Abstract
Multiple myeloma (MM) is a plasma cell dyscrasia characterized by the presence of multiple myelomatous “omas” throughout the skeleton, indicating that there is continuous trafficking of tumor cells to multiple areas in the bone marrow niches. MM may therefore represent one of the best models to study cell trafficking or cell metastasis. The process of cell metastasis is described as a multistep process, the invasion-metastasis cascade. This involves cell invasion, intravasation into nearby blood vessels, passage into the circulation, followed by homing into predetermined distant tissues, the formation of new foci of micrometastases, and finally the growth of micrometastasis into macroscopic tumors. This review discusses the significant advances that have been discovered in the complex process of invasion-metastasis in epithelial carcinomas and cell trafficking in hematopoietic stem cells and how this process relates to progression in MM. This progression is mediated by clonal intrinsic factors that mediate tumor invasiveness as well as factors present in the tumor microenvironment that are permissive to oncogenic proliferation. Therapeutic agents that target the different steps of cell dissemination and progression are discussed. Despite the significant advances in the treatment of MM, better therapeutic agents that target this metastatic cascade are urgently needed.
Introduction
Multiple myeloma (MM) is a plasma cell dyscrasia characterized by the presence of multiple lytic lesions at the time of diagnosis.1,2 The presence of multiple myelomatous “omas” throughout the axial skeleton detected at the time of diagnosis in most patients indicates that there is continuous spread or dissemination of tumor cells from the original site of tumor development to multiple sites in the BM niches, leading to the final development of symptomatic disease. Therefore, MM may represent a good model disease to study cell trafficking or cell metastasis. Although the term “metastasis” is not commonly used to describe dissemination of hematologic malignancies, this review attempts to examine how MM can use a process of cell dissemination that is similar to cell trafficking of hematopoietic stem cell (HSCs) and cell metastasis in solid epithelial carcinomas. These studies can guide our understanding of the biologic changes that occur during progression in MM.
Cell metastasis and cell trafficking
The process of cell metastasis is usually described as a multistep process, often termed as the invasion-metastasis cascade.3-6 This involves several steps of changes that include: (1) cell invasion, (2) intravasation (egress) into nearby blood vessels, (3) passage of the tumor cells into the circulation, followed by (4) homing or extravasation of tumor cells from these vessels into the specific predetermined distant tissues, (5) the formation of new foci of tumor micrometastases, and (6) finally the growth of micrometastatic lesions into macroscopic tumors, a step called “colonization.”
A similar process occurs with cell trafficking of HSCs.7,8 Most HSCs reside in the BM and undergo self-renewal, but some would leave the BM to enter the bloodstream (egress of intravasation). These cells home again to new sites of the BM through sinusoids that express trafficking molecules that support a unique multistep adhesion cascade.7
Tumor growth in MM
Indeed, if we follow the same steps and examine the process of tumor progression in MM, we observe that it follows a similar invasion-metastasis cascade (Figures 1 and 2). The process of initiation of MM is probably from long-lived plasma cells that develop in germinal centers of lymphoid tissues and home to the BM where they survive for years.9 Oncogenic transformations along with support of the microenvironmental niche allow the growth, survival, and proliferation of these cells in the initial sites of the BM niches.9
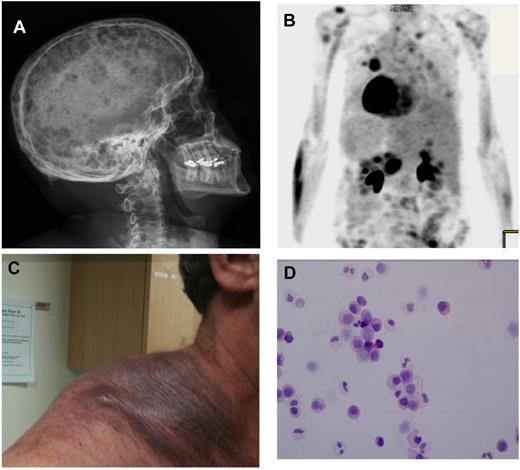
Clinical presentations of cell dissemination and metastasis in MM. (A) Skeletal survey showing multiple lytic lesions in the skull of a patient diagnosed with symptomatic multiple myeloma (MM). These multiple lesions represent multiple sites of growth of MM cells within the BM niches in the skull. (B) A PET scan showing multiple areas of enhancement in a patient with extramedullary MM, indicating that MM cells can metastasize to areas outside the BM in a subgroup of patients with extramedullary MM. (C) Extramedullary MM presenting as a large subcutaneous mass on the shoulder of a patient with advanced disease. (D) Circulating tumor plasma cells observed in a patient with MM demonstrating that a small number of tumor cells are continuously circulating in the peripheral blood leading to cell dissemination. This patient does not have plasma cell leukemia.
Clinical presentations of cell dissemination and metastasis in MM. (A) Skeletal survey showing multiple lytic lesions in the skull of a patient diagnosed with symptomatic multiple myeloma (MM). These multiple lesions represent multiple sites of growth of MM cells within the BM niches in the skull. (B) A PET scan showing multiple areas of enhancement in a patient with extramedullary MM, indicating that MM cells can metastasize to areas outside the BM in a subgroup of patients with extramedullary MM. (C) Extramedullary MM presenting as a large subcutaneous mass on the shoulder of a patient with advanced disease. (D) Circulating tumor plasma cells observed in a patient with MM demonstrating that a small number of tumor cells are continuously circulating in the peripheral blood leading to cell dissemination. This patient does not have plasma cell leukemia.
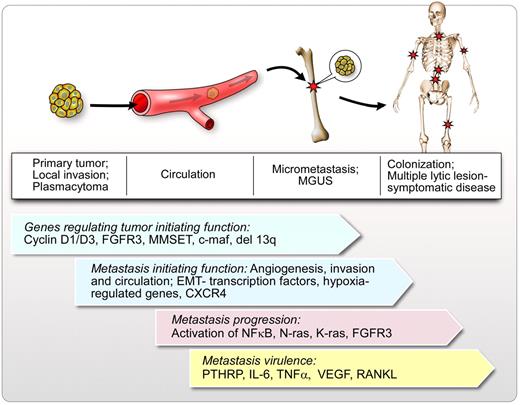
Model of metastasis and dissemination in MM. In this schematic figure, the initial site of tumor growth of clonal plasma cells is represented by a solitary plasmacytoma (these are not clinically detected in most cases). In a small group of patients, solitary plasmacytomas do not disseminate. However, in the majority of patients, local invasion occurs, which allows some cells to egress into the peripheral circulation (circulating tumor cells) followed by specific homing into BM niches and local micrometastasis. Micrometastasis is represented by the clinical condition of MGUS. MGUS can progress to macrometastasis or colonization after a long latency period, leading to symptomatic disease with multiple lytic lesions, anemia, hypercalcemia, and renal failure. Genes regulating tumor initiation, metastasis initiation, metastasis progression, and metastasis virulence are represented in the figure. This is not a complete list of genes that could regulate cell trafficking in MM but represents some of the known regulators in cell dissemination and progression in MM.
Model of metastasis and dissemination in MM. In this schematic figure, the initial site of tumor growth of clonal plasma cells is represented by a solitary plasmacytoma (these are not clinically detected in most cases). In a small group of patients, solitary plasmacytomas do not disseminate. However, in the majority of patients, local invasion occurs, which allows some cells to egress into the peripheral circulation (circulating tumor cells) followed by specific homing into BM niches and local micrometastasis. Micrometastasis is represented by the clinical condition of MGUS. MGUS can progress to macrometastasis or colonization after a long latency period, leading to symptomatic disease with multiple lytic lesions, anemia, hypercalcemia, and renal failure. Genes regulating tumor initiation, metastasis initiation, metastasis progression, and metastasis virulence are represented in the figure. This is not a complete list of genes that could regulate cell trafficking in MM but represents some of the known regulators in cell dissemination and progression in MM.
An example of such localized tumor proliferation without distant metastasis in MM is represented in solitary plasmacytomas, where localized progression and oncogenic proliferation occur without evidence of distant dissemination. This phenomenon may present clinically as solitary plasmacytomas in a minority of patients,10,11 but it probably remains undetected in most cases. This may represent the first original site of tumor initiation in MM (Figure 2). In most patients with this disease, these cells do not remain localized but disseminate and engraft multiple areas of the axial skeleton.10,11 The continuous trafficking process leads to micrometastasis, which is represented by monoclonal gammopathy of undetermined significance (MGUS), a common disease that precedes overt MM in many cases. MGUS progresses to overt MM at a slow rate of 1% per year, but it almost always precedes MM, indicating that indeed micrometastasis slowly leads to overt colonization and infiltration of the BM.12-14 In long-term follow-up studies of patients with MGUS, there was an incremental increase in the rate of progression of MGUS to overt MM.15 The cumulative probability of progression was 12% at 10 years, 25% at 20 years, and 30% at 25 years.15 Most intriguingly, in a nationwide population-based prospective study, all cases that presented with MM and had prior blood samples available showed the presence of MGUS that preceded MM.16 MGUS was present in all the cases up to 8+ years before MM diagnosis. In approximately half the study population, the M-protein concentration level showed a yearly increase before MM diagnosis.16
Finally, with spread and growth of these lesions, the clinical manifestations of MM begin to appear with anemia, hypercalcemia, renal failure, and multiple lytic lesions12-14 (Figure 2). In some rare cases, MM present as macrofocal disease,17 a term used to define cases where there are multiple skeletal lesions with or without soft tissue masses and less than 10% involvement with BM plasma cells. This condition further confirms the notion that MM is indeed a metastatic disease. In more advanced cases of MM, the number of circulating tumor cells increases significantly because of rapid infiltration of BM niches and leads to acquisition of independence on the microenvironment and the development of plasma cell leukemia.18 Similarly, in cases of extramedullary involvement, the tumor cells do not only home to the BM niches but home to other organs, including subcutaneous sites or to the liver, gut, lungs, and rarely central nervous system. These conditions lead to multiple plasmacytomas (metastatic lesions) in these organs as shown in Figure 1 (plasmacytomas of the skin). Extramedullary MM represents an entity in which the clonal plasma cells have lost their dependence on the BM milieu for growth. This entity occurs de novo in approximately 7% to 19% of newly diagnosed patients and another 6% to 20% in patients over the course of their disease.19-21 However, some studies have shown that the incidence is much higher after allogeneic stem cell transplantation, with up to 32% of cases in one series. More interestingly, the numbers are much higher in late stages; in an autopsy series, up to 70% of patients showed involvement in extraskeletal sites of disease including 40% showing liver involvement.20,22
The BM niches
Stem cell niches or BM niches have been described as anatomic and functional dimensions that specifically enable stem cells to self-renew.8,23 This concept was first proposed by Schofield almost 30 years ago, where he described it as a specialized microenvironment that housed stem cells.24 At least 2 distinct niches supporting stem cells have been identified in the BM: the osteoblastic or endosteal niche and the vascular niche,25,26 although these distinctions are currently being challenged.27 Quiescent HSCs reside in the endosteal niche indicating that this niche might contain the most dormant HSCs and therefore serve as a quiescent-storage niche or a self-renewing niche. Osteoblasts and HSCs are closely associated in this niche, leading to a reciprocal relationship between the 2 types of cells that lead to the production of various growth factors, such as the receptor activator of NF-κB ligand (RANKL) and Notch activation26 and the regulation of the number of HSCs. Once these cells are ready for proliferation, they detach from the endosteal niche and migrate toward the center of the BM where they are in contact with endothelial cells in the vascular niche from where they reestablish hematopoiesis. The proximity of these cells to the blood vessels would enable them to monitor the concentration of various signals and factors that are in the blood circulation and regulate the hematopoietic system.8,23
Whether a similar process occurs in MM is not well defined. Our group has shown that MM cells could be seen interacting with the endothelium of the calvarial BM vasculature within minutes after intravenous injection into the tail vein of SCID mice using intravital confocal microscopy28 (Figure 3). MM cells were found closely associated with the BM vasculature. At very early time points after injection, we saw no overt interaction between the MM cells and BM osteoblasts. This is different from normal HSCs/progenitor cells that home in close proximity to both the vasculature and endosteal surface immediately after injection. However, these studies did not address whether a more primitive progenitor MM cell could be located in the endosteal niche and provide support of the more proliferative late-stage tumor cells present in the vascular niche. Such studies would be critical in examining in-depth the regulators of minimal residual disease and progenitor cells in MM.
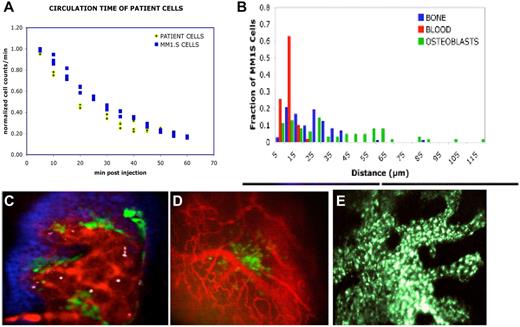
In vivo tracking of tumor cell trafficking in a MM mouse model. (A) Depletion of CD138+ patient cells from the circulation occurs with the same kinetics as MM.1S cell line. MM.1S (n = 4) or MM patient sample cells (n = 5) were labeled with fluorescent cytoplasmic or membrane dyes, injected into mice, and immediately the proportion of cells remaining in the circulation was measured by in vivo flow cytometry and plotted against time (adapted from Figure 3 of Runnels et al154 with permission). (B) MM cells position themselves in proximity to the vasculature. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine–stained MM.1S cells were injected intravenously into Col2.3-GFP mice at a dose of 100 000 cells per mouse. Immediately before imaging, the mice were injected with the vascular marker Quantum Dots 800. The mice were imaged within 2, 6, and 72 hours after MM cell injection. Z stacks were acquired from multiple regions in the calvaria of the mice. Distances were measured and tabulated between MM cells and osteoblasts or endosteal surface for the first 6 hours after MM cell injection. (Adapted from Figure 3 of Runnels et al154 with permission). (C) Imaging at 72 hours after MM cell injection. The image demonstrates the relationship of the MM cells (white) to the vasculature (red), osteoblasts (green), and bone (blue) during the first 72 hours after cell injection. Scale bars represent 100 μm. (Adapted from Figure 3 of Runnels et al154 with permission). (D) Imaging shows vessel formation around an area of GFP-positive MM cells growing in a cluster in close association to blood vessels. Immediately before imaging, the mice were injected with the vascular marker Quantum Dots 800. The MM1S cells are GFP-positive (green color). Scale bars represent 100 μm. (E) Primary plasma cells injected from a patient with plasma cell leukemia and allowed to engraft and proliferate for 8 weeks. A green-fluorescently labeled anti-CD138 antibody was injected intravenously just before imaging to allow imaging of the cells.
In vivo tracking of tumor cell trafficking in a MM mouse model. (A) Depletion of CD138+ patient cells from the circulation occurs with the same kinetics as MM.1S cell line. MM.1S (n = 4) or MM patient sample cells (n = 5) were labeled with fluorescent cytoplasmic or membrane dyes, injected into mice, and immediately the proportion of cells remaining in the circulation was measured by in vivo flow cytometry and plotted against time (adapted from Figure 3 of Runnels et al154 with permission). (B) MM cells position themselves in proximity to the vasculature. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine–stained MM.1S cells were injected intravenously into Col2.3-GFP mice at a dose of 100 000 cells per mouse. Immediately before imaging, the mice were injected with the vascular marker Quantum Dots 800. The mice were imaged within 2, 6, and 72 hours after MM cell injection. Z stacks were acquired from multiple regions in the calvaria of the mice. Distances were measured and tabulated between MM cells and osteoblasts or endosteal surface for the first 6 hours after MM cell injection. (Adapted from Figure 3 of Runnels et al154 with permission). (C) Imaging at 72 hours after MM cell injection. The image demonstrates the relationship of the MM cells (white) to the vasculature (red), osteoblasts (green), and bone (blue) during the first 72 hours after cell injection. Scale bars represent 100 μm. (Adapted from Figure 3 of Runnels et al154 with permission). (D) Imaging shows vessel formation around an area of GFP-positive MM cells growing in a cluster in close association to blood vessels. Immediately before imaging, the mice were injected with the vascular marker Quantum Dots 800. The MM1S cells are GFP-positive (green color). Scale bars represent 100 μm. (E) Primary plasma cells injected from a patient with plasma cell leukemia and allowed to engraft and proliferate for 8 weeks. A green-fluorescently labeled anti-CD138 antibody was injected intravenously just before imaging to allow imaging of the cells.
The role of the endosteal niche in regulating cell numbers is not confined to HSCs. Indeed, recent studies provided direct evidence that osteoblasts play a central role in bone metastases.29 The study showed that metastatic prostate cancer cells preferentially home to the osteoblastic niche in the BM, where they compete with normal HSCs for niche support. In this study, the tumors directly competed with HSCs for occupancy of the endosteal HSC niche. Using conditional osteoblast knockout tissues, fewer metastatic cells were able to home to the BM. Conversely, increasing the number of HSC niches with parathyroid hormone promoted metastasis.29
Preferential homing to the BM in MM
The mechanisms by which MM cells and many other metastatic tumors preferentially home to the BM are not well understood. Bone metastasis represents nearly 70% of cases of metastasis in breast and prostate cancer and approximately 15% to 30% of patients with carcinomas of the lung, colon, stomach, bladder, uterus, rectum, thyroid, or kidney.30 Most hematologic malignancies preferentially traffic to the BM and lymph nodes.30
One hypothesis to this preferential trafficking or homing to the BM is that these cells hijack the same homing behavior of HSCs.23,31,32 Similarly, molecules that play critical roles in HSC niche selection are thought to be used by metastatic cells' homing to the BM, including chemoattractants, such as stromal derived factor-1 (SDF-1, also called CXCL12), attachment factors (annexin II), and regulators of cell growth, and vascular recruitment (IL-6) and VEGF.30 Other factors that contribute to the selective homing to the BM include the high blood flow in areas of red marrow accounting for the predilection of metastases for those sites.30 The bone is also a large repository for immobilized growth factors, including TGF-β, insulin-like growth factors I and II (IGF), fibroblast growth factors, platelet-derived growth factors, bone morphogenetic proteins, and calcium.30 In addition, parathyroid hormone-related protein is also a critical regulator of bone metastasis and the regulation of stem cell homing to the BM.33-37
The steps of invasion-metastasis
The process of invasion and intravasation (or egress)
In solid tumors, the process of metastasis is initiated with a first step termed epithelial-mesenchymal transition (EMT). In this process, transformed epithelial cells acquire the abilities to invade, resist apoptosis, and disseminate.38-40 This process is hijacked from embryonic morphogenesis and wound healing where there is activation of several transcriptional factors, including Snail, Slug, Twist, and ZEB1 and ZEB 2. These transcriptional regulators orchestrate the EMT process and allow the process of invasion and metastasis to occur.38-43 This process leads to inhibition of E-cadherin.44 Loss of E-cadherin is one of the best-characterized regulators of invasion and metastasis. It is a key cell-to-cell adhesion molecule that is frequently down-regulated or mutated leading to inactivation in human carcinomas, indicating that it is a key suppressor of metastasis.5,45 In addition, some miRNAs can specifically regulate the process of EMT transition.46 For example, miRNA-200 promotes EMT-inducing transcription factors.47 This EMT process is not only restricted to epithelial tumors. Other studies have shown that other nonepithelial tumor types, such as sarcomas and neuroectodermal tumors, show activation of the EMT program.45,48
In addition, hypoxia plays a critical role in the process of EMT and metastasis. Hypoxia contributes to progression and metastasis by activating transcriptional programs that promote cell survival, motility, and tumor angiogenesis.49,50 Recent studies show that each step of the metastasis process, from the initial EMT to the ultimate colonization, can be regulated by hypoxia, suggesting a master regulator role of hypoxia in metastasis.51
In HSC cell trafficking, egress of HSCs from the endosteal niche to the vascular niche is regulated by c-kit/stromal cell factor, CXCR4/SDF-1, MMP-9, and G-CSF.52 In addition, the regulation of CXCR4/SDF-1 and MMP2/MMP9 is critical for the release of HSCs into the peripheral blood.52 The influence of CXCL12 on detachment/egress of cells from their tissue niches was studied in models of mobilization of HSCs.53-55 Mobilization may be enhanced by disrupting the SDF-1/CXCR4 axis, by decreasing the concentration of endogenous SDF-1 (eg, after infusion of G-CSF or cyclophosphamide) in the BM as performed for stem cell transplantation,54 or the cleavage and inactivation of SDF-1 by proteases in the BM.55 Direct measurement of oxygen levels has revealed that the BM is, in general, quite hypoxic (1%-2% O2).56 The endosteal niche is usually hypoxic, and only oxygen-independent cells are able to survive. Hypoxia induces quiescence of these cells and resistance to therapeutic agents. In addition, osteoblasts secrete high levels of SDF-1 (chemokine ligand of CXCR4) leading to adhesion of HSCs to these cells.
In MM, the most identified factors regulating cell trafficking of MM cells include the CXCR4/SDF-1 axis, IGF-1, and intracellular regulators downstream of CXCR4, including Rho and Rac.57-59 Of these, the CXCR4/SDF-1 axis plays a critical role in regulating migration and adhesion of MM cells. CXCR4 regulates both homing and mobilization of MM cells. Plerixafor (AMD3100; Genzyme) induced disruption of the interaction of MM cells with the BM reflected by mobilization of MM cells into the circulation in vivo, with kinetics that differed from that of HSCs.58 Similar to the role of hypoxia in EMT activation and cell dissemination in epithelial cancers, we recently showed that hypoxia leads to inactivation of E-cadherin and activation of the transcription factors regulating EMT, including Snail and Twist,60 indicating that this process is activated in MM, just as it is in epithelial tumors. Similarly, other studies have shown that the BM in MM is hypoxic.61-64
Circulation in the peripheral blood
Prior studies in solid tumors have shown that circulating tumor cells are most vulnerable during their passage in the peripheral blood circulation because of the lack of adhesion to the extracellular matrix and environmental cells as well as immune surveillance and shear stress in the circulation.5 This may lead to anoikis, which is a form of apoptosis triggered by loss of adhesion to substratum.65,66 It is not known how long circulating tumor cells last in the circulation; it may be a few minutes or several hours in the circulation.5 This short transit may allow the cells to escape immune surveillance. In addition, another mechanism by which cells can avoid anoikis and immune surveillance involves tumor cells attaching themselves to platelets through tissue factor and/or L- and P-selectins to form microemboli or microthrombi that carry them to the target organs.67
In MM, studies have demonstrated the presence of a small number of circulating plasma cells in more than 70% of patients with MM.68 The presence of circulating plasma cells was prognostic in these patients. Similar results were observed in earlier stages of the disease, including smoldering MM and MGUS, indicating that they can have a value in predicting risk of progression.69 Studies to determine the phenotypic and genotypic characteristics of these circulating cells in MM are ongoing.70 One question that has not been answered is whether these cells represent specific subclones of MM that are present in the BM and that have a higher propensity to circulate and home to new niches. Another question is whether these cells have stem cell–like features. Studies in epithelial tumors have shown that EMT transition is linked to acquisition of a stem cell–like phenotype.42
The process of extravasation or homing
The extravasation of blood-circulating stem cells into extravascular tissues appears to invoke a multistep adhesion cascade similar to that initially described in the intravasation process.71 However, the process may be different as the new host microenvironment is not adapted for the growth and protection of the migrating cells.5 For example, neo-angiogenesis has not occurred in the new host microenvironment. Migration of cells through the blood to the BM niches is not a passive process, but rather an active process of navigation that involves multiple adhesion and chemokine receptors. Homing involves tethering of the cells by E- and P-selectin that are associated with P-selectin glycoprotein ligand-1 and CD44 on HSCs.72 This tethering occurs as an interaction of endothelial cells with circulating HSCs. This leads to rolling of the HSCs on the endothelium and activation of the SDF-1/CXCR4 axis, followed by VLA-4/VCAM-1 activation. This activation leads to transmigration of the HSCs into the BM vascular niche through activation of the SDF-1/CXCR4 axis. Other molecules that could also be playing a role in homing include LFA-1, VLA-5, and activation of metalloproteases MMP2/9 in HSCs.72-74 Moreover, chemokines and integrins interact in a complex signaling cascade. For example, SDF-1 has been reported to induce firm adhesion and migration by inducing activation of integrins, such as LFA-1, VLA-4, and VLA-5 on HSCs.53,75-77 Interestingly, adhesion molecules may also trigger signals for both enhanced CXCR4 expression and increased function.78
As discussed before, the SDF-1/CXCR4 axis plays a critical role in homing of MM cells to the BM.57,58 CXCR4 is essential for the migration of MM cells in vitro and their homing to the BM in vivo. CXCR4 knockdown led to significant inhibition of migration to SDF-1 in MM cell lines and primary CD138+ cells. In addition, Rho and Rac are critical regulators of MM migration, homing, and adhesion.59 In addition, the invasive potential of MM cells involves the action of MMP-9. MM cells were shown to constitutively produce MMP-9.79,80 Similar results were observed with a VLA-4 (α4β1) inhibitory antibody28 or with a selective antibody Natalizumab (Biogen Idec).81 Similarly, P-selectin glycoprotein ligand-1 knockdown or inhibition by a small pan-selectin inhibitor (GMI-1070; Glycomimetics) showed significant inhibition of rolling and homing in vitro and in vivo.82 Similarly, we showed another interaction of the SDF-1/CXCR4 with downstream GTPases, including RhoA and Rac1.59 Other regulators of homing in MM include integrin-β7, VEGF, and IGF-1.83-86
In cases of extramedullary MM and plasma cell leukemia, plasma cells can change the expression of their homing and adhesion molecules to allow them to home to other organs. Although this process is not well defined, some studies have shown that plasma cells in extramedullary MM show decreased expression of adhesion molecules, such as VLA-4, CD44, loss of CD56, as well as disruption of the CXCR4/SDF-1 axis.
Although their role in homing in MM is not well understood, there are 2 other critical receptors that regulate adhesion in MM. These are CD138 or syndecan and CD44. MM tumors are characterized by the high expression of syndecan-1 (CD138), a heparan sulfate proteoglycan present on the myeloma cell surface and shed into the tumor microenvironment.87,88 High levels of shed syndecan-1 in the serum of patients are an indicator of poor prognosis, and numerous studies have implicated syndecan-1 in promoting the growth and progression of this cancer. Another proteoglycan is CD44. Expression of CD44v9-containing isoforms (CD44v9) on MM plasma cells correlates with unfavorable prognosis.89,90 CD44v9-mediated plasma cell binding resulted in a significant induction of IL-6 secretion by BM stromal cells.89,90 Further studies to better define the role of these important molecules in regulating cell dissemination and adhesion in MM are needed.
Micrometastasis and the premetastatic niche
The new host microenvironment is not well adapted to the cancer cells that metastasized into it.5,6,45 Therefore, significant changes in the stroma, endothelial cells, ECM constituents, cytokines, and chemokines need to occur to allow for the growth and survival of these metastastic cells. Preparation of the metastatic niche occurs even before the first metastastic cell arrives. Based on this intriguing concept of a premetastatic niche, cancer cells in the original site release systemic signals to specific niches in preparation for metastasis.91,92 Some of these signals include cytokines and chemokines or cellular elements. For example, lysyl oxidase has been shown to induce a premetastatic niche by up-regulating fibronectin from resident tissue fibroblasts. Similarly, cellular elements, such as hematopoietic progenitor cells (VEGFR1-positive) are mobilized from the BM to these premetastatic sites to prepare the microenvironment by secreting MMP-9 and activating integrins and SDF-1.5 Most interestingly, rerouting of these hematopoietic progenitor cells leads to alterations in distant metastasic sites in lung carcinoma.91,92
Other carriers of information include microvesicles, microparticles, exosomes, or platelets as well as endothelial progenitor cells, all of which have been shown to alter the premetastatic niche in different studies.93-99 Exosomes are small nanometer-sized (50-100 nm) vesicles of endocytic origin, which are released in the extracellular milieu by several cell types.100-109 Previous studies have shown the intriguing role of exosomes in tumor progression because of the ability of tumor cell-derived exosomes to modulate and mold the host microenvironment, thereby promoting tumor cell growth and disease progression.110-113
Although preparation of the premetastatic niche has not been studied in MM, we recently showed in preliminary data that stromal cells present in contact with MM cells secrete exosomes that modulate the growth and dissemination potential of MM cells.114 Our study showed that MM-derived BM stromal cells release exosomes, which are transferred to tumor cells, thereby resulting in modulation of tumor growth in vivo. However, studies to define tumor-derived exosomes and their role in preparing the premetastatic niche in MM have not been performed.
Colonization
Micrometastasis or its analogous stage of MGUS in MM may persist for years without evidence of progression. This could be the result of a steady state of viability in the absence of any net gain or loss in overall cell number.115 The cells can achieve this steady state by inducing quiescence (stem cell–like feature) or through external suppression of tumor growth by the surrounding host microenvironment.115 At later stages, a change in the microenvironment then allows tumor growth.5
Interestingly, in solid tumors, the process of colonization is not very effective and is considered the most rate-limiting step for metastasis to occur.5 It is estimated that 80% of cells can survive the circulation and extravasate to distant organs; however, only 3% or less can achieve micrometastasis and only less than 0.02% can achieve colonization and macroscopic metastasis.5 This indicates that only a small fraction of micrometastatic cells acquire genetic or epigenetic changes that lead to active growth and colonization. We may postulate that a similar process occurs in MM; therefore, it is not surprising, then, that patients with MGUS (micrometastasis) only have 1% chance of progression per year to overt MM.
For these cells to acquire the ability for active proliferation, they may induce cell-autonomous changes or nonautonomous changes, such as the recruitment of progenitor cells that make the host site more permissive. For example, tumor cells at the original site may secrete osteopontin or SDF-1 or IL-11 to induce changes in the distant metastatic niche.5 Interestingly, autonomous and external genetic and epigenetic changes can affect whether micrometastatic cells in the bone or in the lung can grow to clinically detectable macrometastasis. For example, in breast cancer, IL-11 and Jagged1 can drive osteolysis and induce significant changes in the metastatic niche in the BM but have no effect on a metastatic niche in the lung or brain.5 Therefore, distinct tissue microenvironments impose dramatically different organ-specific requirements for metastatic colonization.5,116 It would also indicate that the same cancer cell type has to use a different molecular program to metastasize to the BM compared with the lung or brain. Similarly, different tumor types, such as breast cancer and MM, would use different molecular programs to metastasize to the BM.5
Indeed, different gene signatures for egress and colonization have been identified in solid tumors. These include metastasis initiation genes, metastasis progression genes, and metastasis virulence genes.117 Metastasis virulence genes are responsible for the tumor cells to adapt to the new microenvironment, emerge from dormancy, and perform organ-specific colonization. Although many gene signatures have been performed in MM, none has been examined in their role for specific tumor initiation or tumor colonization. Examining subgroups of MM, and their patterns of spread and involvement of the BM, as well as their time to progression to overt MM or extramedullary MM could identify insight into these subgroups of genes. For example, patients with p53 mutation have a higher propensity to develop extramedullary disease with rapid tumor progression.118-120
The role of the microenvironment in cell metastasis
More than 120 years ago, Stephen Paget postulated the hypothesis of the “seed-and-soil.”6 Although many factors regulating cell metastasis are autonomous, they may not be sufficient, and a permissive microenvironment is required for frank malignancy to emerge. Indeed, studies have shown that the tumor microenvironment is a key regulator in many steps of the invasion-metastasis cascade, including tumor oncogenesis, egress, protection in the circulation, preparation of the metastatic niche, organ-specific homing, and the permissive role of the microenvironment in tumor colonization.5
Changes in a tissue microenvironment have been suggested to precede and promote the initiation of genetic events by creating a “premalignant” state that is characterized by disruption of quiescence-inducing signals or increasing proliferative signals. This hypothesis has been validated in several models, including those altering TGF-β signaling in tissue fibroblasts or Rb deletion in the BM leading to myeloid progenitor expansion.121,122 Another study showed that deletion of Dicer1 in mouse osteoprogenitors disrupts the integrity of hematopoiesis leading to myelodysplasia and acute myelogenous leukemia that have intact Dicer1.123 These studies support the concept of niche-mediated oncogenesis.
Other examples of microenvironmental contributions to neoplasia include mast cell contribution to Nf1-induced neurofibromas, mesenchymal cell alteration of epithelial tumor growth kinetics, stromal CD4+ T-lymphocytes promoting mammary carcinoma invasion by stimulating tumor-associated macrophages.5,45 Similarly, perturbation of Hedgehog signaling or caveolin-1 specifically within the stroma alters tumor progression in neighboring carcinoma cells.5,45 These studies provide proof of bidirectional interactions that occur between tumor cells and the nearby microenvironment that is permissive for tumor initiation and progression, establishing a positive feedback loop that may be self-amplifying.5,45
In MM, the role of the BM microenvironment has been extensively studied in many models in vitro and in vivo. The BM provides signals that influence the behavior of MM cells (eg, tumor cell growth, survival, migration, and drug resistance). For example, adhesion of MM cells to stromal cells, endothelial cells, and ECM proteins such as fibronectin and laminin, lead to enhanced MM cell growth and survival and confer protection against drug-induced apoptosis.124,125 These sequelae are the result of cell-cell contact, as well as NFκB-dependent transcription and secretion of IL-6, a major factor in the growth, survival and drug resistance of MM.124,125 Activated stroma triggers the paracrine and autocrine production and secretion of a variety of cytokines and growth factors into the MM BM microenvironment (Figure 4), including IL-6, IGF-1, TNF-α, SDF-1α, TGF-β, basic fibroblast growth factor, MIP-1α, stem cell factor, HGF, IL-1β, IL-3, IL-10, IL-15, and IL-21, as well as Ang-1 and matrix metalloproteinases (eg, MMP-2 and MMP-9). MM cell adhesion to fibronectin also protects tumor cells from DNA-damaging drugs (eg, anthracyclines and alkylating agents) by inducing cell-adhesion-mediated drug resistance.126,127
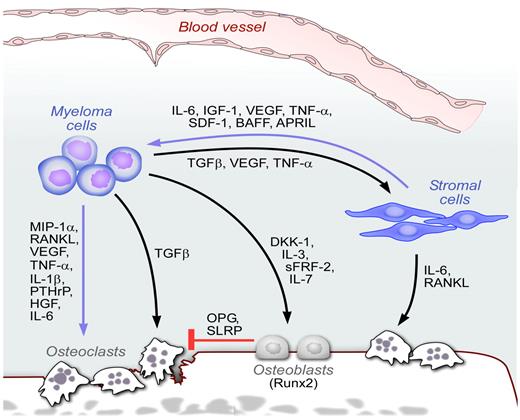
The BM niche in MM. Schematic representation of the BM niches in MM. MM cells interact with many cellular elements in the BM, including osteoclasts, osteoblasts, stromal cells, and endothelial cells. Multiple cytokines and chemokines are secreted in response to these cell-cell interactions, leading to enhanced tumor growth, inhibition of osteoblasts, and increased osteoclast activity.
The BM niche in MM. Schematic representation of the BM niches in MM. MM cells interact with many cellular elements in the BM, including osteoclasts, osteoblasts, stromal cells, and endothelial cells. Multiple cytokines and chemokines are secreted in response to these cell-cell interactions, leading to enhanced tumor growth, inhibition of osteoblasts, and increased osteoclast activity.
The cellular elements of the BM interact with MM cells directly or indirectly through secretion of stimulatory cytokines and chemokines that induce survival, growth advantage, and drug resistance. These microenvironmental cells include mesenchymal stem cells, osteoclasts, osteoblasts, and vascular endothelial cells.128 These cells can induce cell growth through direct cell-cell interactions or through secretion of growth and/or antiapoptotic factors, such as IL-6, IGF-1, VEGF, TNF-α, and SDF-1.128-131 Increased osteoclast activity is triggered by a variety of osteoclast-activating factors produced by both tumor as well as stromal cells. These factors include MIP-1α and RANKL, VEGF, TNFα, IL-1β, parathyroid hormone-related protein, HGF, and IL-6 (Figure 4). In turn, osteoclast activity modulates MM cell growth and survival.132,133 In addition, MM patients have impaired osteoblast differentiation. Deregulation of several molecules contributes to this effect, including Runx2/Cbfa1, Wingless-type (Wnt), and IL-3. Specifically, MM cells block activity and function of the transcription factor Runx2/Cbfa1 in human BM osteoblast progenitors through direct VLA-4/VCAM-1–mediated contact or IL-7 secretion,134-136 and increase osteoclastogenesis via enhanced secretion of RANKL in osteoprogenitor cells.137 Moreover, studies also suggest the importance of the canonical Wnt signaling pathway in MM bone disease. Specifically, the Wnt-signaling antagonist dickkopf-1 (DKK1), an inhibitor of osteoblast differentiation, is significantly overexpressed in patients with MM presenting with lytic bone lesions (Figure 4). Unlike other cancers with bone metastasis, such as prostate and breast cancer, MM represents one of the only diseases with pure lytic lesions indicating that changes that occur is mesenchymal stem cells and osteoblasts may be unique to MM and not shared with other cancers. Finally, plasmacytoid dendritic cells in the BM microenvironment have been shown to both mediate immune deficiency characteristic of MM and promote MM cell growth, survival, and drug resistance.138
Clinical applications and therapeutic targeting
Potential markers of prognosis with cell dissemination or cell metastasis include the measurement of circulating miRNAs or circulating tumor cells.5 Indeed, the levels of several individual miRNAs (including miR-10b, miR-21, miR-31, miR-126, miR-335, and miR-373) have been correlated with metastatic outcome in carcinoma patients.139,140 Circulating tumor cells have shown correlation with tumor progression in MGUS and smoldering MM as well as poor prognosis in MM as described previously.
One of the major limitations in the design of antimetastatic therapeutic agents is the fact that, by the time patients are diagnosed with cancer, many of them already harbor metastatic disease. Therefore, successful antimetastatic agents must be capable of impairing the proliferation and survival of already disseminated carcinoma cells, rather than just blocking dissemination from the primary site. Some of the agents that were previously developed as antimetastatic agents failed because of their inability to prevent growth of the metastatic lesions. Agents that are in preclinical and clinical development, such as Src inhibitors (dasatinib, Bristol-Myers Squibb),141 or the acute expression of miR-31142 could potentially regulate metastasis, even after dissemination.
Another major strategy is targeting the microenvironment that is permissive to tumor growth. Such agents include bisphosphonates, anti-RANK antibodies (denosumab, Amgen), and TGF-β inhibitors, such as SD-208 (Scios) and LY2157299 (Eli-Lilly).5 Antiangiogenic agents that target endothelial cells can also regulate the process of metastasis. However, recent studies that have shown that antiangiogenic agents may indeed paradoxically increase metastatic propensity by inducing more hypoxia in the tumor cells.143
In MM, several agents have been developed that not only target the tumor clone but also the microenvironment. Indeed, MM may be considered one of the success areas for targeting the microenvironment.131,144,145 The therapeutic success of bortezomib (Millennium) and lenalidomide (Celgene), 2 of the most active agents in MM, is not only based on their direct tumor activity but also on their role in targeting the BM microenvironment.144,145 Bortezomib inhibits MM cell growth triggered by stromal adhesion, as well as production and secretion of cytokines that mediate MM cell growth and survival. In addition, bortezomib inhibits osteoclast activity and induces osteoblast activity. Bortezomib induces mesenchymal stem cells to preferentially undergo osteoblastic differentiation.146 Similarly, immunomodulatory drugs, such as thalidomide and lenalidomide, have antiangiogenic activity and immunomodulatory activity, to antitumor immunity mediated by IFN-γ and IL-2 as well as augmented NK cell cytotoxicity.144,145
Another novel strategy is to target hypoxic cells present in the BM microenvironment. In the murine 5T33MM model, MM cells localize in an extensively hypoxic niche compared with the naive BM.147 The investigators of this study showed that hypoxia could be used as a treatment target for MM by evaluating the effects of a new hypoxia-activated pro-drug TH-302 (Threshold) in vitro and in vivo.147 In severely hypoxic conditions, TH-302 was activated and induced apoptosis in MM cells. A phase 1/2 study of TH-302 in patients with relapsed MM is ongoing.
Other agents that only target the microenvironment include bisphosphonates, anti–DKK-1 inhibitors, anti-RANKL inhibitors (denosumab; Amgen), anti-CS1 antibodies (elotuzumab; Bristol-Myers Squibb), and anti-CXCR4 inhibitors (plerixafor or BMS-936564; Bristol-Myers Squibb).148,149 Bisphosphonates are often used for the management of MM patients with bone lesions. The most significant data that indicates that bisphosphonates have an effect on survival of patients with MM came from a recent randomized study of the Medical Research Council IX.150 This study showed a significant improvement of survival in patients who receive zolendronic acid, indicating that bisphosphonates do not only prevent lytic lesions but can also help control MM tumor growth. Denosumab, a fully human monoclonal antibody to RANKL, was developed to treat patients with skeletal diseases.151 Further clinical trials of this agent are ongoing in MM. In addition, a neutralizing anti-DKK1 inhibitor antibody (BHQ880; Novartis) was tested in vivo and demonstrated reduced osteolytic bone resorption and increased bone formation and helped control MM growth in mice in vivo.152 The direct inhibitor of CXCR4 (plerixafor) has been tested in vitro and in vivo and showed mobilization of MM cells, de-adhesion of the tumor cells from the stroma, and their chemosensitization in animal models.58 Clinical trials of chemosensitization with plerixafor or BMS-936564 have been developed in acute myeloid leukemia and MM.
Another critical strategy in the treatment of MM is the timing of initiation of therapy. Based on the analogy of MM being a metastatic disease by the time patients are diagnosed, it is not surprising that, even with the best combinations of agents that are currently available, cure has not been achieved in most patients. Therefore, it may be that we are initiating therapy at very late stages when disease has already led to macrometastasis. Indeed, several trials are now ongoing to examine the treatment of patients with smoldering MM before the initial clinical presentations of criteria of symptomatic disease. A randomized phase 3 study has shown a significant progression-free survival in patients with smoldering MM who were treated with lenalidomide and dexamethasone compared with those in the control arm.153 Other studies using BHQ880 or elotuzumab in smoldering MM are ongoing.
In conclusion, this review shows the significant advances that have been discovered in the complex process of invasion-metastasis and how it relates to the process of progression in MM from plasmacytoma to MGUS to MM. This progression is mediated by clonal intrinsic factors that mediate tumor invasiveness and metastatic virulence, as well as factors present in the tumor microenvironment that are permissive to oncogenic initiation, progression, and dissemination. The advantages of using MM as a model disease for cell metastasis are that most patients present with multiple lesions at the time of diagnosis, indicating that the process of cell dissemination or “metastasis” is activated in almost all cases of patients with MM. Furthermore, MM progresses through well-defined stages of plasmactyomas, MGUS, active MM, and late plasma cell leukemia. The accessibility of samples of tumor cells and microenvironmental cells from BM biopsies of these patients at the different stages of the disease make MM a great model to examine the process of invasion-metastasis. Despite the significant advances in the treatment of MM, more biologic studies are needed to examine in-depth this process of progression in MM and design better therapeutic agents that target the different steps of this metastatic cascade. In the future, patients with extramedullary disease and plasma cell leukemia may need specific clinical trials to better develop agents that target their complex process of cell trafficking and cell dissemination.
Acknowledgments
The author thanks Dr Kenneth Anderson for his critical review of the manuscript, Steven Moskowitz (Advanced Medical Graphics) for the design of the figures, Dr Robert Schlossman and Ludmila Flores for providing images for Figure 1, and all the members of her laboratory and clinical team and her colleagues in the Multiple Myeloma program at Dana-Farber Cancer Institute.
This work was supported in part by the National Institutes of Health (R01CA133799, R01CA125690, and R01CA154648).
National Institutes of Health
Authorship
Contribution: I.M.G. wrote the manuscript.
Conflict-of-interest disclosure: I.M.G. is on the advisory board of Millennium, Novartis, Onyx, and Bristol-Myers Squibb and has research support from Millennium, Bristol-Myers Squibb, Noxxon, Novartis, Onyx, and Genzyme.
Correspondence: Irene M. Ghobrial, Department of Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 1B, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.