Abstract
In a randomized, phase 3 study, superior complete/near-complete response (CR/nCR) rates and extended progression-free survival were demonstrated with bortezomib-thalidomide-dexamethasone (VTD) versus thalidomide-dexamethasone (TD) as induction therapy before, and consolidation after, double autologous stem cell transplantation for newly diagnosed myeloma patients (intention-to-treat analysis; VTD, n = 236; TD, n = 238). This per-protocol analysis (VTD, n = 160; TD, n = 161) specifically assessed the efficacy and safety of consolidation with VTD or TD. Before starting consolidation, CR/nCR rates were not significantly different in the VTD (63.1%) and TD arms (54.7%). After consolidation, CR (60.6% vs 46.6%) and CR/nCR (73.1% vs 60.9%) rates were significantly higher for VTD-treated versus TD-treated patients. VTD consolidation significantly increased CR and CR/nCR rates, but TD did not (McNemar test). With a median follow-up of 30.4 months from start of consolidation, 3-year progression-free survival was significantly longer for the VTD group (60% vs 48% for TD). Grade 2 or 3 peripheral neuropathy (8.1% vs 2.4%) was more frequent with VTD (grade 3, 0.6%) versus TD consolidation. The superior efficacy of VTD versus TD as induction was retained despite readministration as consolidation therapy after double autologous transplantation. VTD consolidation therapy significantly contributed to improved clinical outcomes observed for patients randomly assigned to the VTD arm of the study. The study is registered at www.clinicaltrials.gov as #NCT01134484.
Introduction
Traditionally, the most important consolidation therapy for transplant-eligible patients with multiple myeloma (MM) has been considered to be high-dose melphalan plus autologous stem cell transplantation (ASCT).1-4 This is based on the demonstrated ability of high-dose melphalan to overcome resistance to standard-dose conventional chemotherapy and to improve the response rates offered by traditional induction therapies.1-4 Studies of ASCT have shown the prognostic relevance of maximal response to both conventional induction therapy and high-dose therapy with autologous stem cell support.5 Attainment of complete response (CR) or at least very good partial response (VGPR) before and after ASCT is one of the strongest predictors of long-term clinical outcomes6,7 and represents a major end point of current treatment strategies incorporating ASCT upfront.
The novel agents thalidomide, bortezomib, and lenalidomide have recently been introduced as part of induction therapy for newly diagnosed MM.8 Incorporation of these newer drugs into primary therapy before ASCT improved the rate of high-quality responses compared with traditional therapies.9-17 Recent studies have demonstrated that ASCT is complementary with novel agent–based induction therapies and further enhances the degree of tumor cell mass reduction, even in the context of high rates of CR or VGPR yielded by incorporation of novel agents into primary induction therapy.9-17
Over the past few years, the treatment paradigm for transplant-eligible MM patients has continued to evolve with the investigational use of the novel agents as consolidation and maintenance therapies after ASCT. Preliminary results suggest that novel agents after transplantation may further increase the rate of high-quality responses and improve both progression-free survival (PFS) and overall survival (OS).8,18-27
We designed a randomized, phase 3 study to assess the superior efficacy of bortezomib, thalidomide, and dexamethasone (VTD) versus thalidomide and dexamethasone (TD) as induction therapy before double ASCT for newly diagnosed MM patients.13 A second randomization after ASCT between VTD and TD was not planned because the efficacy of the 2 regimens as consolidation therapy was not a primary study end point. However, because it was probable that the anticipated superior efficacy of VTD over TD as induction therapy would be retained in subsequent treatment phases, the study was designed such that patients who were initially randomized to receive VTD or TD as induction therapy subsequently were given the same triplet or doublet regimens as consolidation therapy. Thus, a secondary study end point was the efficacy and safety of consolidation therapy with VTD or TD. In a previously reported intention-to-treat (ITT) analysis, it was confirmed that the VTD arm was superior over the TD arm in terms of CR and CR/near-complete response (nCR) rates after all treatment phases, including induction and consolidation therapy.13 We also demonstrated that patients randomized to receive VTD induction and who subsequently received VTD consolidation therapy after double ASCT had a reduced risk of relapse or progression and prolonged PFS than those assigned to the TD arm of the study.13
However, in our initial report, the relative contribution of different treatment phases, including consolidation, to improved clinical outcomes for patients randomized to the VTD arm was not well defined. Thus, the aim of the present analysis was to specifically assess the efficacy and safety of consolidation therapy with VTD or TD, and to evaluate whether VTD consolidation therapy had a favorable impact on improved clinical outcomes observed for patients randomized to the VTD arm of the study.
Methods
Patients
As previously reported, 480 patients were enrolled in this phase 3, open-label study at 73 centers of the Gruppo Italiano Malattie Ematologiche dell'Adulto Myeloma Network in Italy between May 2006 and April 2008.13 The study is still underway but is not recruiting participants; the cut-off date for inclusion of data in the current report was March 31, 2011. Key inclusion criteria were age 18 to 65 years, previously untreated symptomatic and measurable MM, and adequate hematologic, renal, cardiac, and hepatic function.13 Patients with peripheral neuropathy (PN) of grade 2 or higher according to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0, a history of venous thromboembolism, or previous diagnosis of thrombophylic alterations were ineligible. The study was approved by the independent ethics committee or institutional review board at all participating institutions and was done in accordance with International Conference on Harmonization guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent.
Study design
The treatment protocol was as previously reported.13 Patients were randomized in a 1:1 ratio to receive induction therapy with VTD or TD. Stratification was by international staging system (ISS) disease stage.28 Induction treatment consisted of three 21-day cycles of bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11, thalidomide 100 mg daily for the first 14 days and 200 mg daily thereafter, and dexamethasone 40 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 (VTD), or the same doses of thalidomide plus dexamethasone 40 mg on days 1 to 4 and 9 to 12 (TD). Patients then received double ASCT to support 2 sequential courses of melphalan 200 mg/m2 given 3 to 6 months apart. Thalidomide 100 mg daily and dexamethasone on days 1 to 4 every 28 days were administered from recovery of hematopoiesis after the first transplantation to the day before the second transplantation.
Two 35-day cycles of consolidation treatment were commenced 3 months after the second transplantation, regardless of response to ASCT. In the VTD arm, consolidation therapy was composed of bortezomib 1.3 mg/m2 on days 1, 8, 15, and 22, thalidomide 100 mg daily, and dexamethasone 40 mg on days 1, 2, 8, 9, 15, 16, 22, and 23. In the TD arm, thalidomide was given at 100 mg daily and dexamethasone at 40 mg on days 1 to 4 and 20 to 23. Patients then received maintenance therapy with dexamethasone 40 mg on days 1 to 4 every 28 days until disease progression, relapse, or undue toxicity.
Reasons for study drug dose reduction (bortezomib: 1.3 to 1.0 to 0.7 mg/m2; thalidomide: 100 to 50 mg/daily) included predefined hematologic and nonhematologic toxic events, as previously reported.13 Bortezomib-related peripheral sensory neuropathy and/or neuropathic pain was managed according to established guidelines. For thalidomide-related grade 2 PN, the dose was reduced by 50%; for grade 3 or higher PN, dosing was held until resolution to grade 2 or lower, and then restarted at a lower dose. Acyclovir prophylaxis to prevent varicella zoster virus reactivation was recommended for patients receiving VTD.
Laboratory and clinical investigations to assess response were performed as previously detailed.13 Response was assessed at day 64 after induction, at day 90 after each of the 2 courses of melphalan 200 mg/m2, and at day 71 after consolidation. During maintenance therapy or follow-up, response was assessed every 90 days until disease progression. Response and progression were reported by investigators according to criteria of the European Group for Blood and Marrow Transplantation,29 with the addition of categories for nCR30 and VGPR.31 Responses were monitored by an external contract organization and were centrally reassessed by the study coordinating team. Patients with CR who lacked confirmation from bone marrow biopsy samples were centrally downgraded to VGPR.
Safety was monitored until 30 days after the last dose of study drug. Adverse events (AEs), as graded by investigators according to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0, were externally monitored and centrally reassessed.
The primary study end point was the rate of CR/nCR to induction therapy. The efficacy and safety of consolidation therapy were secondary end points. Additional secondary end points included time to progression or relapse (TTP), PFS, and OS.
Statistical analysis
For the primary study end point, we calculated that a sample size of 450 patients (225 per group) was needed to provide 80% power to detect a significant increase in the rate of CR/nCR after induction from 15% with TD induction therapy to 27% with VTD induction therapy. For the purposes of the current study, we performed a per-protocol analysis of 321 patients who completed the allocated treatment. All tests were 2-sided with P values of less than .05 deemed significant. The efficacy of consolidation therapy with VTD or TD was evaluated by comparing within each treatment arm the rates of CR and CR/nCR before starting consolidation therapy with those assessed after consolidation. The frequencies of high-quality responses after consolidation therapy were also compared between the VTD-treated and TD-treated groups. Comparisons between rates of response and treatment groups, with estimates of 95% confidence intervals (CIs), were made using the χ2 test. Rates of AEs were compared between treatment groups with the χ2 test. The probabilities of improving from less than nCR before consolidation therapy to CR and CR/nCR after consolidation therapy with VTD or TD were evaluated using the McNemar test, which compares paired binary data before and after a specific event.
A landmark analysis, with the landmark set at the start of consolidation therapy, was used to estimate TTP, PFS, and OS. Times to these outcomes were estimated using the Kaplan-Meier method. Between-group comparisons of TTP, PFS, and OS were done with the log-rank test.
A multivariate Cox regression analysis was done to identify factors significantly affecting PFS, with calculation of hazard ratios (HR) and 95% CI. A univariate Cox regression analysis was performed to investigate the impact of VTD versus TD as consolidation therapy on PFS in subgroups of patients with different prognosis.
This study is registered at www.clinicaltrials.gov as #NCT01134484 and at EudraCT as #2005-003723-39 (www.clinicaltrialsregister.eu).
Results
Patients
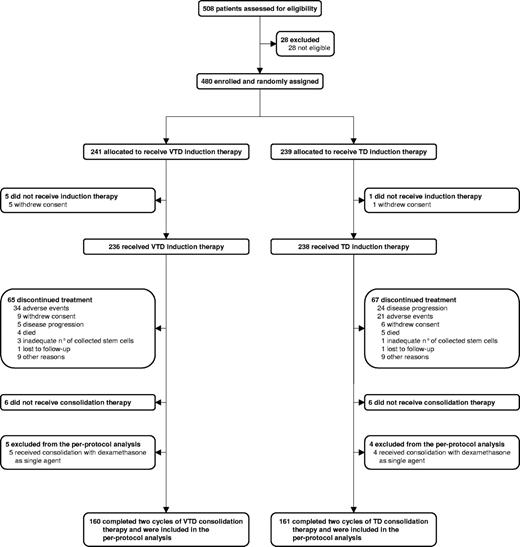
As previously reported, 474 patients of 480 who were randomly assigned to the 2 arms of the study started induction therapy with VTD (n = 236) or TD (n = 238; Figure 1).13 A total of 330 patients (n = 165 in each arm) completed the allocated treatment.13 A total of 321 of these 330 patients were included in this per-protocol analysis; the remaining 9 patients were excluded because they received consolidation therapy with dexamethasone as single agent (Figure 1). For 5 patients randomized to the VTD arm, this was the result of protocol violation in 3 patients and physician's decision in 2 patients who developed grade 3 PN during VTD induction therapy, with subsequent resolution. For 4 patients randomized to the TD arm, this was the result of a protocol violation in a single patient and physician's decision in 3 patients, based on previous AEs during TD induction therapy, including deep vein thrombosis (n = 2) and pancreatitis (n = 1). Therefore, the per-protocol population included 160 of 236 (68%) patients who started induction therapy with VTD and 161 of 238 (68%) patients who started induction therapy with TD.
Demographics and disease characteristics at baseline were well balanced between the 2 groups (Table 1), with no differences seen with respect to key characteristics with a potential influence on clinical outcomes. Overall, the main baseline characteristics of the per-protocol population were comparable with those of patients included in the ITT analysis, with the exception of age (median 57.2 years for the per-protocol population vs 58.4 years for the remaining patients, P = .030) and albumin level (median 4.0 g/L vs 3.7 g/L, P = .0004); however, neither age nor albumin level was a significant predictor of clinical outcomes in both univariate and multivariate analyses.13 Median follow-up for surviving patients was 30.4 months (interquartile range [IQR] = 25.3-37.6) from the start of consolidation therapy.
Demographic and disease characteristics at baseline of patients receiving the 2 cycles of consolidation therapy with VTD or TD
| . | VTD (n = 160) . | TD (n = 161) . |
|---|---|---|
| Age, y | ||
| Median (IQR) | 57.4 (51.2-61.3) | 56.8 (50.5-61.6) |
| Mean (SD) | 55.6 (7.3) | 55.5 (7.6) |
| Sex | ||
| Male | 96 (60.0%) | 95 (59.0%) |
| Female | 64 (40.0%) | 66 (41.0%) |
| Myeloma subtype | ||
| IgG | 98 (61.2%) | 99 (61.5%) |
| IgA | 30 (18.7%) | 34 (21.1%) |
| Light chain | 31 (19.4%) | 25 (15.5%) |
| Other | 1 (0.6%) | 3 (1.9%) |
| ISS disease stage | ||
| I | 79 (49.4%) | 75 (46.6%) |
| II | 56 (35.0%) | 62 (38.5%) |
| III | 25 (15.6%) | 24 (14.9%) |
| β2-microglobulin (mg/L) | ||
| Median (IQR) | 3.0 (2.2-4.3) | 3.2 (2.3-4.7) |
| Mean (SD) | 3.7 (2.3) | 3.8 (2.1) |
| Albumin (g/L) | ||
| Median (IQR) | 39.1 (34.9-44.0) | 40.0 (35.0-43.0) |
| Mean (SD) | 38.9 (6.4) | 39.1 (6.2) |
| Creatinine (Mmol/L) | ||
| Median (IQR) | 88.1 (70.4-97.6) | 88.4 (70.7-97.9) |
| Mean (SD) | 89.2 (26.7) | 88.7 (26.2) |
| Hemoglobin (g/L) | ||
| Median (IQR) | 110.4 (97.2-125.0) | 113.1 (99.3-129.1) |
| Mean (SD) | 111.1 (19.2) | 114.2 (19.9) |
| Platelets (×109/L) | ||
| Median (IQR) | 230.5 (192.5-282.5) | 235.0 (199.3-278.7) |
| Mean (SD) | 242.3 (80.3) | 240.9 (75.5) |
| Bone marrow plasma cells | ||
| Median (IQR) | 50.0 (35.5-70.0) | 50 (30-70) |
| Mean (SD) | 52.7 (22.2) | 51.9 (24.8) |
| FISH analysis for cytogenetic abnormalities* | ||
| Absence of del(13q), t(4;14), or del(17p) | 66 (44.6%) | 76 (51.3%) |
| Presence of del(13q)** | 71 (48.0%) | 63 (42.6%) |
| Presence of t(4;14) and/or del(17p) | 38 (25.7%) | 38 (25.7%) |
| . | VTD (n = 160) . | TD (n = 161) . |
|---|---|---|
| Age, y | ||
| Median (IQR) | 57.4 (51.2-61.3) | 56.8 (50.5-61.6) |
| Mean (SD) | 55.6 (7.3) | 55.5 (7.6) |
| Sex | ||
| Male | 96 (60.0%) | 95 (59.0%) |
| Female | 64 (40.0%) | 66 (41.0%) |
| Myeloma subtype | ||
| IgG | 98 (61.2%) | 99 (61.5%) |
| IgA | 30 (18.7%) | 34 (21.1%) |
| Light chain | 31 (19.4%) | 25 (15.5%) |
| Other | 1 (0.6%) | 3 (1.9%) |
| ISS disease stage | ||
| I | 79 (49.4%) | 75 (46.6%) |
| II | 56 (35.0%) | 62 (38.5%) |
| III | 25 (15.6%) | 24 (14.9%) |
| β2-microglobulin (mg/L) | ||
| Median (IQR) | 3.0 (2.2-4.3) | 3.2 (2.3-4.7) |
| Mean (SD) | 3.7 (2.3) | 3.8 (2.1) |
| Albumin (g/L) | ||
| Median (IQR) | 39.1 (34.9-44.0) | 40.0 (35.0-43.0) |
| Mean (SD) | 38.9 (6.4) | 39.1 (6.2) |
| Creatinine (Mmol/L) | ||
| Median (IQR) | 88.1 (70.4-97.6) | 88.4 (70.7-97.9) |
| Mean (SD) | 89.2 (26.7) | 88.7 (26.2) |
| Hemoglobin (g/L) | ||
| Median (IQR) | 110.4 (97.2-125.0) | 113.1 (99.3-129.1) |
| Mean (SD) | 111.1 (19.2) | 114.2 (19.9) |
| Platelets (×109/L) | ||
| Median (IQR) | 230.5 (192.5-282.5) | 235.0 (199.3-278.7) |
| Mean (SD) | 242.3 (80.3) | 240.9 (75.5) |
| Bone marrow plasma cells | ||
| Median (IQR) | 50.0 (35.5-70.0) | 50 (30-70) |
| Mean (SD) | 52.7 (22.2) | 51.9 (24.8) |
| FISH analysis for cytogenetic abnormalities* | ||
| Absence of del(13q), t(4;14), or del(17p) | 66 (44.6%) | 76 (51.3%) |
| Presence of del(13q)** | 71 (48.0%) | 63 (42.6%) |
| Presence of t(4;14) and/or del(17p) | 38 (25.7%) | 38 (25.7%) |
Data are number (%) unless otherwise stated.
VTD indicates bortezomib with thalidomide plus dexamethasone; TD, thalidomide plus dexamethasone; IQR, interquartile range; SD, standard deviation; ISS, international staging system; and FISH, fluorescent in situ hybridization
148 patients on VTD and 148 on TD were available for assessment.
Regardless of absence or presence of t(4;14) and/or del(17p).
Response to treatment phases before starting consolidation therapy
Patients who were included in the per-protocol analysis and were randomized to the VTD arm of the study had significantly higher rates of CR and CR/nCR to both induction therapy and the first ASCT than those assigned to the TD arm (Table 2). At the landmark of starting consolidation therapy after the second ASCT, the rates of CR and CR/nCR for VTD-treated patients were 48.7% and 63.1%, respectively (Table 2). The corresponding values for TD-treated patients were 40.4% and 54.7% (Table 2). The difference between the 2 groups was not statistically different for both CR (P = .131) and CR/nCR (P = .123) comparisons.
Response to different treatment phases in the per-protocol population, according to central assessment
| . | VTD (n = 160) . | TD (n = 161) . | P . |
|---|---|---|---|
| After induction therapy | |||
| CR | 36 (22.5%, 16.0-29.0) | 9 (5.6%, 2.0-9.1) | < .0001 |
| CR/nCR | 53 (33.1%, 25.8-40.4) | 22 (13.7%, 8.3-19.0) | < .0001 |
| VGPR or better | 100 (62.5%, 55.0-70.0) | 50 (31.1%, 23.9-38.2) | < .0001 |
| PR or better | 154 (96.2%, 93.3-99.2) | 140 (87.0%, 81.7-92.1) | .003 |
| MR or SD | 6 (3.7%, 0.8-6.7) | 21 (13.0%, 7.8-18.2) | .003 |
| After first ASCT | |||
| CR | 70 (43.8%, 36.1-51.4) | 49 (30.4%, 23.3-37.5) | .014 |
| CR/nCR | 91 (56.9%, 49.2-64.5) | 66 (41.0%, 33.4-48.6) | .004 |
| VGPR or better | 131 (81.9%, 75.9-87.8) | 117 (72.7%, 65.8-79.6) | .049 |
| PR or better | 156 (97.5%, 95.1-100) | 156 (96.9%, 94.2-99.6) | .742 |
| MR or SD | 4 (2.5%, 0.1-0.5) | 5 (3.1%, 0.04-5.8) | .742 |
| After second ASCT | |||
| CR | 78 (48.7%, 41.0-56.5) | 65 (40.4%, 32.8-47.9) | .131 |
| CR/nCR | 101 (63.1%, 55.6-70.6) | 88 (54.7%, 47.0-62.3) | .123 |
| VGPR or better | 138 (86.2%, 80.9-91.6) | 131 (81.4%, 75.3-87.4) | .235 |
| PR or better | 157 (98.1%, 96.0-100) | 157 (97.5%, 95.1-99.9) | .709 |
| MR or SD | 3 (1.9%, 0.0-4.0) | 4 (2.5%, 0.1-4.9) | .709 |
| After consolidation therapy | |||
| CR | 97 (60.6%, 53.0-68.2) | 75 (46.6%, 38.9-54.3) | .012 |
| CR/nCR | 117 (73.1%, 66.2-80.0) | 98 (60.9%, 53.3-68.4) | .020 |
| VGPR or better | 147 (91.9%, 87.6-96.1) | 142 (88.2%, 83.2-93.2) | .272 |
| PR or better | 156 (97.5%, 95.1-99.9) | 160 (99.4%, 98.2-100) | .174 |
| MR or SD | 1 (0.6%, 0-1.8) | 1 (0.6%, 0-1.8) | .996 |
| PD | 3 (1.9%, 0-4.0) | .081 |
| . | VTD (n = 160) . | TD (n = 161) . | P . |
|---|---|---|---|
| After induction therapy | |||
| CR | 36 (22.5%, 16.0-29.0) | 9 (5.6%, 2.0-9.1) | < .0001 |
| CR/nCR | 53 (33.1%, 25.8-40.4) | 22 (13.7%, 8.3-19.0) | < .0001 |
| VGPR or better | 100 (62.5%, 55.0-70.0) | 50 (31.1%, 23.9-38.2) | < .0001 |
| PR or better | 154 (96.2%, 93.3-99.2) | 140 (87.0%, 81.7-92.1) | .003 |
| MR or SD | 6 (3.7%, 0.8-6.7) | 21 (13.0%, 7.8-18.2) | .003 |
| After first ASCT | |||
| CR | 70 (43.8%, 36.1-51.4) | 49 (30.4%, 23.3-37.5) | .014 |
| CR/nCR | 91 (56.9%, 49.2-64.5) | 66 (41.0%, 33.4-48.6) | .004 |
| VGPR or better | 131 (81.9%, 75.9-87.8) | 117 (72.7%, 65.8-79.6) | .049 |
| PR or better | 156 (97.5%, 95.1-100) | 156 (96.9%, 94.2-99.6) | .742 |
| MR or SD | 4 (2.5%, 0.1-0.5) | 5 (3.1%, 0.04-5.8) | .742 |
| After second ASCT | |||
| CR | 78 (48.7%, 41.0-56.5) | 65 (40.4%, 32.8-47.9) | .131 |
| CR/nCR | 101 (63.1%, 55.6-70.6) | 88 (54.7%, 47.0-62.3) | .123 |
| VGPR or better | 138 (86.2%, 80.9-91.6) | 131 (81.4%, 75.3-87.4) | .235 |
| PR or better | 157 (98.1%, 96.0-100) | 157 (97.5%, 95.1-99.9) | .709 |
| MR or SD | 3 (1.9%, 0.0-4.0) | 4 (2.5%, 0.1-4.9) | .709 |
| After consolidation therapy | |||
| CR | 97 (60.6%, 53.0-68.2) | 75 (46.6%, 38.9-54.3) | .012 |
| CR/nCR | 117 (73.1%, 66.2-80.0) | 98 (60.9%, 53.3-68.4) | .020 |
| VGPR or better | 147 (91.9%, 87.6-96.1) | 142 (88.2%, 83.2-93.2) | .272 |
| PR or better | 156 (97.5%, 95.1-99.9) | 160 (99.4%, 98.2-100) | .174 |
| MR or SD | 1 (0.6%, 0-1.8) | 1 (0.6%, 0-1.8) | .996 |
| PD | 3 (1.9%, 0-4.0) | .081 |
Data are number (%, 95% CI).
VTD indicates bortezomib with thalidomide plus dexamethasone; TD, thalidomide plus dexamethasone; CR, complete response; nCR, near-complete response; VGPR, very good partial response; PR, partial response; MR, minimal response; SD, stable disease; and PD, progressive disease.
Response improvement after consolidation therapy
After consolidation therapy, the rates of CR (60.6% vs 46.6%, P = .012) and CR/nCR (73.1% vs 60.9%, P = .020) were significantly higher with VTD versus TD consolidation therapy (Table 2). Overall, consolidation therapy with VTD affected an increase in the rates of CR and CR/nCR, averaging 11.9% and 10%, respectively. The corresponding enhanced rates after TD consolidation therapy were 6.2% each. The difference between VTD-treated and TD-treated groups was statistically significant in favor of VTD consolidation therapy for both CR and CR/nCR comparisons.
The McNemar test confirmed the favorable impact of VTD consolidation therapy on enhanced rates of CR (P = .0009) and CR/nCR (P = .004; Table 3). Seventy-two of 78 patients who were in CR before starting consolidation therapy with VTD maintained CR after consolidation, whereas 6 patients downgraded to less than CR. Among the 82 patients who had not achieved CR after double ASCT, 25 (30.5%) upgraded to CR after VTD consolidation therapy, and 20 (24.4%) improved their response status to less than CR. Of the remaining 37 patients, 33 maintained and 4 downgraded their preconsolidation response status. Patients' responses before and after VTD consolidation therapy are detailed in Table 4.
Analysis by use of the McNemar test of patients who upgraded or downgraded their response status after consolidation therapy with VTD or TD
| Response status before consolidation . | Response status after consolidation . | |||||
|---|---|---|---|---|---|---|
| VTD . | TD . | |||||
| CR (N) . | < CR (N) . | Total (N) . | CR (N) . | < CR (N) . | Total (N) . | |
| CR | 72 | 6 | 78 | 59 | 6 | 65 |
| < CR | 25 | 57 | 82 | 16 | 80 | 96 |
| Total | 97 | 63 | 160 | 75 | 86 | 161 |
| P | .0009 | .0525 | ||||
| Response status before consolidation . | Response status after consolidation . | |||||
|---|---|---|---|---|---|---|
| VTD . | TD . | |||||
| CR (N) . | < CR (N) . | Total (N) . | CR (N) . | < CR (N) . | Total (N) . | |
| CR | 72 | 6 | 78 | 59 | 6 | 65 |
| < CR | 25 | 57 | 82 | 16 | 80 | 96 |
| Total | 97 | 63 | 160 | 75 | 86 | 161 |
| P | .0009 | .0525 | ||||
| Response status before consolidation . | CR/nCR (N) . | < nCR (N) . | Total (N) . | CR/nCR (N) . | < nCR (N) . | Total (N) . |
|---|---|---|---|---|---|---|
| CR/nCR | 95 | 6 | 101 | 77 | 11 | 88 |
| < nCR | 22 | 37 | 59 | 21 | 52 | 73 |
| Total | 117 | 43 | 160 | 98 | 63 | 161 |
| P | .0037 | .1102 | ||||
| Response status before consolidation . | CR/nCR (N) . | < nCR (N) . | Total (N) . | CR/nCR (N) . | < nCR (N) . | Total (N) . |
|---|---|---|---|---|---|---|
| CR/nCR | 95 | 6 | 101 | 77 | 11 | 88 |
| < nCR | 22 | 37 | 59 | 21 | 52 | 73 |
| Total | 117 | 43 | 160 | 98 | 63 | 161 |
| P | .0037 | .1102 | ||||
N indicates number of patients; VTD, bortezomib with thalidomide plus dexamethasone; TD, thalidomide plus dexamethasone; CR. complete response; < CR, less than CR; nCR, near-CR; < nCR, less than near-CR.
Patients' response status before and after VTD and TD consolidation therapy
| Response status before starting consolidation with VTD . | Response status after consolidation with VTD, N . | Total . | |||||
|---|---|---|---|---|---|---|---|
| CR . | nCR . | VGPR . | PR . | MR/NR/SD . | PD . | ||
| CR | 72 | 2 | 1 | 0 | 0 | 3 | 78 |
| nCR | 11 | 10 | 2 | 0 | 0 | 0 | 23 |
| VGPR | 13 | 5 | 17 | 2 | 0 | 0 | 37 |
| PR | 1 | 3 | 10 | 5 | 0 | 0 | 19 |
| MR or NR or SD | 0 | 0 | 0 | 2 | 1 | 0 | 3 |
| Total | 97 | 20 | 30 | 9 | 1 | 3 | 160 |
| Response status before starting consolidation with VTD . | Response status after consolidation with VTD, N . | Total . | |||||
|---|---|---|---|---|---|---|---|
| CR . | nCR . | VGPR . | PR . | MR/NR/SD . | PD . | ||
| CR | 72 | 2 | 1 | 0 | 0 | 3 | 78 |
| nCR | 11 | 10 | 2 | 0 | 0 | 0 | 23 |
| VGPR | 13 | 5 | 17 | 2 | 0 | 0 | 37 |
| PR | 1 | 3 | 10 | 5 | 0 | 0 | 19 |
| MR or NR or SD | 0 | 0 | 0 | 2 | 1 | 0 | 3 |
| Total | 97 | 20 | 30 | 9 | 1 | 3 | 160 |
| Response status before starting consolidation with TD . | Response status after consolidation with TD, N . | Total . | |||||
|---|---|---|---|---|---|---|---|
| CR . | nCR . | VGPR . | PR . | MR/NR/SD . | PD . | ||
| CR | 59 | 1 | 4 | 1 | 0 | 0 | 65 |
| nCR | 6 | 11 | 4 | 2 | 0 | 0 | 23 |
| VGPR | 7 | 9 | 27 | 0 | 0 | 0 | 43 |
| PR | 2 | 2 | 9 | 13 | 0 | 0 | 26 |
| MR or NR or SD | 1 | 0 | 0 | 2 | 1 | 0 | 4 |
| Total | 75 | 23 | 44 | 18 | 1 | 0 | 161 |
| Response status before starting consolidation with TD . | Response status after consolidation with TD, N . | Total . | |||||
|---|---|---|---|---|---|---|---|
| CR . | nCR . | VGPR . | PR . | MR/NR/SD . | PD . | ||
| CR | 59 | 1 | 4 | 1 | 0 | 0 | 65 |
| nCR | 6 | 11 | 4 | 2 | 0 | 0 | 23 |
| VGPR | 7 | 9 | 27 | 0 | 0 | 0 | 43 |
| PR | 2 | 2 | 9 | 13 | 0 | 0 | 26 |
| MR or NR or SD | 1 | 0 | 0 | 2 | 1 | 0 | 4 |
| Total | 75 | 23 | 44 | 18 | 1 | 0 | 161 |
N indicates number of patients; VTD, bortezomib with thalidomide plus dexamethasone; CR, complete response; nCR, near-CR; VGPR, very good partial response; PR, partial response; MR, minimal response; NR, no response; SD, stable disease; and TD, thalidomide plus dexamethasone.
Among patients in the TD group, the probability of improving from less than CR before consolidation therapy to CR after consolidation was borderline significant (P = .052) and from less than nCR to CR/nCR was not significant (P = .110; Table 3). Fifty-nine of 65 patients who were in CR before starting consolidation therapy maintained CR after consolidation with TD, whereas 6 patients downgraded to less than CR. Among 96 patients who had not achieved CR after double ASCT, 16 (16.7%) upgraded to CR after TD consolidation therapy, and an additional 22 patients (22.9%) improved their response status. Of the remaining 58 patients, 52 maintained and 6 downgraded their preconsolidation response status. Patients' responses before and after TD consolidation therapy are detailed in Table 4.
Overall, the probability of upgrading from less than CR before consolidation therapy to CR after consolidation was significantly higher in patients receiving VTD (25 of 82 patients, 30.5%) than in those receiving TD (16 of 96 patients, 16.7%; P = .030). Most of the patients who improved to CR after VTD consolidation therapy were in nCR (44%) or VGPR (52%) before starting consolidation therapy.
Response improvement during maintenance therapy
Overall, a total of 41 patients (n = 16, 10%, in the VTD-treated group; n = 25, 15.5%, in the TD-treated group) who failed CR after consolidation therapy achieved negative immunofixation during maintenance therapy with dexamethasone. Of these, 9 of 16 patients in the VTD-treated group and 11 of 25 in the TD-treated group (P = .443) were in nCR before starting maintenance therapy.
Landmark analysis of outcomes from the start of consolidation therapy
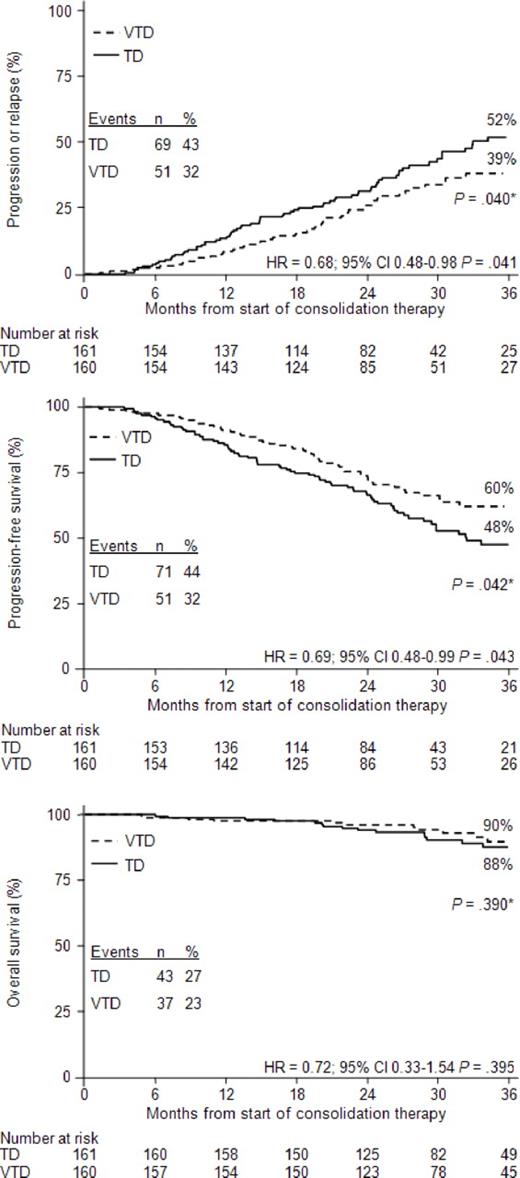
The estimated 3-year probability of progression or relapse from the start of consolidation therapy was 39% in the VTD group versus 52% in the TD group (P = .040; Figure 2A). TTP was significantly longer with VTD versus TD (median not reached vs 33 months; HR = 0.68, 95% CI, 0.48-0.98, P = .041).
Analysis of outcomes from the start of consolidation therapy with VTD or TD. Kaplan-Meier curves for TTP (A), PFS (B), and OS (C) from the landmark of starting consolidation therapy. *P value according to log-rank test.
Analysis of outcomes from the start of consolidation therapy with VTD or TD. Kaplan-Meier curves for TTP (A), PFS (B), and OS (C) from the landmark of starting consolidation therapy. *P value according to log-rank test.
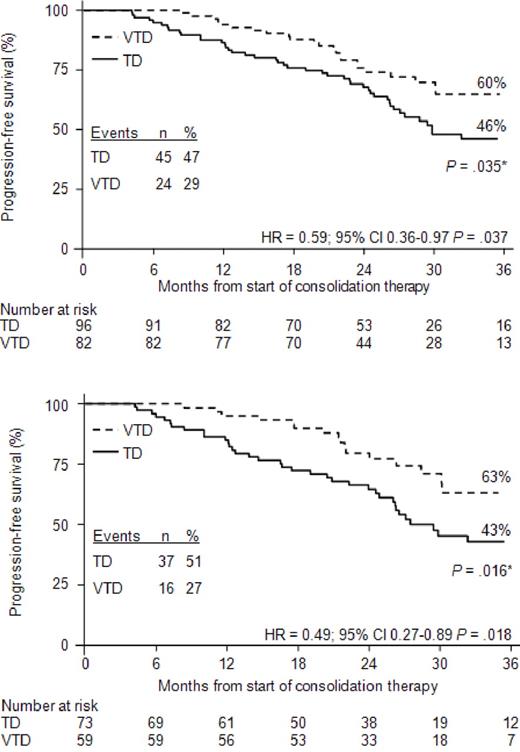
The estimated 3-year PFS rate was 60% in the VTD group versus 48% in the TD group (P = .042; Figure 2B). Overall, PFS was significantly longer with VTD versus TD (median not reached vs 32 months). Compared with TD consolidation therapy, the relative risk of progression or death was reduced by 31% with VTD consolidation (HR = 0.69, 95% CI, 0.48-0.99, P = .043). With a median follow-up of 43 months from the start of induction therapy, the estimated 5-year PFS rate was 62% for the 160 patients in the VTD-treated group versus 49% for the 161 patients in the TD-treated group (P = .045 according to log-rank test; HR = 0.69, 95% CI, 0.48-0.99, P = .042). Analysis of PFS according to response at the landmark of starting consolidation therapy showed that patients who most benefited from VTD consolidation therapy were those who did not achieve CR (HR = 0.59, 95% CI, 0.36-0.97, P = .037; Figure 3A) and CR/nCR (HR = 0.49, 95% CI, 0.27-0.89, P = .018; Figure 3B) after double ASCT. In both of these subgroups of patients, the rates of progression or death were significantly higher in the TD-treated versus the VTD-treated subgroups (47% vs 29% for patients not achieving CR, P = .016, Figure 3A; 51% vs 27% for patients not achieving CR/nCR, P = .006, Figure 3B). Compared with the TD-treated subgroup, the relative risk of progression or death for the VTD-treated subgroup was reduced by 41% and 51% in patients not achieving CR and CR/nCR, respectively.
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy. The figure shows PFS for patients who had not achieved CR (A) or CR/nCR (B) after double ASCT. *P value according to log-rank test.
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy. The figure shows PFS for patients who had not achieved CR (A) or CR/nCR (B) after double ASCT. *P value according to log-rank test.
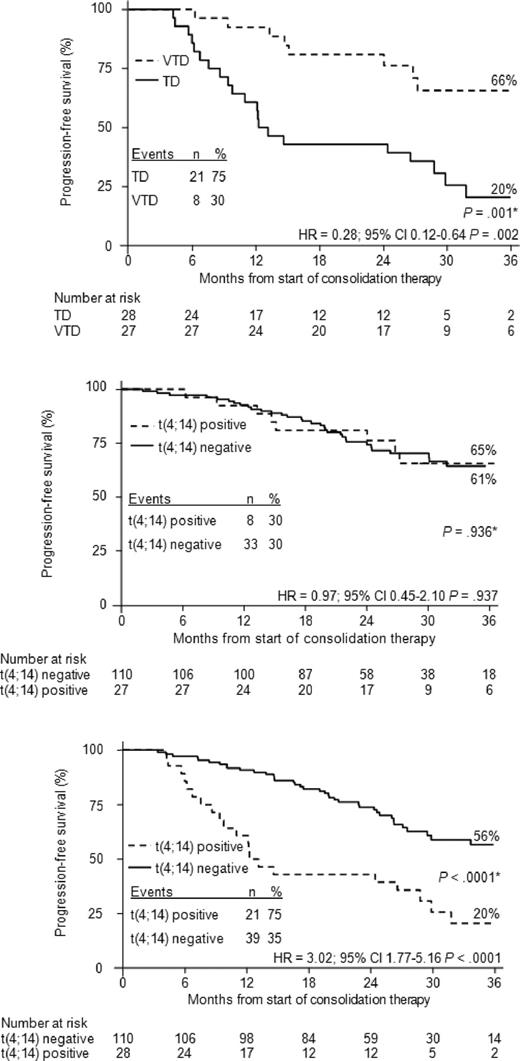
PFS remained significantly longer in the VTD group than in the TD group across subgroups of patients with conventionally defined poor prognostic variables, including t(4;14) and/or del(17p) positivity (P = .002), presence of del(13q) (P = .006), elevated lactate dehydrogenase (P = .007), high serum β2-microglobulin concentration (P = .022), and ISS stages 2 and 3 (P = .023; Table 5). The impact of absence of cytogenetic abnormalities or presence of del(13q) but lack of t(4;14) and del(17p) or positivity for t(4;14) and/or del(17p) on PFS was subsequently evaluated in the 2 treated groups. In the VTD arm, PFS curves were almost superimposable regardless of absence or presence of cytogenetic abnormalities, including the presence of the high-risk profile characterized by positivity for t(4;14) and/or del(17p), which was associated with a 3-year PFS estimate in the 59% range (P = .713; Figure 4A). In contrast, patients who received TD consolidation therapy and carried t(4;14) and/or del(17p) had a significantly worse outcome (3-year PFS: 19%) compared with the other 2 groups of patients (P < .0001; Figure 4B). A separate outcome analysis of patients with t(4;14) but not del(17p), regardless of presence or absence of del(13q), showed that VTD consolidation therapy was associated with a significantly greater 3-year probability of PFS versus TD consolidation therapy (66% vs 20%; P = .001; Figure 5A). PFS curves for patients treated with VTD consolidation therapy and stratified according to presence or absence of t(4;14) were almost superimposable (3-year estimates: 65% vs 61%, respectively; P = .936; Figure 5B). In contrast, the poor prognosis associated with t(4;14) was retained in patients who received TD consolidation therapy (median PFS from the landmark: 12 months vs 44 months in patients with and without t(4;14), respectively; P < .0001; Figure 5C). The limited frequency of del(17p) positivity (VTD: n = 11, 6.9%; TD: n = 10, 6.2%) precluded a careful analysis of the impact of this high-risk cytogenetic abnormality on PFS.
Cox regression analysis of PFS from start of consolidation therapy in subgroups of patients with poor prognosis
| Subgroup . | VTD . | TD . | Hazard ratio . | 95% CI . | P* . |
|---|---|---|---|---|---|
| t(4;14) and/or del(17p) positive | 38 | 38 | 0.37 | 0.19-0.70 | .002 |
| del(13q) positive** | 71 | 63 | 0.48 | 0.29-0.81 | .006 |
| LDH > 190 U/L | 137 | 144 | 0.59 | 0.40-0.86 | .007 |
| β2-microglobulin > 3.5 mg/L | 59 | 67 | 0.56 | 0.34-0.92 | .022 |
| ISS stage 2-3 | 80 | 86 | 0.58 | 0.36-0.93 | .023 |
| Subgroup . | VTD . | TD . | Hazard ratio . | 95% CI . | P* . |
|---|---|---|---|---|---|
| t(4;14) and/or del(17p) positive | 38 | 38 | 0.37 | 0.19-0.70 | .002 |
| del(13q) positive** | 71 | 63 | 0.48 | 0.29-0.81 | .006 |
| LDH > 190 U/L | 137 | 144 | 0.59 | 0.40-0.86 | .007 |
| β2-microglobulin > 3.5 mg/L | 59 | 67 | 0.56 | 0.34-0.92 | .022 |
| ISS stage 2-3 | 80 | 86 | 0.58 | 0.36-0.93 | .023 |
VTD indicates bortezomib with thalidomide plus dexamethasone; TD, thalidomide plus dexamethasone; LDH, lactate dehydrogenase; and ISS, international staging system.
Wald χ2 test; hazard ratios, 95% CIs, and P values refer to the treatment effects in the stated subgroups.
Regardless of absence or presence of t(4;14) and/or del(17p).
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy according to the presence or absence of cytogenetic abnormalities. The figure shows PFS for patients with no cytogenetic abnormality, or with del(13q) positivity but lack of t(4;14) and del(17p), or t(4;14) and/or del(17p) positivity who received VTD consolidation therapy (A) or TD consolidation therapy (B). *P value according to log-rank test.
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy according to the presence or absence of cytogenetic abnormalities. The figure shows PFS for patients with no cytogenetic abnormality, or with del(13q) positivity but lack of t(4;14) and del(17p), or t(4;14) and/or del(17p) positivity who received VTD consolidation therapy (A) or TD consolidation therapy (B). *P value according to log-rank test.
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy according to t(4;14) positivity or negativity. The figure shows PFS for patients with t(4;14) positivity, but del(17p) negativity, receiving VTD or TD (A), for patients with or without t(4;14) positivity, but del(17p) negativity, receiving VTD (B), and for patients with or without t(4;14) positivity, but del(17p) negativity, receiving TD (C). *P value according to log-rank test.
Kaplan-Meier curves for PFS from the landmark of starting consolidation therapy according to t(4;14) positivity or negativity. The figure shows PFS for patients with t(4;14) positivity, but del(17p) negativity, receiving VTD or TD (A), for patients with or without t(4;14) positivity, but del(17p) negativity, receiving VTD (B), and for patients with or without t(4;14) positivity, but del(17p) negativity, receiving TD (C). *P value according to log-rank test.
A multivariate analysis of variables influencing PFS confirmed the independent value of VTD as consolidation therapy. Additional variables significantly associated with extended PFS included absence of t(4;14) and del(17p), and low serum β2-microglobulin concentration (Table 6).
Multivariate analysis of variables affecting PFS from start of consolidation therapy
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| β2-microglobulin < 3.5 mg/L | 0.42 | 0.29-0.61 | < .0001 |
| Absence of t(4;14) and del(17p) | 0.49 | 0.34-0.73 | < .0001 |
| Consolidation with VTD | 0.61 | 0.42-0.89 | .010 |
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| β2-microglobulin < 3.5 mg/L | 0.42 | 0.29-0.61 | < .0001 |
| Absence of t(4;14) and del(17p) | 0.49 | 0.34-0.73 | < .0001 |
| Consolidation with VTD | 0.61 | 0.42-0.89 | .010 |
VTD indicates bortezomib with thalidomide plus dexamethasone.
No difference in OS from landmark was seen between the 2 treatment groups, the estimated 3-year rates being 90% and 88% for the VTD and TD arms, respectively (Figure 2C).
Treatment exposure and AEs during consolidation therapy
All patients in the VTD and TD groups completed the 2 cycles of consolidation therapy within a median of 71 days (IQR = 70-74 days) and 70 days (IQR = 70-72 days), respectively. Patients randomized to VTD consolidation received 93%, 93%, and 96% of planned doses of bortezomib, thalidomide, and dexamethasone, respectively. In the TD arm, patients received 97% and 94% of planned doses of thalidomide and dexamethasone, respectively.
The rates of AEs observed during consolidation therapy with VTD or TD were much lower than during induction therapy with the same regimens.13 During consolidation therapy, the frequencies of all-grade (16.2% vs 4.9%, P = .001), including grade 2 and 3 PN (8.1% vs 2.4%) and all-grade thrombocytopenia (5.5% vs 0%, P = .002), were significantly higher in patients receiving VTD than in those treated with TD (Table 7). There were no other significant differences between treatment groups in the overall frequency of AEs or the frequency of grade 3 or 4 AEs. Overall, the rate of grade 3 PN in the VTD-treated group was 0.6% (1 patient with preexisting, albeit not severe, neuropathy that worsened during VTD consolidation) versus 0% in the TD group; no grade 4 neuropathy was observed. With the exception of 3 patients in whom the first onset of grade 2 PN occurred during VTD consolidation therapy and of 3 additional patients (n = 2 in the VTD group; n = 1 in the TD group) in whom preexisting mild neuropathy worsened to grade 2 during consolidation therapy, grade 2 PN reoccurred during consolidation after resolution of prior neurologic toxicity.
Rates of AEs of all grades (≥ 3% in either group) and all grade 3 and 4 AEs reported during consolidation therapy with VTD or TD
| AE, n (%) . | VTD (n = 160) . | TD (n = 161) . | P . |
|---|---|---|---|
| All-grade AEs | |||
| PN | 26 (16.2%) | 8 (4.9%) | .001 |
| Infections (excluding herpes zoster) | 24 (15.0%) | 25 (15.5%) | .895 |
| Herpes zoster | 5 (3.1%) | 6 (3.7%) | .767 |
| Gastrointestinal events (excluding constipation) | 20 (12.5%) | 11 (6.8%) | .086 |
| Fever | 15 (9.3%) | 9 (5.5%) | .197 |
| Constipation | 11 (6.8%) | 6 (3.7%) | .208 |
| Skin rash | 8 (5.0%) | 5 (3.1%) | .389 |
| Thrombocytopenia | 9 (5.5%) | 0 | .002 |
| Any grade 3 or 4 AE | 17 (10.6%) | 15 (9.3%) | .696 |
| Nonhematologic grade 3 or 4 AEs | 15 (9.3%) | 14 (8.6%) | .832 |
| Gastrointestinal events (excluding constipation) | 3 (1.8%) | 1 (0.6%) | .311 |
| Infections (excluding herpes zoster) | 2 (1.2%) | 5 (3.1%) | .255 |
| Fever | 1 (0.6%) | 2 (1.2%) | .566 |
| Herpes zoster | 1 (0.6%) | 1 (0.6%) | .996 |
| Constipation | 1 (0.6%) | 1 (0.6%) | .996 |
| Skin rash | 1 (0.6%) | 1 (0.6%) | .996 |
| PN | 1 (0.6%) | 0 | .315 |
| Deep vein thrombosis | 1 (0.6%) | 1 (0.6%) | .996 |
| Hepatic | 1 (0.6%) | 0 | .315 |
| Pancreatitis | 1 (0.6%) | 0 | .315 |
| Hyperglycemia | 0 | 2 (1.2%) | .157 |
| AE, n (%) . | VTD (n = 160) . | TD (n = 161) . | P . |
|---|---|---|---|
| All-grade AEs | |||
| PN | 26 (16.2%) | 8 (4.9%) | .001 |
| Infections (excluding herpes zoster) | 24 (15.0%) | 25 (15.5%) | .895 |
| Herpes zoster | 5 (3.1%) | 6 (3.7%) | .767 |
| Gastrointestinal events (excluding constipation) | 20 (12.5%) | 11 (6.8%) | .086 |
| Fever | 15 (9.3%) | 9 (5.5%) | .197 |
| Constipation | 11 (6.8%) | 6 (3.7%) | .208 |
| Skin rash | 8 (5.0%) | 5 (3.1%) | .389 |
| Thrombocytopenia | 9 (5.5%) | 0 | .002 |
| Any grade 3 or 4 AE | 17 (10.6%) | 15 (9.3%) | .696 |
| Nonhematologic grade 3 or 4 AEs | 15 (9.3%) | 14 (8.6%) | .832 |
| Gastrointestinal events (excluding constipation) | 3 (1.8%) | 1 (0.6%) | .311 |
| Infections (excluding herpes zoster) | 2 (1.2%) | 5 (3.1%) | .255 |
| Fever | 1 (0.6%) | 2 (1.2%) | .566 |
| Herpes zoster | 1 (0.6%) | 1 (0.6%) | .996 |
| Constipation | 1 (0.6%) | 1 (0.6%) | .996 |
| Skin rash | 1 (0.6%) | 1 (0.6%) | .996 |
| PN | 1 (0.6%) | 0 | .315 |
| Deep vein thrombosis | 1 (0.6%) | 1 (0.6%) | .996 |
| Hepatic | 1 (0.6%) | 0 | .315 |
| Pancreatitis | 1 (0.6%) | 0 | .315 |
| Hyperglycemia | 0 | 2 (1.2%) | .157 |
Data are number (%).
AE indicates adverse event; VTD, bortezomib with thalidomide plus dexamethasone; TD, thalidomide plus dexamethasone; and PN, peripheral neuropathy.
Discussion
When discussing treatment strategies for patients with MM, the terms consolidation and maintenance therapy are often used synonymously, although they identify 2 treatment phases with different goals. Consolidation therapy is, by definition, short-term and intended to further enhance the rate and quality of response obtained with the previous treatment phase(s). Maintenance therapy is generally long-term and typically aims to reduce the risk of progression or relapse and to prolong OS. To our knowledge, this is the first full report from a phase 3 trial primarily aimed at comparing a triplet versus a doublet novel agent–based induction therapy in which the efficacy and safety of the same triplet or doublet regimens given as consolidation therapy, which was a secondary end point, were specifically assessed. For these purposes and because the superior rate of high-quality responses to VTD versus TD consolidation was previously demonstrated on an ITT basis, a per-protocol analysis of those patients who completed the allocated treatment seemed to be the more appropriate method.
The results of this per-protocol analysis confirmed the superior efficacy of VTD versus TD consolidation therapy.13 Our results for the ITT population appear generally applicable to the transplant-eligible population of MM patients as a whole, given the representative nature of our patient population of those receiving transplantation in clinical practice. The superiority of VTD over TD as consolidation therapy is noteworthy because the per-protocol analysis was restricted to those patients who were more compliant to therapy or whose disease was inherently more sensitive to treatment. This is shown by the higher rates of response after all treatment phases seen in both groups in the present analysis compared with those previously reported for the ITT population,13 as well as by comparable rates of high-quality responses after the second ASCT in the VTD-treated and TD-treated groups, which differs from what was found in the ITT population.13 Exclusion from the per-protocol analysis of patients who discontinued treatment because of progressive disease (who were more frequently included in the TD arm; Figure 1) or because of AEs or other reasons (in whom response was deeper in the VTD arm compared with the TD arm) explains the discrepancies seen in terms of high-quality response rates at the landmark of starting consolidation therapy between the per-protocol population and ITT population of patients. Overall, among the 144 patients who were excluded from the per-protocol analysis, the rate of CR/nCR was 3 times higher in the VTD arm compared with the TD arm (39.4% vs 12.3%; P < .0001). Nevertheless, the efficacy of consolidation therapy was significantly enhanced with the addition of bortezomib to TD, as shown by the higher rates of CR (60.6% vs 46.6%) and CR/nCR (73.1% vs 60.9%). In particular, the probability of upgrading from less than CR to CR after consolidation therapy was approximately 2 times higher with VTD versus TD. The favorable impact of VTD, but not TD, consolidation on enhanced rates of CR and CR/nCR was further confirmed using the McNemar test. These findings support the conclusion that the superiority of VTD over TD when administered as induction therapy in newly diagnosed patients13 was retained when VTD was readministered as consolidation therapy after ASCT. These results appear generally applicable to the overall population of patients who are able to receive consolidation therapy after transplantation, based on the similarity of the patients' characteristics in our per-protocol and ITT analyses, and the consistent superiority of VTD over TD as induction and consolidation in these outcome analyses.
Compared with TD as consolidation therapy, VTD was associated with extended TTP and PFS from the landmark of starting consolidation therapy. Use of the landmark analysis for these outcomes allowed us to more carefully define the specific contribution from VTD consolidation to improved clinical outcomes previously reported for the ITT population of patients randomized to the VTD arm of the study.13 Differences in outcomes with VTD versus TD consolidation therapy remained significant when the 9 patients who received dexamethasone as single-agent consolidation were included in the landmark analyses. Consistent with the demonstrated superior efficacy of VTD compared with TD consolidation therapy in terms of enhanced rates of high-quality responses, the reduced risk of progression or death associated with VTD versus TD consolidation was particularly evident for patients who had not achieved CR and CR/nCR after double ASCT. In these patient subgroups, the relative reduction in the risk of progression or death with VTD versus TD consolidation therapy was 41% and 51%, respectively. Studies aimed at detecting the presence or absence of minimal residual disease by multiparametric flow cytometry or molecular techniques before and after consolidation therapy may help to more carefully assess the role of consolidation therapy in patients with conventionally defined CR after ASCT. Positive results of univariate analyses performed in this per-protocol population were further confirmed by a multivariate regression analysis that identified VTD consolidation as an important and independent variable favorably affecting PFS.
Importantly, the superior PFS with VTD versus TD consolidation therapy was retained in poor-prognosis patients at high risk of relapse or death, including those with advanced ISS disease stage and an adverse cytogenetic profile characterized by the presence of t(4;14) and/or del(17p). Conflicting data regarding the clinical outcomes of patients with adverse cytogenetic abnormalities treated with bortezomib-based regimens and ASCT have been reported.32,33 In our per-protocol population of patients with presence of t(4;14) and/or del(17p), or who carried t(4;14) but lacked del(17p), those receiving VTD consolidation therapy had a 63% and 72% relative reduction in the risk of progression or death, respectively, compared with the TD-treated group. Importantly, the triplet VTD regimen overcame the poor prognosis associated with the presence of a high-risk cytogenetic profile, which conversely retained its adverse impact on PFS in TD-treated patients.
Data from other reports are consistent with our findings of the clinical benefit of consolidation therapy after ASCT. In a randomized trial, bortezomib as single-agent consolidation therapy was compared with no consolidation in a population of bortezomib-naive patients.26 In several phase 2 studies, the role of consolidation therapy with conventional cytotoxic drugs or the novel agents thalidomide, bortezomib, and lenalidomide, alone or in combination, was explored in patients with no prior exposure to any of the novel agents or who had received the new drugs as part of induction therapy.18,23-25 In all these trials, consolidation therapy was reported to increase the rate of high-quality responses,23-26 even to the molecular level.24 In several studies, prolonged PFS and OS with consolidation therapy were also reported.18,26
At the time of the present analysis, no difference in OS between patients receiving VTD or TD consolidation therapy was recorded. This finding could have 2 possible explanations. First, the short follow-up period did not allow us to appreciate different survival distributions, a hypothesis supported by results of several previous trials in which many years from start of therapy were required before divergences between survival curves could be detected.34 Second, OS was not a primary study end point. Therefore, the study was not designed with the statistical power to demonstrate a survival benefit with VTD versus TD consolidation after double ASCT. Finally, proving an OS benefit at this time is probably difficult because of the rapidly increasing availability of effective salvage therapies at the time of relapse, which might favorably influence the course of the disease, an issue not addressed in the current analysis. Although the issue of possible emergence of more resistant clones at the time of relapse in the VTD arm was beyond the aim of the current analysis, no statistically significant difference between the 2 treatment groups was seen in terms of OS after relapse.
A substantial reduction in toxicity was seen during or after the 2 cycles of VTD consolidation therapy compared with that previously reported for VTD induction therapy.13 In particular, the reduced dose of thalidomide compared with the induction phase and the different schedule of bortezomib administration (once-weekly vs twice-weekly during induction) were chosen to reduce the risk of neurologic toxicity, particularly of grade 3 and 4. While this goal was achieved, with only one case of grade 3 PN with VTD consolidation after a preexisting mild neurologic toxicity, the rate of grade 2 neurologic toxicity remained significantly higher with VTD versus TD consolidation therapy. Subcutaneous administration of full-dose bortezomib has been recently reported to be associated with reduced PN35 and would potentially allow a higher dose-intensity and/or more prolonged consolidation therapy. Whether twice-weekly subcutaneous bortezomib administration and/or more than 2 cycles of treatment might ultimately result in improved activity and lesser toxicity compared with those herein reported remains an open issue.
In conclusion, specific response and landmark outcome analyses performed in the per-protocol population demonstrate that VTD consolidation therapy significantly contributed to improved clinical outcomes observed for patients randomly assigned to the VTD arm of the study.13 The role of consolidation therapy after ASCT in MM warrants further investigation in ongoing prospective randomized clinical trials specifically designed to address this issue.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicoletta Testoni, Carolina Terragna, Giulia Marzocchi, and Sandra Durante for doing cytogenetic and molecular analyses; Mauro Fiacchini for valuable support in statistical analysis of the data; Katia Vitali for supporting data analysis; Francesca Miselli for help in data collection; and Jane Saunders and Stephen Hill of FireKite for editorial assistance in the development of this report, which was supported by Millennium Pharmaceuticals Inc and Janssen Global Services.
This work was supported and sponsored by Seràgnoli Institute of Hematology at the University of Bologna, Bologna, Italy, and supported in part by Janssen providing bortezomib free of charge, by the University of Bologna (Ricerca Fondamentale Orientata; M.C.) for substudies within the main study, and Fondazione Dal Monte and Bologna (M.C.) for substudies within the main study.
Authorship
Contribution: M.C. was the principal investigator, designed the study, analyzed and interpreted data, and wrote the manuscript; L.P., E.Z., G.P., and A. Brioli analyzed and interpreted data and reviewed the report; A. Pezzi provided all statistical analyses and reviewed the report; and all remaining authors designed the study, recruited patients, and reviewed the report.
Conflict-of-interest disclosure: M.C. has received honoraria and served on speakers' bureaus for Janssen, Celgene, and Novartis; and has been a consultant for Janssen, Celgene, Novartis, and Millennium Pharmaceuticals. M.T.P. has received honoraria from Janssen and Celgene. P.M. has received honoraria and served as consultant for Janssen and Celgene. A. Palumbo has served on an advisory committee for Celgene and Janssen and has received honoraria from Celgene, Janssen, Merck, and Amgen. E.Z. has received honoraria from Janssen and Celgene. M.B. has served on an advisory committee for Celgene and Janssen and has received honoraria from Celgene and Janssen. The remaining authors declare no competing financial interests.
A complete list of investigators of the GIMEMA Italian Myeloma Network can be found in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Michele Cavo, Università degli Studi di Bologna, Istituto di Ematologia “Seràgnoli,” Policlinico S. Orsola-Malpighi, via Massarenti 9, 40138, Bologna, Italy; e-mail: michele.cavo@unibo.it.