| 20-49.9 | 67 | 36 | 31 |
| 50-99.9 | 35 | 10 | 25 |
| 100+ | 27 | 1 | 26 |
| Missing | 1 | 1 | 0 |
| Median (range) | 17.6 (1.2-298.0) | 10.5 (1.2-153.0) | 41.8 (1.3-298.0) |
| . | Total no. . | t(8;21) . | inv(16) . |
|---|---|---|---|
| Patients | 278 | 163 | 115 |
| Age, y | |||
| 15-29 | 67 | 33 | 34 |
| 30-39 | 58 | 33 | 25 |
| 40-49 | 72 | 40 | 32 |
| 50-59 | 60 | 44 | 16 |
| 60+ | 21 | 13 | 8 |
| Median (range) | 42 (15-70) | 45 (15-70) | 38 (16-64) |
| Sex | |||
| Female | 118 | 69 | 49 |
| Male | 160 | 94 | 66 |
| Diagnosis | |||
| De novo | 270 | 158 | 112 |
| Secondary | 8 | 5 | 3 |
| Performance Status (WHO) | |||
| 0 | 198 | 120 | 78 |
| 1 | 67 | 39 | 28 |
| 2 | 6 | 2 | 4 |
| 3 | 5 | 2 | 3 |
| 4 | 5 | 0 | 2 |
| WBC, × 109/L | |||
| < 10 | 99 | 76 | 23 |
| 10-19.9 | 49 | 39 | 10 |
| 20-49.9 | 67 | 36 | 31 |
| 50-99.9 | 35 | 10 | 25 |
| 100+ | 27 | 1 | 26 |
| Missing | 1 | 1 | 0 |
| Median (range) | 17.6 (1.2-298.0) | 10.5 (1.2-153.0) | 41.8 (1.3-298.0) |
The median follow-up time for survival was 36 months (range, 2-79 months). The CR rates for all t(8;21) and inv(16) patients entered in the trial were 97% and 92%, with 5-year CIR of 18% and 23%, respectively. For the MRD study, data on 71 morphologically relapsed patients [38 t(8;21) and 35 inv(16)] were available for analysis. Comparison of CBF patients in the MRD study with those not in it (278 vs 83 not included) showed no difference in baseline characteristics, Mylotarg randomization, or RFS between the 2 groups.
Impact of log reduction and log copy numbers at remission after course 1 induction chemotherapy
The impact of log reduction and log copy numbers (transcript levels) at remission on relapse risk was assessed by univariate and multivariate analyses in t(8;21) and inv(16) patients separately. Multivariate analyses were adjusted for age, WBC, secondary AML, performance status, and sex.
t(8;21).
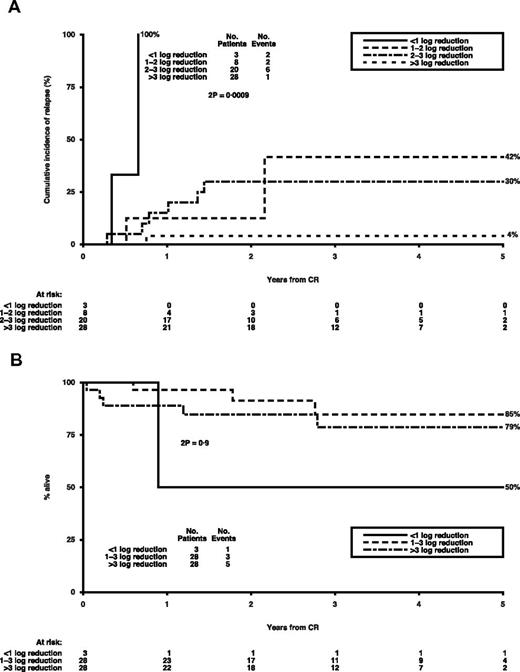
For t(8;21) patients, the hazard ratios (HRs) according to relative log reduction in fusion transcripts and normalized level of RUNX1-RUNX1T1 transcripts after induction are shown in Table 2. To determine a significant level for each of the 4 variables, further analysis was carried out on the BM and PB log reduction split in < 1, 1 or 2, 2 or 3, and > 3 groups and BM and PB transcript levels at cut-offs of 10, 50, 100, 500, and 1000 copies. The impact of BM log reduction on relapse risk is shown in Figure 1A; a > 3 log reduction is highly significant and is associated with a CIR of only 4% in the 47% of patients achieving it. With respect to BM copy numbers at remission, a level of < 100 copies identified 47% of patients with a CIR of 7% (representing 15% of all relapsing patients), whereas in PB, a cut-off level of 1000 copies was also prognostic, identifying 78% of patients with a CIR of 15%, compared with 50% in the remaining 22%. However, adjusted regression analysis using the 3 variables (BM log reduction and BM and PB copies) revealed that the BM log reduction was the most prognostic (P = .008). Adjusted analyses of survival from CR showed no significant effect of BM log reduction after induction (adjusted HR per log reduction = 1.09; 0.60-1.95; P = .8; Figure 1B).
Impact of BM and PB log reduction and log copy numbers at remission, on relapse risk: unadjusted and adjusted HRs per log decrease
| Measure . | Unadjusted HR per log decrease . | Adjusted HR per log decrease . |
|---|---|---|
| t(8;21) | ||
| Marrow log reduction | 0.36 (0.18-0.74) P = .005 | 0.33 (0.15-0.73) P = .004 |
| Marrow log copy level | 2.27 (1.43-3.58) P = .0005 | 2.84 (1.61-5.00) P = .0002 |
| Blood log reduction | 0.70 (0.45-1.09) P = .12 | 0.65 (0.40-1.08) P = .09 |
| Blood log copy level | 1.46 (1.09-1.97) P = .01 | 1.57 (1.12-2.22) P = .007 |
| inv(16) | ||
| Marrow log reduction | 0.33 (0.16-0.68) P = .003 | 0.30 (0.12-0.77) P = .02 |
| Marrow log copy level | 2.07 (1.20-3.58) P = .009 | 1.78 (0.98-3.21) P = .06 |
| Blood log reduction | 0.40 (0.21-0.76) P = .005 | 0.32 (0.15-0.71) P = .002 |
| Blood log copy level | 2.16 (1.39-3.34) P = .0006 | 2.37 (1.46-3.85) P = .0002 |
| Measure . | Unadjusted HR per log decrease . | Adjusted HR per log decrease . |
|---|---|---|
| t(8;21) | ||
| Marrow log reduction | 0.36 (0.18-0.74) P = .005 | 0.33 (0.15-0.73) P = .004 |
| Marrow log copy level | 2.27 (1.43-3.58) P = .0005 | 2.84 (1.61-5.00) P = .0002 |
| Blood log reduction | 0.70 (0.45-1.09) P = .12 | 0.65 (0.40-1.08) P = .09 |
| Blood log copy level | 1.46 (1.09-1.97) P = .01 | 1.57 (1.12-2.22) P = .007 |
| inv(16) | ||
| Marrow log reduction | 0.33 (0.16-0.68) P = .003 | 0.30 (0.12-0.77) P = .02 |
| Marrow log copy level | 2.07 (1.20-3.58) P = .009 | 1.78 (0.98-3.21) P = .06 |
| Blood log reduction | 0.40 (0.21-0.76) P = .005 | 0.32 (0.15-0.71) P = .002 |
| Blood log copy level | 2.16 (1.39-3.34) P = .0006 | 2.37 (1.46-3.85) P = .0002 |
Outcomes of log reduction in BM at remission in t(8;21) patients.P values are by log-rank test. (A) Cumulative incidence of relapse (CIR) by log reduction. (B) Survival from CR by log reduction.
Outcomes of log reduction in BM at remission in t(8;21) patients.P values are by log-rank test. (A) Cumulative incidence of relapse (CIR) by log reduction. (B) Survival from CR by log reduction.
inv(16).
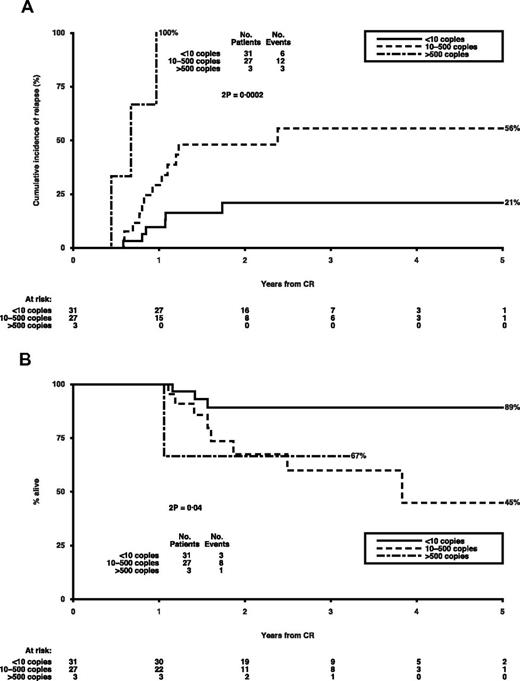
Similar analyses were carried out for patients with inv(16) at remission after course 1; the unadjusted and adjusted HR per log decrease are shown in Table 2. There was no critical BM log reduction level with respect to relapse risk, whereas other significant variables included BM copy numbers (> 100), PB log reduction (< 1 vs ≥ 1), and PB transcript levels. Of these, the most prognostic was the PB copy numbers, with a level of < 10 copies being associated with a CIR of 21% (Figure 2A), in the 51% of patients achieving it, representing 29% of all relapsing patients. Moreover, higher PB copy numbers had a significant adverse impact on survival after CR (adjusted HR per log reduction = 1.75; 1.04-2.94; P = .02; Figure 2B).
Outcomes by CBFB-MYH11 copy numbers in PB at remission in inv(16) patients.P values are by log-rank test. (A) Cumulative incidence of relapse by copy numbers. (B) Survival from CR by copy numbers
Outcomes by CBFB-MYH11 copy numbers in PB at remission in inv(16) patients.P values are by log-rank test. (A) Cumulative incidence of relapse by copy numbers. (B) Survival from CR by copy numbers
Impact of log reduction and log copy numbers after courses 2 and 3
Applying the same methodology as used for course 1, there was no additional prognostic value of BM and PB log reduction or log copy numbers after course 2 in both groups of CBF AML than that already established after course 1.
t(8;21).
After course 3, the 2 most prognostic factors for relapse risk were 4 log reduction in BM (< 4 log reduction, n = 20; CIR 49% vs > 4 log reduction, n = 18; CIR 13%; adjusted HR = 0.10; 0.01-0.83; P = .01) and BM copy number (copies > 500, n = 3; CIR 100% vs copies < 500, n = 55; CIR 28%; adjusted HR = 22.15; 3.75-130.76; P < .0001).
inv(16).
The most prognostic factor after course 3 for relapse risk was a PB copy number of 10 (copies < 10, n = 36; CIR 36% vs copies > 10, n = 9; CIR 78%, adjusted HR = 3.07; 1.07-8.80; P = .03).
Impact of GO on MRD at remission and consolidation
The effect of GO, which we previously reported to be beneficial in the CBF subset,29 on transcript levels after course 1 is shown in Table 3. Of 58 patients with t(8;21) log reduction data, 29 entered the GO induction randomization. There was some evidence that there were greater log reductions in transcripts in patients given GO (P = .04 for trend), but none for the 22 of 61 inv(16) patients entering GO randomization. After course 3, there was no difference in molecular response with respect to GO randomization in both subtypes (data not shown). When the effect of GO on relapse and survival was considered, the effect in this set of patients was consistent with the figures seen overall in the CBF leukemias.29
Effect of GO on transcript levels in CBF AML patients after course 1 of treatment
| . | GO . | No GO . | P . |
|---|---|---|---|
| t(8;21) < 1 log reduction | 0 | 1 | .04 |
| t(8;21) 1-3 log reduction | 5 | 10 | |
| t(8;21) > 3 log reduction | 9 | 4 | |
| inv(16) < 10 copies | 6 | 5 | 1.0 |
| inv(16) 10-500 copies | 3 | 5 | |
| inv(16) > 500 copies | 2 | 1 |
| . | GO . | No GO . | P . |
|---|---|---|---|
| t(8;21) < 1 log reduction | 0 | 1 | .04 |
| t(8;21) 1-3 log reduction | 5 | 10 | |
| t(8;21) > 3 log reduction | 9 | 4 | |
| inv(16) < 10 copies | 6 | 5 | 1.0 |
| inv(16) 10-500 copies | 3 | 5 | |
| inv(16) > 500 copies | 2 | 1 |
Impact of chemotherapy regimens on MRD
The numbers were small for a reliable comparison of chemotherapy regimens: FLAG-Ida appeared to provide a greater log reduction than DA or ADE after course 1 (data not shown), but this difference did not translate into significantly improved overall survival for FLAG-Ida patients.
Impact of BM and PB copy numbers during follow-up
The number of observations after course 4 was quite small (total of 82), and these data were therefore combined with those obtained during the follow-up period, defined as > 4 weeks after completion of course 4. Serial monitoring in BM and PB was carried out until relapse or closure of the study. A key aim of serial monitoring during remission was to establish critical MRD thresholds in BM and PB to predict relapse. Relapse risk was assessed at different BM and PB cut-off transcript levels by Mantel-Byar analysis in both groups of CBF AML patients.
t(8;21).
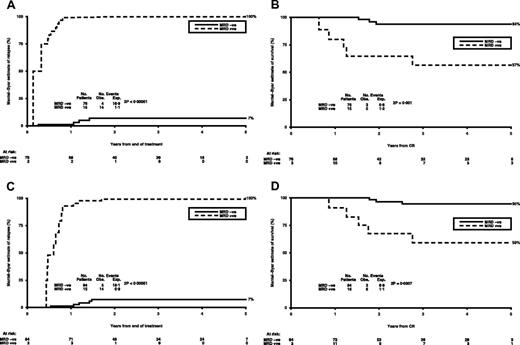
Mantel-Byar analysis of relapse after treatment identified that positivity at a rate of > 500 RUNX1-RUNX1T1 copies was highly prognostic, identifying 15 of 78 patients with a high risk of relapse (Mantel-Byar estimates of relapse 100% vs 7%, P < .0001; Figure 3A). There was a significant difference in survival for these patients, with Mantel-Byar estimates of survival at 5 years of 94% vs 57% (HR = 40.18; 5.80-278.3; P = .001; Figure 3B). For PB, copy numbers of > 100 identified 15 patients of 86 with a Mantel-Byar estimate of relapse of 100%, compared with 7% in the MRD-negative group (P < .0001; Figure 3C) translating into survival from relapse of 59% vs 95% (P = .0007; Figure 3D). Of 127 remission patients, 11 had persistent MRD levels < 500 copies in the BM (ie, PCR positive), of whom 2 also had intermittent transcript levels < 50 copies in the PB; these patients have stayed in clinical remission with a median follow-up of 4.5 years (range, 2-8 years). Thus, in a small number of patients with t(8;21), low levels of MRD, as specified in this study, which did not increase on serial monitoring, were consistent with durable clinical remission.
Sequential MRD monitoring during follow-up in t(8;21) patients. (A) Mantel-Byar estimate of relapse (%) in patients with > 500 RUNX1-RUNXITI copies in BM. (B) Mantel-Byar estimate of survival (%) from CR in patients with > 500 RUNX1-RUNXITI copies in BM. (C) Mantel-Byar estimate of relapse (%) in patients with > 100 RUNX1-RUNXITI copies in PB. (D) Mantel-Byar estimate of survival (%) from CR in patients with > 100 RUNX1-RUNXITI copies in PB.
Sequential MRD monitoring during follow-up in t(8;21) patients. (A) Mantel-Byar estimate of relapse (%) in patients with > 500 RUNX1-RUNXITI copies in BM. (B) Mantel-Byar estimate of survival (%) from CR in patients with > 500 RUNX1-RUNXITI copies in BM. (C) Mantel-Byar estimate of relapse (%) in patients with > 100 RUNX1-RUNXITI copies in PB. (D) Mantel-Byar estimate of survival (%) from CR in patients with > 100 RUNX1-RUNXITI copies in PB.
inv(16).
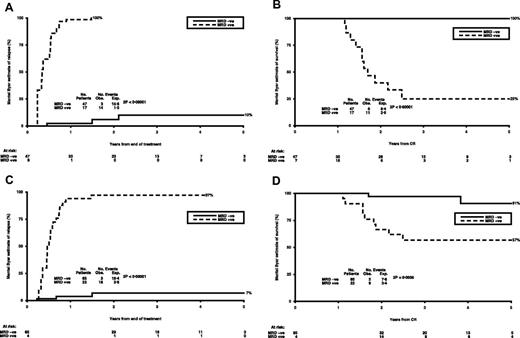
Patients with > 10 CBFB-MYH11 copies in the BM had a Mantel-Byar estimate of relapse of 90%, which increased to 100% in the 17 of 53 patients who had copies of > 50, compared with 10% in patients with fewer than 50 copies (P < .0001; Figure 4A). Survival from remission was 100% in those patients (n = 47) who did not reach the positive threshold (50 copies) compared with only 25% in patients (n = 17) who were positive (P < .0001; Figure 4B). Similarly, the 22 patients with > 10 copies in the PB had a Mantel-Byar estimate of relapse of 97%, compared with a relapse risk of 7% in those with < 10 copies (P < .0001; Figure 4C). This translated into survival from remission of 57% versus 91% (P = .0005; Figure 4D). Thus, in PB, any positive MRD level > 10 CBFB-MYH11 copies was associated with clinical relapse.
Sequential MRD monitoring during follow-up in inv(16) patients. (A) Mantel-Byar estimate of relapse (%) in patients with > 50 CBFB-MYH11 copies in BM. (B) Mantel-Byar estimate of survival (%) from CR in patients with > 50 CBFB- MYH11 copies in BM. (C) Mantel-Byar estimate of relapse (%) in patients with > 10 CBFB- MYH11 copies in PB. (D) Mantel-Byar estimate of survival (%) from CR in patients with > 10 CBFB- MYH11 copies in PB.
Sequential MRD monitoring during follow-up in inv(16) patients. (A) Mantel-Byar estimate of relapse (%) in patients with > 50 CBFB-MYH11 copies in BM. (B) Mantel-Byar estimate of survival (%) from CR in patients with > 50 CBFB- MYH11 copies in BM. (C) Mantel-Byar estimate of relapse (%) in patients with > 10 CBFB- MYH11 copies in PB. (D) Mantel-Byar estimate of survival (%) from CR in patients with > 10 CBFB- MYH11 copies in PB.
The evaluation of sequential quantitative RT-PCR monitoring to predict relapse
The predictive value of sequential monitoring by quantitative RT-PCR in BM or PB could be demonstrated in 11 t(8;21) and 18 inv(16) patients who had rising MRD levels culminating in clinical relapse. The median times of detectable PCR positivity to hematologic relapse in BM and PB in t(8;21) patients were 4.9 months (range, 0.3-11 months) and 4.5 months (range, 2.3-7.3 months), respectively. Corresponding values for inv(16) patients were 3 months for both BM and PB (BM range, 1.3-9.6 months; PB range, 1.1-5.8 months). In the remaining 42 relapsed patients, inability to predict impending relapse was mostly the result of a lack of scheduled serial samples, as the intervals between BM or PB sampling for MRD analysis were > 3 months.
Kinetics of relapse
The kinetics of leukemia relapse in BM and PB in patients in whom serial monitoring data were available are shown in Figure 5. Relapse kinetics varied between both patient and disease types. In t(8;21) patients, the median increments in RUNX1-RUNX1T1 transcripts in BM and PB were 0.5 log/m (r = 0.2-1.5) and 0.6 log/m (r = 0.3-1.5), respectively, whereas in inv(16) patients, the corresponding median increment in CBFB-MYH11 transcripts was 0.5 log/m in both BM and PB (BM: r = 0.1-1.7; PB: r = 0.0-2.5).
Kinetics of relapse. Graphs represent the rate of rise of normalized RUNX1-RUNXITI and CBFB-MYH11 transcript levels in serial samples before hematologic relapse. (A) t(8;21) BM. (B) t(8;21) PB. (C) inv(16) BM. (D) inv(16) PB.
Kinetics of relapse. Graphs represent the rate of rise of normalized RUNX1-RUNXITI and CBFB-MYH11 transcript levels in serial samples before hematologic relapse. (A) t(8;21) BM. (B) t(8;21) PB. (C) inv(16) BM. (D) inv(16) PB.
Paired BM and PB MRD analysis
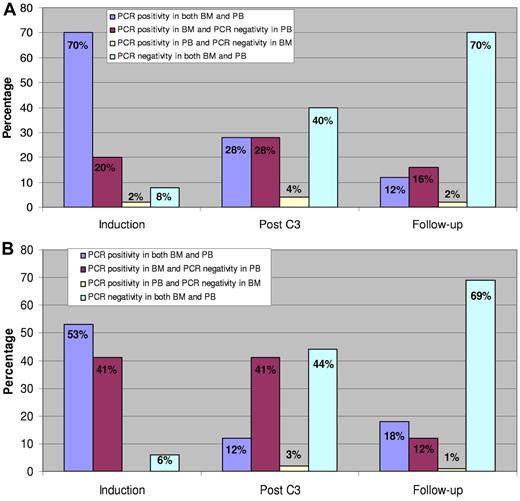
To assess whether PB could be used for MRD monitoring, we analyzed paired BM and PB samples collected after induction (course 1), during consolidation (course 3), and during follow-up. For t(8;21) patients, there were 394 paired samples (81 after C1, 53 after C3, and 260 at follow-up) and for inv(16) patients, 369 paired samples (51 after C1, 61 after C3, and 257 at follow-up). With respect to RUNX1-RUNX1T1and CBFB-MYH11 transcripts, the pairs were categorized into 4 groups as follows: PCR positivity in both BM and PB, PCR positive in BM and negative in PB, PCR positive in PB and negative in BM, and PCR negative in both BM and PB. The distribution in percentages among the groups at the various time points is shown in Figure 6. In both CBF AML subtypes, differences in transcript copies between BM and PB (ie, BM-positive and PB-negative) were larger during treatment and smaller during follow-up. The percentages of paired samples during follow-up belonging to the group with PCR positive in BM and PCR negative in the corresponding PB were 16% for t(8;21) and 12% for inv(16) patients, respectively. Conversely, there were very few samples that were PCR-positive in PB and PCR-negative in BM (2% in t(8;21) and 1% in inv(16) patients).
Quantitative RT-PCR analysis in paired BM and PB samples after induction, during consolidation, and at follow-up. Corresponding pairs were categorized according to their quantitative RT-PCR values into 4 groups. The distribution in percentages among the groups at the 3 time points is illustrated by the columns. (A) t(8;21). (B) inv(16).
Quantitative RT-PCR analysis in paired BM and PB samples after induction, during consolidation, and at follow-up. Corresponding pairs were categorized according to their quantitative RT-PCR values into 4 groups. The distribution in percentages among the groups at the 3 time points is illustrated by the columns. (A) t(8;21). (B) inv(16).
We also compared transcript levels in paired BM and PB samples at diagnosis, after induction (C1), during consolidation (C3) and at first PCR positivity during follow-up. A significant correlation between BM and PB MRD levels at these time points was found in both groups of patients, using both Spearman and Pearson analysis (Table 4).
Correlation coefficients between paired BM and PB transcript copies at specific time points
| . | Diagnosis . | After 1 course . | After 3 courses . | After 4 courses and follow-up . |
|---|---|---|---|---|
| t(8;21) | 0.65 | 0.79 | 0.42 | 0.92 |
| Spearman | P < .0001 | P < .0001 | P = .005 | P < .0001 |
| Log [t(8;21)] | 0.64 | 0.78 | 0.50 | 0.74 |
| Pearson | P < .0001 | P < .0001 | P = .0005 | P = .009 |
| Inv(16) | 0.40 | 0.45 | 0.33 | 0.95 |
| Spearman | P = .01 | P = .007 | P = .05 | P < .0001 |
| Log [inv(16)] | 0.17 | 0.49 | 0.44 | 0.84 |
| Pearson | P = .3 | P = .0002 | P = .01 | P < .0001 |
| . | Diagnosis . | After 1 course . | After 3 courses . | After 4 courses and follow-up . |
|---|---|---|---|---|
| t(8;21) | 0.65 | 0.79 | 0.42 | 0.92 |
| Spearman | P < .0001 | P < .0001 | P = .005 | P < .0001 |
| Log [t(8;21)] | 0.64 | 0.78 | 0.50 | 0.74 |
| Pearson | P < .0001 | P < .0001 | P = .0005 | P = .009 |
| Inv(16) | 0.40 | 0.45 | 0.33 | 0.95 |
| Spearman | P = .01 | P = .007 | P = .05 | P < .0001 |
| Log [inv(16)] | 0.17 | 0.49 | 0.44 | 0.84 |
| Pearson | P = .3 | P = .0002 | P = .01 | P < .0001 |
Discussion
The aim of MRD monitoring in leukemias is to assess the effectiveness of treatment and to identify patients who are at high risk of relapse from those who are potentially cured. Specific gene targets can be accurately and reliably quantified using quantitative RT-PCR, thus facilitating the prospective clinical evaluation of MRD monitoring. However, in CBF AML, studies using quantitative RT-PCR techniques have been limited by relatively small numbers of patients and a lack of MRD data at specific time points during and after chemotherapy, especially in patients with t(8;21). Our MRD study in 163 t(8;21) and 115 inv(16) patients from a relatively young age group (median age, 42 years) represents, thus far, the largest cohort of CBF AML patients treated uniformly on the same chemotherapy regimens in the MRC AML-15 trial.
Several studies have shown that using nested RT-PCR in patients with t(8;21), RUNX1-RUNX1T1 transcripts could be detected after chemotherapy, autologous and allogeneic bone marrow transplantation in many patients in long-term remission.34,35 The phenomenon has been ascribed to quiescent populations of stem cells, monocytes and B cells harboring the fusion gene.36 Similarly, in AML with inv(16), MRD studies using nested RT-PCR assays have reported persistence of residual disease in some long-term remitters, although most patients in prolonged remission remain PCR-negative.37-39 Consequently, quantitative RT-PCR methods were developed to quantify RUNX1-RUNX1T1 transcripts during remission in patients with t(8;21).20,21
Using a sensitive quantitative RT-PCR assay, we looked at relapse risk, with respect to both the reduction in leukemia load from diagnosis and the absolute residual disease levels, as measured by the RUNX1-RUNX1T1and CBFB-MYH11 transcript copies, on achieving remission after course 1 induction chemotherapy. In t(8;21) patients, we showed that BM log reduction and BM (> 500 copies) and PB (> 1000 copies) were predictive of relapse risk, with a > 3 log reduction in bone marrow being the most prognostic variable on multivariate analysis. In inv(16) patients, no BM log reduction threshold for relapse risk could be established, but BM (> 100) and PB (> 10) copy numbers were predictive of which PB transcripts level was the most important prognostic variable. Assessment of MRD after course 3 (consolidation) was also shown to be informative. In t(8;21) patients, achievement of at least a 4 log reduction in fusion transcripts in the BM was associated with a CIR of only 10%, whereas conversely detection of MRD exceeding 500 RUNX1-RUNX1T1 transcripts invariably predicted relapse. In the inv(16) group, PB MRD was most prognostic, with a CBFB-MYH11 copy number > 10 being associated with a CIR of 78%.
We showed that, after consolidation chemotherapy and during remission follow-up, BM and PB transcript levels were highly predictive of relapse risk. In the t(8;21) group, the Mantel-Byar estimates of relapse in patients with a BM RUNXI-RUNXITI copy number > 500 (15 of 91 patients) or a PB copy number > 100 (15 of 99) were both 100%. Similarly, in inv(16), the Mantel-Byar estimate of relapse in patients with BM CBFB-MYH11 transcript copies > 50 (16 of 77 patients) was 100%, whereas that in patients with PB copies > 10 (22 of 87) was 97%. Five-year survival in the MRD-positive patients in both t(8;21) and inv(16), as defined by transcript copy numbers, was significantly worse, compared with that in MRD-negative patients. However, it is worth noting that salvage rates in the relapsed t(8;21) patients were nearly 60%. In addition to demonstrating that critical MRD thresholds in both BM and PB could be established, above which relapse appears inevitable, we also showed that rising MRD levels on sequential monitoring during follow-up, which were not associated with BM or PB changes, accurately predicted hematologic relapse. It is also worth noting that in t(8;21) patients, low levels of MRD in both BM and PB were compatible with durable remission, consistent with the results previously described using qualitative RT-PCR. In contrast to inv(16) patients, molecular MRD negativity is not a prerequisite for long term remission in t(8;21) patients.
One of the aims of the AML-15 trial was to assess the effect of GO in AML patients, and we have previously reported that the addition of GO significantly improved the survival of CBF AML patients.29 We therefore looked at MRD levels after courses 1 and 3 in these patients; there was some evidence that GO reduces molecular positivity after course 1 in t(8;21) but none for inv(16) patients. However, these results need to be interpreted with caution, as the number of evaluable patients is quite small. There was no difference in molecular responses after course 3 with respect to GO randomization. Marked molecular responses in CBF AML patients treated with GO and chemotherapy have been reported previously.40 The results in our set of patients are in keeping with those seen overall in CBF AML.29
Several studies have evaluated the prognostic value of MRD monitoring by quantitative RT-PCR in t(8;21) patients undergoing chemotherapy.24-28 In a study of 21 patients, Leroy et al reported that significant predictors of relapse were a > 3 log reduction in the BM and absolute transcript levels after induction and also high MRD levels after consolidation therapy.25 Similarly Weisser et al studied 45 patients and showed that the quality of molecular response after both induction and consolidation chemotherapy was predictive of relapse risk, RFS, and OS.28 In our study of 163 patients, we were able to confirm the prognostic value of BM log reduction and absolute MRD levels after both induction and consolidation chemotherapy. However, in our patients, although a > 3 log reduction after induction was prognostically important on multivariate analysis and predicted a relapse risk of only 4% compared with more than 30% for those who did not reach this threshold (HR per log reduction = 0.33; 0.15-0.73; P = .004), it did not significantly impact on survival after CR, with a 5-year survival of 79%, which is no better than in the group with a 1-3 log reduction. This observation, together with 5-year survival rates of nearly 60% in relapsed t(8;21) patients, can be explained by the fact that patients with CBF AML can be effectively salvaged after relapse.41
Similarly, the prognostic value of MRD monitoring by quantitative RT-PCR in inv(16) patients has been described in several studies.22,23 Cut-off CBFB-MYH11 copy numbers of 10-12 after completion of chemotherapy have been identified that could separate patients with high and low risk of relapse.27,42,43 More recently, Corbacioglu et al, in a study of 53 CBFB-MYH11-positive AML patients, have reported clinically relevant MRD checkpoints during consolidation chemotherapy and early follow-up that allowed the identification of patients with high risk of relapse and also the negative impact of high MRD levels on RFS and OS.18 They also showed that transcript levels below a threshold of 10 copies (normalized to β2-microglobulin × 106) during follow-up were a prerequisite for long-term remission. In our study of 115 patients with inv(16), we were able to confirm that MRD levels based on CBFB-MYH11 copy numbers after induction, during consolidation, and after chemotherapy were highly predictive of relapse risk. We also showed that a high MRD level in PB after induction adversely affected survival after CR.
One important aspect of this study was to assess the suitability of PB sampling compared with BM for MRD monitoring. Data on the applicability of PB for MRD assessment in CBF AML are sparse. In a small number of t(8;21) patients studied, a strong correlation between paired BM and PB MRD levels has been reported.25 In inv(16) patients, a study by Boeckx et al on 54 paired BM/PB follow-up samples showed no clear correlation between BM and PB MRD levels, and these were generally higher in BM than in PB.44 In contrast, Corbacioglu et al analyzed 198 paired BM and PB samples during chemotherapy and follow-up and found that 90% of the paired samples during follow-up showed either comparable or identical negative MRD levels.18 In only 11% of samples, the PCR was positive in the BM, with a negative result in the corresponding PB. In our study, we analyzed 394 paired BM and PB samples in t(8;21) patients and 369 paired samples in inv(16) patients. In both groups of patients, differences in transcript copies between BM and PB (BM PCR-positive, PB PCR-negative group) were larger during treatment and smaller during follow-up, together with an increasing number of paired BM and PB samples with a negative PCR during follow-up. The percentage of paired samples with a positive PCR in the BM and negative PCR in the PB during follow-up in t(8;21) patients was 16%. In the corresponding group in inv(16) patients, the percentage was 12%. This result is very similar to that reported by Corbacioglu et al.18 Interestingly, there were very few paired samples during follow-up that were PCR positive in PB and negative in BM (2% in t(8;21) and 1% in inv(16) patients). In addition, we showed that there was a significant correlation between BM and PB transcript levels at diagnosis, during treatment, and follow-up in both groups of CBF AML patients.
Information on the optimal schedules for MRD monitoring in CBF AML, including sampling source, is scanty, and published data are somewhat conflicting. The relapse kinetics in the 4 common AML subtypes with molecular targets (NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11) have all been shown to be strikingly different.11,18,19,26,45,46 A study by Ommen et al applying a mathematical model to investigate optimal MRD monitoring by quantitative RT-PCR in CBF AML highlighted that CBFB-MYH11–positive AML relapses relatively more slowly than AML with RUNX1-RUNX1T1.46 The authors proposed that, in inv(16) patients, PB monitoring every 6 months would be sufficient to predict relapse, whereas for t(8;21) patients, BM sampling every 4 months would be required. In contrast, the recent study in CBFB-MYH11–positive AML by Corbacioglu et al showed that the kinetics of relapse are far more rapid than previously described, and our results accord with theirs.18 We show that in inv(16) patients the kinetics of relapse in BM and PB were quite rapid with a median time from molecular positivity to clinical relapse of 3 months, whereas for t(8;21) patients, the median times to hematologic relapse were slightly longer (PB 4.5 months, BM 4.9 months) than in inv(16). Furthermore, in both t(8;21) and inv(16) patients, BM and PB were comparable for MRD detection during follow-up. We propose that optimal schedules for MRD monitoring in CBF AML should include assessment after induction, during consolidation, and at 3 monthly intervals in BM and/or PB, at least during the first 18 months of follow-up. Our data show that, during follow-up, MRD monitoring should ideally be carried out in paired BM and PB samples, to avoid missing a positive PCR result in BM with a negative PCR in the corresponding PB, in ∼ 10%-15% of patients. However, in the majority of patients, PB sampling was equally informative for MRD monitoring and can therefore be used as a suitable alternative to BM.
In conclusion, MRD monitoring by quantitative RT-PCR in CBF AML allows risk stratification based on treatment responses after induction and consolidation chemotherapy, and sequential monitoring during follow-up can accurately predict relapse, thus opening the way to risk-directed therapy. Furthermore, we were able to establish MRD threshold levels in both BM and PB during follow-up, which were highly predictive of clinical relapse. In patients who have molecular relapse or rising MRD levels on serial monitoring, there may be a window of opportunity of ∼ 3-4 months, which may allow for preemptive treatment, including investigational agents and potential allografting. Furthermore, patients who are KIT mutation positive may be candidates for a trial of tyrosine kinase inhibitors,47 which, if successful, can also serve as a useful bridge to transplantation. Although monitoring of MRD in CBF AML may allow specific treatment to be tailored to individual patients, it is questionable whether a preemptive approach would be beneficial, given the rapidity of clinical relapse and the good responses achievable with salvage therapy. Thus, the role of early therapeutic intervention based on MRD detection to improve outcome in AML subtypes, other than APL, remains to be established in future clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all consultant hematologists who participated in the AML 15 trial MRD study for providing diagnostic and follow-up samples and Dr David Grimwade for critical review of the manuscript.
This work was supported by the United Kingdom Leukemia and Lymphoma Research.
Authorship
Contribution: J.A.L.Y., R.K.H., and A.K.B. initiated and designed the study, analyzed the data, and wrote the manuscript; M.A.O. and S.B.D. collected and analyzed the samples; K.W. designed the study; and all authors initially reviewed the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John A. Liu Yin, Department of Haematology, Manchester Royal Infirmary, Oxford Road, Manchester, M13 9WL, United Kingdom; e-mail: john.yin@cmft.nhs.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal