Abstract
RUNX1 gene alterations are associated with acquired and inherited hematologic malignancies that include familial platelet disorder/acute myeloid leukemia, primary or secondary acute myeloid leukemia, and chronic myelomonocytic leukemia. Recently, we reported that RUNX1-mediated silencing of nonmuscle myosin heavy chain IIB (MYH10) was required for megakaryocyte ploidization and maturation. Here we demonstrate that runx1 deletion in mice induces the persistence of MYH10 in platelets, and a similar persistence was observed in platelets of patients with constitutional (familial platelet disorder/acute myeloid leukemia) or acquired (chronic myelomonocytic leukemia) RUNX1 mutations. MYH10 was also detected in platelets of patients with the Paris-Trousseau syndrome, a thrombocytopenia related to the deletion of the transcription factor FLI1 that forms a complex with RUNX1 to regulate megakaryopoiesis, whereas MYH10 persistence was not observed in other inherited forms of thrombocytopenia. We propose MYH10 detection as a new and simple tool to identify inherited platelet disorders and myeloid neoplasms with abnormalities in RUNX1 and its associated proteins.
Introduction
Alterations in RUNX1 are associated with several acquired and inherited hematologic malignancies that include familial platelet disorder with propensity to develop acute myeloid leukemia (FPD/AML; OMIM 601399), primary or secondary acute myeloid leukemia (AML), and chronic myelomonocytic leukemia (CMML). FPD/AML is an autosomal dominant disorder characterized by a mild to moderate thrombocytopenia, a normal platelet size and morphology, an abnormal platelet aggregation, and heterozygous germline mutations or deletions in RUNX1.1 Acquired RUNX1 mutations have been detected in 15% to 30% of CMML2-4 and 12% of AML. The frequency of RUNX1 alterations is underestimated for at least 2 reasons. First, intragenic deletions, demonstrated to be causal in some FPD/AML pedigrees, are not identified by common gene sequencing that is limited to coding exons. Second, in most FPD/AML pedigrees, gene sequencing is performed only when an AML/MDS occurrence is observed, which prevents identification of most FPD/AML pedigrees with thrombocytopenia alone. The improved detection of RUNX1 alterations is necessary for better molecular characterization of AML and CMML, and also for earlier diagnosis of FPD/AML.
We recently observed that the expression of nonmuscle myosin heavy chain IIB (MYH10) was almost completely silenced during the ploidization process and terminal maturation of megakaryocytes because of RUNX1-mediated negative regulation of the MYH10 gene. RUNX1 knockdown in human/mice was associated with a decreased ploidy of megakaryocytes and an increased expression of MYH10, whereas other myosins were rather down-regulated.5 We show here that the biochemical detection of MYH10 in platelets can be used as a marker to screen for RUNX1 gene inactivation in inherited as well as acquired myeloid diseases.
Methods
Patients
The study was approved by the Local Research Ethics Committee from Assistance Publique–Hôpitaux de Paris and Institut National de la Santé et de la Recherche Médicale. Blood samples from patients and healthy subjects were collected with informed consent in accordance with the Declaration of Helsinki.
Samples
Granulocytes, red blood cells (RBCs), and mononuclear cells were separated by standard techniques. CD14 (monocytes), CD3 (T lymphocytes), and CD19 (B lymphocytes) cells were separated by double-positive selection using a magnetic cell-sorting system (AutoMACS; Miltenyi Biotec). The platelet-rich plasma was prepared by centrifugation at 170g for 10 minutes. Platelets were pelleted by centrifugation at 2100g for 10 minutes. Remaining RBCs (GPA+) were depleted using immunomagnetic beads (Miltenyi Biotec).
Immunoblotting
A total of 30 μg of protein was resolved by SDS-PAGE and transferred to nitrocellulose membranes. Blots were incubated with a rabbit anti-MYH10 antibody (Cell Signaling) and reprobed with an antibody against HSC70 (Sigma-Aldrich) followed by HRP-linked secondary antibodies and, respectively, Super Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific) for MYH10 detection and Immobilon Western Chemiluminescent HRP Substrate (Millipore Corporation) for HSC70 detection.
Sequence and CGH array analysis
Gene sequencing was performed using BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) and analyzed on the Applied Biosystems 3130xl Genetic Analyzer. The genomic profile was assessed by comparative genomic hybridization (CGH) arrays (Agilent AMADID 021850). All data and protocols have been submitted to ArrayExpress at the European Bioinformatics Institute 8 with the accession number E-MTAB-1121.
Results and discussion
RUNX1-mediated silencing of MYH10 during normal megakaryopoiesis5 suggested that, contrary to normal platelets, MYH10 should be expressed in platelets of runx1 KO mice as well as in platelets of patients with RUNX1 gene alterations. Therefore, we induced runx1 knockout in runx1f/f/(Mxe−Cre+) mice by pIpC injections.5 As expected, contrary to control mice (runx1f/f/(Mxe−Cre−)), MYH10 protein was detected in the platelets of KO (runx1f/f/(Mxe−Cre+)) animals (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
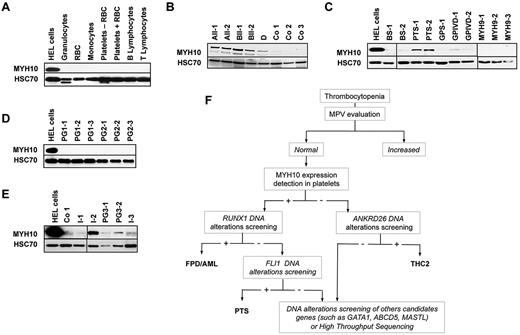
MYH10 expression was then studied in human platelets from patients and healthy subjects. Immunoblotting experiments failed to detect any MYH10 protein in healthy donor platelets. We also tested the expression of MYH10 in other blood cells as platelets can be contaminated on purification, especially in pathologic samples. MYH10 protein was not detected in RBCs, granulocytes, monocytes, and T and B cells sorted from healthy donor peripheral blood (Figure 1A). In contrast, MYH10 expression was easily detected in platelets of 5 patients from 3 FPD/AML pedigrees (A, B, and D; supplemental Table 1) compared with 3 control samples (Co1-3; Figure 1B). MYH10 was easily detected in a dividing immature megakaryocytic/erythroid cell line, HEL, used as a positive control.
MYH10 detection in platelets from patients with inherited thrombocytopenia. (A-E) Immunoblot analysis of MHYH10 expression. In all panels, HEL cells were used as positive controls and HSC70 as loading control. (A) Indicated peripheral blood cell populations from a healthy donor. Platelets − RBC indicates platelets after depletion of RBCs; and Platelets + RBC, platelets without RBC depletion. One representative of 2 experiments is shown. (B) Platelets of FPD/AML patients (AII-1 and AII-2 with R174Q RUNX1 mutation; BII-2 and BIII-1 with R139X RUNX1 mutation; and D with RUNX1 monoallelic deletion) and healthy donors as controls (Co1-3). (C) Platelets of patients with well-identified inherited thrombocytopenia. BS indicates Bernard-Soulier syndrome; GPS, Gray platelet syndrome; GPIVD, thrombocytopenia associated with GPIV defect; and MYH9, thrombocytopenia associated with MYH9 mutations. (D) Platelets of patients from 2 pedigrees (PG1 and PG2, 3 patients in each) with unexplained thrombocytopenia with increased mean platelet volume. (E) Platelets of patients with unexplained thrombocytopenia with normal mean platelet volume. Two patients from the same pedigree are included (PG3). (F) Flowchart of familial thrombocytopenia diagnosis based on MYH10 detection in platelets.
MYH10 detection in platelets from patients with inherited thrombocytopenia. (A-E) Immunoblot analysis of MHYH10 expression. In all panels, HEL cells were used as positive controls and HSC70 as loading control. (A) Indicated peripheral blood cell populations from a healthy donor. Platelets − RBC indicates platelets after depletion of RBCs; and Platelets + RBC, platelets without RBC depletion. One representative of 2 experiments is shown. (B) Platelets of FPD/AML patients (AII-1 and AII-2 with R174Q RUNX1 mutation; BII-2 and BIII-1 with R139X RUNX1 mutation; and D with RUNX1 monoallelic deletion) and healthy donors as controls (Co1-3). (C) Platelets of patients with well-identified inherited thrombocytopenia. BS indicates Bernard-Soulier syndrome; GPS, Gray platelet syndrome; GPIVD, thrombocytopenia associated with GPIV defect; and MYH9, thrombocytopenia associated with MYH9 mutations. (D) Platelets of patients from 2 pedigrees (PG1 and PG2, 3 patients in each) with unexplained thrombocytopenia with increased mean platelet volume. (E) Platelets of patients with unexplained thrombocytopenia with normal mean platelet volume. Two patients from the same pedigree are included (PG3). (F) Flowchart of familial thrombocytopenia diagnosis based on MYH10 detection in platelets.
To determine whether MYH10 strong expression in platelets was specific for FPD/AML, we collected the platelets of 8 patients with well-identified inherited platelet disorders (supplemental Table 1), including Bernard-Soulier syndrome, Paris-Trousseau syndrome (PTS),6 Gray platelet syndrome,7 mild thrombocytopenia associated with a GPIV (CD36) defect, and MYH9 syndrome (Figure 1C; supplemental Table 1). In these series, MYH10 was detected exclusively in platelets from patients with a PTS, which is characterized by FLI1 haploinsufficiency. Interestingly, FLI1 and RUNX1 transcription factors bind distinct regions on the MYH10 promoter and cooperate during megakaryopoiesis as components of a large transcriptional complex.8,9
We also collected the platelets of 11 patients with unexplained inherited thrombocytopenia (supplemental Table 1). Of 6 who showed an increase in their mean platelet volume, none expressed MYH10 (Figure 1D) and none presented RUNX1 mutations. The 5 other patients, including 3 isolated cases (I-1, I-2, and I-3) and 2 cases from the same pedigree (PG3-1 and PG3-2), had a normal mean platelet volume. MYH10 was detected in the platelets of only one of these 5 patients (I-2; Figure 1E) who was the only one in which RUNX1 gene sequencing identified a mutation (R174Q). In 3 of the 4 other patients, we identified a mutation in ANKRD26 gene, which has also been associated with inherited thrombocytopenia with potential predisposition to acute leukemia (THC2)10 (Figure 1E, I-1, PGS-1, PGS-2; supplemental Table 1). We confirmed these results at the mRNA level using quantitative RT-PCR by showing that the MYH10 transcript was 4- to 7-fold higher in platelets from FPD/AML patients than from controls or TCH2 patients (supplemental Figure 2). According to these results, we established a diagnostic algorithm of unexplained thrombocytopenia (Figure 1F) based on the detection of MYH10 in platelets of thrombocytopenic patients with normal platelet volume, allowing to separate patients with mutations in RUNX1 repressor complex from those with mutations in ANKRD26 or other nonidentified genes. This first screening will drive to diagnose the main platelet pathologies: FPD/AML, PTS, and THC2. In a given pedigree with RUNX1 mutation, the test may be useful to detect rapidly the family members without thrombocytopenia but with the RUNX1 mutation and more importantly family members without RUNX1 mutations to select the best donor for bone marrow transplantation.
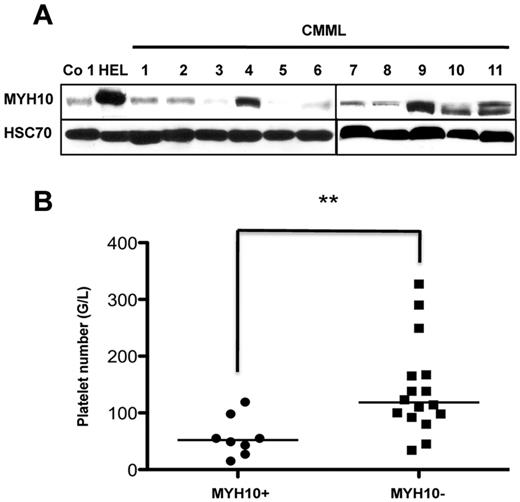
MYH10 protein expression was also examined in the platelets of 24 patients suspected of CMML. The firm diagnosis of CMML was made according to the WHO criteria only in 19 of them (supplemental Table 2). MYH10 protein expression was detected in the platelets of 8 patients, and RUNX1 mutations were detected in 5 of these 8 patients. All patients negative for platelet MYH10 were negative for RUNX1 mutation (Figure 2A; supplemental Table 2). Interestingly, the platelet number was lower in 8 CMML patients expressing MYH10 in their platelets compared with other patients (Figure 2B; supplemental Table 2). No significant correlation was found between the presence of MYH10 and the status of other genes frequently mutated in CMML, including TET2, ASXL1, KRAS, NRAS, CBL, and SRSF2 (supplemental Table 2). To investigate whether the patients expressing MYH10 in platelets without RUNX1 mutation harbor RUNX1 or FLI1 deletion, CGH arrays were performed in 2 of them (no. 15 and no. 16, supplemental Table 2). No deletion in these 2 transcription factors and in other hematopoietic regulators was found, suggesting either the presence of mutation(s) in nonexplored sequences of the gene, or mutation(s) in another member of the RUNX1 repressor complex, or in epigenetic regulators.11
MYH10 detection in platelets from CMML patients. (A) Immunoblot analysis of MYH10 expression in platelets from patients with CMML. HEL cells were used as positive controls, Co 1 platelets as negative control, and HSC70 as loading control. (B) Statistical evaluation of correlation between MYH10 presence in platelets of patients with CMML, RCMD, AML-M4, and reactive monocytosis and thrombopenia degree. MYH10+ indicates patients with the presence of MYH10 in platelets; and MYH10−, patients with the absence of MYH10 in platelets. **P < .007 (Mann-Whitney U test). Statistical analyses were carried out with Prism, Version 5.0 (Graphpad Software).
MYH10 detection in platelets from CMML patients. (A) Immunoblot analysis of MYH10 expression in platelets from patients with CMML. HEL cells were used as positive controls, Co 1 platelets as negative control, and HSC70 as loading control. (B) Statistical evaluation of correlation between MYH10 presence in platelets of patients with CMML, RCMD, AML-M4, and reactive monocytosis and thrombopenia degree. MYH10+ indicates patients with the presence of MYH10 in platelets; and MYH10−, patients with the absence of MYH10 in platelets. **P < .007 (Mann-Whitney U test). Statistical analyses were carried out with Prism, Version 5.0 (Graphpad Software).
All together, the detection of MYH10 expression in platelets indicates a defective silencing of MYH10 gene during the terminal differentiation of megakaryocytes because of a germline or a somatic alteration in either RUNX1 gene or a gene encoding a member of the repressor complex regulating MYH10 gene expression, such as FLI1. MYH10 protein detection in platelets may be used as a biomarker of these gene alterations (eg, as a first-line assay to screen for RUNX1 and FLI1 alterations in autosomal forms of inherited thrombocytopenia) as well as RUNX1 in acquired myeloid malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participation in this study; Dr A. Auvrignon, Pr P. Fenaux, Pr B. Quesnel, Pr F. Dreyfus, Dr C. Berthon, Dr A. Toma, Pr O. Beyne-Rauzy, Pr O. Hermine, Dr S. de Botton, and Pr M. Fontenay for patient samples; Dr D. G. Gilliland and Dr T. Mercher for kindly providing of runx1f/f/(Mxe−Cre+) mice; and Dr G. Meurice for analysis of CGH array data.
This work was supported by Agence Nationale de la Recherche (ANR-GIS Institut des maladies rares and ANR jeunes chercheurs). I.A.-D. was supported by a doctoral fellowship from ARC. D.B. was supported by a postdoctoral fellowship from ANR. R.F., H.R., and W.V. are recipients of a research fellowship from Assistance Publique–Hôpitaux de Paris–Institut National de la Santé et de la Recherche Médicale (contrats d'interface 2009-2012, R.F.; and 2008-2013, H.R.) and from IGR-Institut National de la Santé et de la Recherche Médicale. W.V. and E.S. teams are supported by the Ligue Nationale Contre le Cancer.
Authorship
Contribution: W.V., R.F., and H.R. designed the work; I.A.-D., D.B., V.B., A.R., F.B., M.M., N.D., C.D., Y.C., R.F., and H.R. performed the experiments; V.B., R.I., F.B., G.L., E.S., and R.F. collected the samples; I.A.-D., D.B., R.I., N.D., G.L., E.S., W.V., R.F., and H.R. discussed the results; and R.I., E.S., W.V., R.F., and H.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hana Raslova, Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche 1009, Institut Gustave Roussy, 114 rue Edouard Vaillant, 94805, Villejuif cedex, France; e-mail: hraslova@igr.fr.
References
Author notes
I.A.-D. and D.B. contributed equally to this study.
R.F. and H.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal