Abstract
HM1.24, an immunologic target for multiple myeloma (MM) cells, has not been effectively targeted with therapeutic monoclonal antibodies (mAbs). In this study, we investigated in vitro and in vivo anti-MM activities of XmAb5592, a humanized anti-HM1.24 mAb with Fc-domain engineered to significantly enhance FcγR binding and associated immune effector functions. XmAb5592 increased antibody-dependent cellular cytotoxicity (ADCC) several fold relative to the anti-HM1.24 IgG1 analog against both MM cell lines and primary patient myeloma cells. XmAb5592 also augmented antibody dependent cellular phagocytosis (ADCP) by macrophages. Natural killer (NK) cells became more activated by XmAb5592 than the IgG1 analog, evidenced by increased cell surface expression of granzyme B–dependent CD107a and MM cell lysis, even in the presence of bone marrow stromal cells. XmAb5592 potently inhibited tumor growth in mice bearing human MM xenografts via FcγR-dependent mechanisms, and was significantly more effective than the IgG1 analog. Lenalidomide synergistically enhanced in vitro ADCC against MM cells and in vivo tumor inhibition induced by XmAb5592. A single dose of 20 mg/kg XmAb5592 effectively depleted both blood and bone marrow plasma cells in cynomolgus monkeys. These results support clinical development of XmAb5592, both as a monotherapy and in combination with lenalidomide, to improve patient outcome of MM.
Introduction
Targeted immunotherapy with monoclonal antibodies (mAbs) is an effective and safe method for the treatment of many forms of cancers. However, to date, there is still no mAb-based cancer therapy approved to treat patients with multiple myeloma (MM). Early clinical trials of mAbs targeting CD20 and CD38 have conveyed only very limited benefit, if any, to the treatment of MM.1–3 In recent years, efforts have been made to identify potential therapeutic mAbs by defining alternative or novel MM target antigens, ie, CD40,4,5 IL6R,6 HM1.24,7 CD74,8 TRAIL-R1,9 CS1,10 as well as to conjugate mAbs with classic or novel drugs to specifically kill MM cells, ie, CD56-maytansinoid (DM1),11 CD138-DM1/DM4.12 Development of mAbs with improved cytotoxicity, targeting new and known myeloma specific antigens, continues to be an active research area in novel immunotherapeutics for MM.
HM1.24/CD317/BST2, a type II transmembrane protein of 29-33 kDa, was first identified to be preferentially overexpressed on malignant plasma cells and terminally differentiated B cells.13,14 Subsequent studies further established HM1.24 as an immunologic target on MM.7,15–17 More recently, overexpression of HM1.24 has also been described in a wide variety of invasive or drug-resistant solid tumor cell lines in breast, lung, pancreas, and kidney, as well as lymphoma vasculature,18–22 suggesting the potential for therapy with anti-HM1.24 mAb for these cancers as well. A murine and a humanized mAb against HM1.24 (AHM) exhibited antitumor effects in vitro and in vivo using xenografts of human MM cells and renal carcinomas in mice.7,15,17,19 In addition, inhibition of MM cell growth by AHM mAb was diminished when mice were pretreated with anti-Fcγ receptor (FcγR) III/II Abs, indicating that effector cell functions are critical for AHM mAb-induced anti-MM activity.15 A phase 1 clinical study of AHM in patients with relapsed or refractory MM reported that the mAb did not cause any serious toxicity, although there was no indication of its antitumor activity.23 Natural killer (NK) cell–mediated antibody-dependent cell-mediated cytotoxicity (ADCC) is a critical mechanism of action for many approved therapeutic mAbs.24–26 The importance of the role of interaction between the Fc region of therapeutic antibodies and FcγRs on effector cells is underscored by the clinical data suggesting that the FcγRIIIa polymorphism status of NK cells from cancer patients plays a key role in the clinical outcome of patients receiving rituximab,25 trastuzumab,27 or cetuximab26 ; specifically, patients possessing the higher affinity version of FcγRIIIa achieve much higher response rates. An engineering approach to enhance the affinity of human IgG1-Fc toward FcγRs improved in vitro ADCC activity against tumor cells, mediated by NK cells expressing the various FcγRIIIa polymorphisms.28 Fc-engineered therapeutic anti-CD1929–31 and anti-CD4032 mAbs demonstrated enhanced in vitro and in vivo activity against lymphoma and leukemia. Importantly, early clinical data from a phase 1 trial of the Fc-engineered anti-CD30 antibody XmAb2513 provided encouraging evidence for the safety and antitumor efficacy of this therapeutic strategy.33
XmAb5592 is a humanized anti-HM1.24 mAb with a similarly engineered Fc-domain that specifically increases affinity for Fcγ receptors expressed on various effector cells, and associated cytotoxicity. Here, we evaluate the preclinical activity of XmAb5592 in MM and demonstrate that, compared with an anti-HM1.24 mAb with normal FcγR binding (IgG1 analog), it has much greater anti-MM activity in vitro and in vivo, mediated via superior induction of NK cell activation and degranulation. The anti-MM activity of XmAb5592 shows synergism when combined with lenalidomide pretreatment of effector cells. Its potential for clinical efficacy was also demonstrated by the ability to deplete plasma cells from both blood and bone marrow in nonhuman primates. XmAb5592 represents a promising next-generation immunotherapeutic for MM and several other malignancies.
Methods
Antibodies
Variable region sequences for the parent mouse anti-HM1.24 antibody17 were ligated into the expression vector pTT5 (National Research Council Canada) containing the human IgG1 and κ constant regions. To produce XmAb5592, the Fv was humanized,34 and a potential Asp isomerization site was removed by the substitution D54S in VH-CDR2. The substitutions S239D and I332E were introduced into the human Fc, using standard mutagenesis techniques.28 The IgG1 analog of XmAb5592 (anti-HM1.24 IgG1) and the anti-HM1.24 Fc knockout (anti-HM1.24 Fc-KO) share the Fv with XmAb5592 (Table 1), but for the analog the Fc was wild-type IgG1, and for the Fc-KO substitutions (G236R/L328R) were introduced to eliminate FcγR interactions. Construction of the XmAb isotype control, which has the same Fc as XmAb5592 but an Fv from antirespiratory syncytial virus (RSV) antibody, transfections, and antibody purification were performed as described.29
XmAb5592 has enhanced binding affinities for human FcγRs (Kd values in nM)
| . | Fv domain specificity . | Fc domain mutations . | Binding to human Fcγ Receptors (fold increase relative to IgG1) . | ||||
|---|---|---|---|---|---|---|---|
| FcγRI . | FcγRIIa 131R . | FcγRIIa 131H . | FcγRIIIa 158F . | FcγRIIIa 158V . | |||
| XmAb5592 | HM1.24* | S239D/I332E | 0.08 ± 0.05 (4×) | 141 ± 23 (11×) | 129 ± 29 (14×) | 22 ± 4 (77×) | 8 ± 1 (36×) |
| Anti-HM1.24 IgG1 | HM1.24 | none | 0.32 ± 0.06 | 1473 ± 49 | 1750 ± 976 | 1687 ± 342 | 288 ± 78 |
| Anti-HM1.24 Fc-KO | HM1.24 | G236R/L328R | NB | NB | NB | NB | NB |
| XmAb Isotype control | RSV | S239D/I332E | 0.08 ± 0.05 (4×) | 136 ± 20 (11×) | 137 ± 21 (13×) | 20 ± 3 (84×) | 7 ± 1 (41×) |
| . | Fv domain specificity . | Fc domain mutations . | Binding to human Fcγ Receptors (fold increase relative to IgG1) . | ||||
|---|---|---|---|---|---|---|---|
| FcγRI . | FcγRIIa 131R . | FcγRIIa 131H . | FcγRIIIa 158F . | FcγRIIIa 158V . | |||
| XmAb5592 | HM1.24* | S239D/I332E | 0.08 ± 0.05 (4×) | 141 ± 23 (11×) | 129 ± 29 (14×) | 22 ± 4 (77×) | 8 ± 1 (36×) |
| Anti-HM1.24 IgG1 | HM1.24 | none | 0.32 ± 0.06 | 1473 ± 49 | 1750 ± 976 | 1687 ± 342 | 288 ± 78 |
| Anti-HM1.24 Fc-KO | HM1.24 | G236R/L328R | NB | NB | NB | NB | NB |
| XmAb Isotype control | RSV | S239D/I332E | 0.08 ± 0.05 (4×) | 136 ± 20 (11×) | 137 ± 21 (13×) | 20 ± 3 (84×) | 7 ± 1 (41×) |
Equilibrium dissociation constants (Kd) were determined from Biacore data using Langmuir fitting. Results are from at least 2 separate experiments and are mean ± SD.
NB indicates no detectable binding.
Binding affinity of humanized Fv domain used in these antibodies to HM1.24 ectodomain is 1.5 ± 0.2nM, as determined by Langmuir fitting of Biacore data. The chimeric antibody containing the original murine Fv binds HM1.24 with a Kd of 1.7 ± 0.2nM, as determined using similar methods.
Cell culture, BMSCs, and patient sample processing
All CD138-expressing MM cell lines were either obtained from ATCC, the German Collection of Microorganisms and Cell Cultures (DSMZ), or kindly provided by sources and maintained as previously described.10,35 Primary CD138+ MM cells from patients were obtained after IRB-approved (Dana-Farber Cancer Institute) informed consent protocol, in accordance with the Declaration of Helsinki, using positive selection with CD138 microbeads (Miltenyi Biotec). Residual CD138-negative bone marrow–derived mononuclear cells were cultured in RPMI1640/10% FCS for 3 to 6 weeks to generate bone marrow stromal cells (BMSCs), as previously described.10 Peripheral blood samples were obtained from healthy volunteers or from patients with MM. NK cells (> 85% CD56+CD3−) derived from normal donors or MM patients were isolated directly from fresh whole blood by 30 minutes incubation with NK-cell enrichment cocktail (StemCell Technologies) before Ficoll-Paque density gradient centrifugation.4,10
Flow cytometry
Direct and indirect immunofluorescence analysis was performed using either a Coulter Epics XL with Cytomics RXP (Version 1.0) data acquisition software (Beckman Coulter), or a BD FACSCanto II flow cell analyzer with FACSDiva Version 5.0 acquisition/analysis software (BD Biosciences). Data were analyzed using FlowJo Version 8.6.6 (TreeStar Inc). Fluorescein isothiocyanate (FITC)–labeled XmAb5592, anti-HM1.24 Fc-KO, and XmAb isotype control antibodies were generated using antibody labeling kit (Thermo Scientific). FITC and phycoerythrin (PE)–labeled anti-CD107a and anti-CD56 antibodies were obtained from BioLegend.
Binding to Fc receptors and HM1.24 antigen
Binding to human FcγRs was determined using surface plasmon resonance (SPR) analysis as described.29,32 HM1.24 dissociation constants (Kd) were also determined by SPR analysis by first immobilizing the antibodies on a protein A coupled CM5 biosensor chip to give ∼ 800 RUs, then injecting serial dilutions (200nM to 6.25nM) of HM1.24 antigen into a Biacore 3000 (GE Healthcare) at 30 μL/min for 2 minutes followed by dissociation for 5 minutes. Data were fit and analyzed to obtain Kd values as described.32
ADCC and phagocytosis assays
ADCC for MM cell lines was measured using a lactate dehydrogenase (LDH) activity assay with purified PBMCs as effector cells as described.29,30 Target cells were plated at 10 000 cells per well with 500 000 PBMCs (E/T ratio 50:1). In some experiments, PBMCs were pretreated with 2μM lenalidomide (Selleck Chemicals) for 48 hours before using in ADCC assays. Calcein-AM release assay was used to measure the ADCC activity against CD138+ purified patient MM cells, and/or when purified NK cells were used as effector cells.4,5,10 Macrophage driven antibody-dependent cell-mediated phagocytosis (ADCP) was determined by flow cytometry as described,30 using RPMI8226 or U266B1 cells as targets.
Natural killer cell activation status: CD107a assay
Freshly enriched CD56+ NK cells were incubated with MM cells at a 1:1 ratio in the presence of varying concentrations (10 μg/mL to 0.1 ng/mL) of XmAb5592, anti-HM1.24 IgG1, anti-HM1.24 Fc-KO or XmAb isotype control for 3 hours at 37°C. Cell mix was stained with anti-CD107a–FITC and anti-CD56–PE antibodies (BioLegend), samples were washed twice in PBS/2% FCS, and CD107a+/CD56+ cells enumerated by flow cytometry.
In vivo tumor xenograft models
Six- to 12-week old female C.B-17 severe combined immunodeficient (SCID) mice (Taconic) were injected subcutaneously in the right flank with 107 RPMI8226 human MM cells. Twenty-one days later, mice bearing tumors between 30-100 mm3 were selected and placed into groups with similar mean tumor volume (± 10%, n = 5-15/group). For the dose response study, mice were injected intraperitoneally with 0.9, 3, or 9 mg/kg XmAb5592 or vehicle (PBS) twice per week for 7 doses; and in the comparison study mice were injected intraperitoneally with 9 mg/kg antibody (XmAb5592, anti-HM1.24 IgG1, or anti-HM1.24 Fc-KO) or vehicle twice per week for 7 doses. For the lenalidomide combination study, lenalidomide (25 mg/kg in PBS) was injected intraperitoneally twice a week for a total of 4 doses in 2 of the groups as indicated. Six mg/kg of XmAb5592 or PBS was injected in the groups as indicated twice a week for 8 doses. Tumors were measured twice per week in all the studies with calipers and volumes (mm3) calculated as π/6(length × width × height). The differences between groups were calculated using the nonparametric Mann-Whitney U test with P values < .05 considered significant. Xencor Institutional Animal Care and Use Committee approved all experiments.
Plasma cell depletion study in nonhuman primates
Cynomolgus monkey studies were conducted at Shin Nippon Biomedical Laboratories (USA), and all protocols were approved by their Institutional Animal Care and Use Committee. The study used 2 male and 2 female naive cynomolgus monkeys, 3-5 years old and weighing 2.5-4.5 kg; animals were acclimated to the study rooms for 21 days before study initiation. XmAb5592 was formulated in 10mM sodium phosphate, 150mM sodium chloride, and 0.01% polysorbate 20 (pH 7.0), and administered at 20 mg/kg as a single 1-hour intravenous infusion to the left saphenous vein. Blood samples drawn from the cephalic or femoral veins were collected predose and throughout the duration of the study (1, 2, 3, 4, 7, 14, 21, and 28 days after XmAb5592 injection). Bone marrow aspirates were collected from the humerus predose, and at day 7, 14, and before necropsy at day 28. Immunophenotyping was performed on all samples using cynomolgus monkey cross-reactive, anti–human mouse monoclonal antibodies against CD20 (PE-Cy7 conjugated 2H7; BD Biosciences) and CD38 (FITC-conjugated clone AT-1; StemCell Technologies). Cynomolgus cross-reactive, anti–human CD19 IgG1 antibody29 was PerCP conjugated using labeling kit (Prozyme). Antibodies were added to the samples, incubated, and washed before flow cytometry analysis by gating on live lymphocytes based on forward and side scatter (FSC vs SSC), and then determining the counts of CD38++CD19low/−CD20− cells, expressed as a percentage of the total lymphocytes.
Results
Generation and characterization of XmAb5592
XmAb5592 is a humanized anti-HM1.24 monoclonal antibody with 2 amino acid substitutions (S239D/I332E) in the IgG1 Fc portion of the molecule. The Fv domain was humanized using string optimization34 to generate multiple variants, and the best candidate (XmAb5592) was selected based on HM1.24 binding affinity, thermal and chemical stability, high expression, and low deamidation propensity. XmAb5592 binds to HM1.24 with a Kd value of 1.5nM (Table 1), confirming its high binding affinity to the target antigen. The chimeric antibody containing original murine anti-HM1.24 Fv bound the target with a similar Kd (1.7nM), indicating that humanization of Fv did not impair binding to HM1.24.
The binding affinities (Kd) of XmAb5592 and other control antibodies to human FcγRs were determined by SPR measurement. As expected,28–30,32 compared with the IgG1 analog, XmAb5592 has significantly enhanced binding to all human FcγRs, regardless of their polymorphism (Table 1). The activating receptor FcγRIIIa (CD16) showed the greatest enhancement (77 and 36 fold, respectively, for the 158F and 158V allotypes). An XmAb isotype control antibody, which contains a similarly modified Fc domain but has the Fv derived from an anti-RSV antibody, also showed similar improvements for FcγR binding, confirming its utility as a key XmAb control. This also firmly establishes that the Fc domain containing these 2 mutations is modular, and maintains its FcγR binding characteristics regardless of Fv context. This Fc-domain has been previously shown to increase the affinity for mouse FcγRs relative to the IgG1 analog.29,32 It is expected to retain these characteristics in XmAb5592, allowing the possibility of using murine xenografts to assess the antitumor activity. The anti-HM1.24 Fc-KO, which contains 2 Fc substitutions (G236R/L328R) to eliminate FcγR binding, showed no detectable binding to any of the human FcγRs (Table 1), thus serving as a control devoid of effector function.
XmAb5592 enhances ADCC and ADCP against MM cells
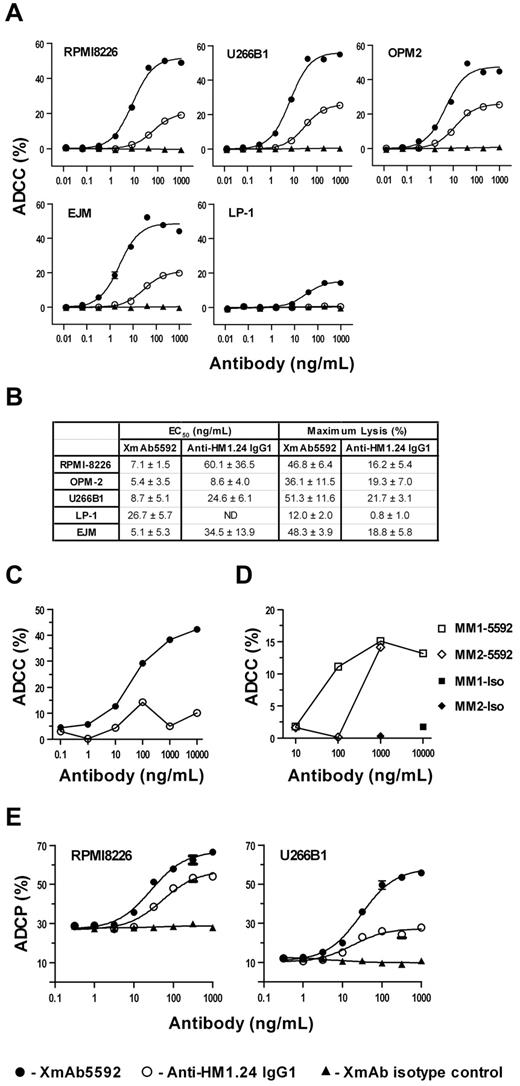
We next determined whether enhanced binding to FcγR-bearing effector cells could be translated to increased XmAb5592 cytotoxic activity compared with the anti-HM1.24 IgG1 analog. The ADCC activity was evaluated against a panel of MM cell lines using PBMCs isolated from healthy donors as effector cells. Relative to its IgG1 analog, XmAb5592 markedly enhanced lysis of MM cell lines (Figure 1A), significantly increasing both efficacy (maximal lysis) and potency (EC50; Figure 1B). EC50 values for XmAb5592 ranged from 5-27 ng/mL, indicating increased potency up to 9-fold. Maximal lysis by XmAb5592 ranged from 12% to 51% and increased more than 2.5 fold for all MM cell lines assayed. XmAb5592-mediated ADCC activity correlated with the expression of HM1.24 on the cell surface of some of these cell lines (supplemental Figures 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); LP-1, with the low HM1.24 expression (∼ 7.8 × 103 molecules/cell) had the lowest lysis, whereas RPMI8226, U266B1 and OPM2 with higher HM1.24 expression (2.6 × 105 to 4.7 × 105 molecules/cell) had comparable higher lysis. Of note, the IgG1 analog had no detectable ADCC activity against LP-1, indicating extended cytotoxicity of XmAb5592 to cells with lower expression of HM1.24 on the surface. The XmAb isotype control antibody induced no detectable cell lysis, confirming that both specific Fv-antigen interaction and FcγR engagement are required to elicit ADCC. XmAb5592 induced strong ADCC activity against additional drug-sensitive and drug-resistant MM cells in the presence of purified NK cells, whereas the XmAb isotype control induced no specific lysis (supplemental Figure 3).
XmAb5592 has significantly improved ADCC and ADCP activity against MM cells. (A) ADCC was measured with an LDH release assay using PBMCs from healthy donors as effector cells, and MM target cells. MM cells, opsonized with serial dilutions of antibodies, were mixed with PMBCs at an effector/target (E/T) ratio of 50:1. Percentage specific lysis was calculated from data obtained in triplicate, and presented as mean ± SD. XmAb5592 significantly augmented ADCC relative to the IgG1 analog against all cell lines, in contrast to XmAb isotype control inducing no cell lysis. (B) The EC50 and maximum specific lysis were calculated from data obtained from 3 independent experiments using 3 separate donor PBMCs. XmAb5592 potency was enhanced up to 9 fold, and efficacy improved up to 15 fold compared with the anti-HM1.24 IgG1 analog. (C-D) Autologous ADCC activity against CD138+ patient MM cells was determined using purified NK cells from the same patient. Calcein-AM labeled patient MM cells were incubated with CD56+ NK cells at an E/T ratio of 10:1. (C) XmAb5592 enhanced potency and efficacy against patient MM cells compared with the IgG1 analog. (D) Specific XmAb5592-induced (open symbol) autologous MM cell lysis was determined in 2 additional patients (MM1 & MM2) with XmAb isotype control (solid symbol) showing no activity. (E) Antibody dependent cellular phagocytosis (ADCP) was determined by flow cytometry using MM cells as targets, and monocyte-derived macrophages (MDM) as effector cells. MM cells were fluorescently labeled with PKH67, opsonized with antibodies, and cocultured with MDMs at an E/T ratio of 4:1 for 4 hours. Percent phagocytosis was determined as the number of triple positive (CD14+/CD11B+/PKH67+) cells divided by the total number of PKH67+ cells. Data were obtained in triplicate and represent the mean ± SD.
XmAb5592 has significantly improved ADCC and ADCP activity against MM cells. (A) ADCC was measured with an LDH release assay using PBMCs from healthy donors as effector cells, and MM target cells. MM cells, opsonized with serial dilutions of antibodies, were mixed with PMBCs at an effector/target (E/T) ratio of 50:1. Percentage specific lysis was calculated from data obtained in triplicate, and presented as mean ± SD. XmAb5592 significantly augmented ADCC relative to the IgG1 analog against all cell lines, in contrast to XmAb isotype control inducing no cell lysis. (B) The EC50 and maximum specific lysis were calculated from data obtained from 3 independent experiments using 3 separate donor PBMCs. XmAb5592 potency was enhanced up to 9 fold, and efficacy improved up to 15 fold compared with the anti-HM1.24 IgG1 analog. (C-D) Autologous ADCC activity against CD138+ patient MM cells was determined using purified NK cells from the same patient. Calcein-AM labeled patient MM cells were incubated with CD56+ NK cells at an E/T ratio of 10:1. (C) XmAb5592 enhanced potency and efficacy against patient MM cells compared with the IgG1 analog. (D) Specific XmAb5592-induced (open symbol) autologous MM cell lysis was determined in 2 additional patients (MM1 & MM2) with XmAb isotype control (solid symbol) showing no activity. (E) Antibody dependent cellular phagocytosis (ADCP) was determined by flow cytometry using MM cells as targets, and monocyte-derived macrophages (MDM) as effector cells. MM cells were fluorescently labeled with PKH67, opsonized with antibodies, and cocultured with MDMs at an E/T ratio of 4:1 for 4 hours. Percent phagocytosis was determined as the number of triple positive (CD14+/CD11B+/PKH67+) cells divided by the total number of PKH67+ cells. Data were obtained in triplicate and represent the mean ± SD.
The ADCC activity of XmAb5592 against MM patient derived CD138+ primary MM cells was next evaluated, using NK cells derived from the same patient (autologous). This more closely mimics the in vivo clinical setting in patients. XmAb5592 induced significantly enhanced ADCC compared with the IgG1 analog in a dose dependent manner; maximal lysis seen with XmAb5592 was 40 ± 2.2% versus only 5 ± 2.5% for the IgG1 version at 1 μg/mL (Figure 1C). XmAb5592 similarly induced autologous lysis against additional MM patient cells (Figure 1D), with no ADCC seen for the XmAb isotype control. Primary tumor cells are generally more resistant, and ADCC activity seen with XmAb5592 therefore underscores the utility of this Fc engineered therapeutic compared with the lack of significant activity seen with the IgG1 analog.
We also assessed the impact of XmAb5592 on macrophage phagocytosis, as it is an important contributor to the cytotoxic activity of therapeutic antibodies.30,36 ADCP assays were done with monocyte-derived macrophages as effectors, and using RPMI8226 or U266B1 MM cell lines as target cells. With both cell lines, XmAb5592 displayed ∼ 2-fold greater potency relative to the IgG1 analog (Figure 1E). Maximal phagocytosis increased from 55% to 67% for RPMI8226 cells, and from 28% to 56% for U266B1 cells, when using XmAb5592 versus the IgG1 analog. As expected, the XmAb isotype control did not induce any macrophage phagocytic activity. Both XmAb5592 and the IgG1 analog showed no detectable complement-dependent cytotoxic (CDC) activity against RPMI8226 cells when incubated in the presence of human serum complement for 2 hours (data not shown).
XmAb5592 induces potent ADCC and activates NK degranulation in the coculture of MM-BMSCs
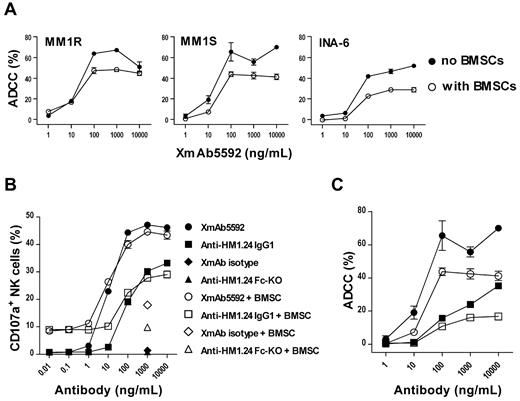
Since the bone marrow (BM) microenvironment protects MM cells against cell death, we next determined whether XmAb5592 still triggered ADCC lysis against MM cells in the presence of BMSCs. Strong XmAb5592-induced ADCC activity was seen against MM1S or MM1R target cells in the absence or presence of BMSCs (Figure 2A), suggesting that it will be effective against MM cells in the BM microenvironment. Significantly, XmAb5592 retains ADCC activity against IL6-dependent INA-6 cells in the presence of BMSCs, although overall lysis is reduced. This apparent reduction in killing in the presence of BMSCs is likely because of a generalized effect of these cells on MM survival.
XmAb5592 induces strong MM cell lysis by degranulation of NK cells even in the presence of BMSCs. (A) XmAb5592-mediated lysis against MM1S, MM1R, and INA-6 MM cells was measured by calcein-AM release ADCC assay, in the presence or absence of BMSCs. Data were obtained in triplicate and represent the mean ± SD. (B) MM1S target cells and NK effector cells were incubated with serial dilutions of antibodies in the presence and absence of BMSCs, followed by dual-color flow cytometry analysis to determine the percentage of CD107a+ NK cells. Data were obtained in triplicate and represent the mean ± SD. XmAb5592 induced ∼ 10-fold more NK degranulation than the IgG1 analog in a dose dependent manner, regardless of the presence of BMSCs. (C) Calcein-AM release based ADCC assay was simultaneously performed (as in panel B) for XmAb5592 and the IgG1 analog treatment groups, in the presence or absence of BMSCs.
XmAb5592 induces strong MM cell lysis by degranulation of NK cells even in the presence of BMSCs. (A) XmAb5592-mediated lysis against MM1S, MM1R, and INA-6 MM cells was measured by calcein-AM release ADCC assay, in the presence or absence of BMSCs. Data were obtained in triplicate and represent the mean ± SD. (B) MM1S target cells and NK effector cells were incubated with serial dilutions of antibodies in the presence and absence of BMSCs, followed by dual-color flow cytometry analysis to determine the percentage of CD107a+ NK cells. Data were obtained in triplicate and represent the mean ± SD. XmAb5592 induced ∼ 10-fold more NK degranulation than the IgG1 analog in a dose dependent manner, regardless of the presence of BMSCs. (C) Calcein-AM release based ADCC assay was simultaneously performed (as in panel B) for XmAb5592 and the IgG1 analog treatment groups, in the presence or absence of BMSCs.
During granzyme-B/perforin dependent NK cell degranulation process, CD107a (lysosome-associated membrane protein 1) becomes transiently mobilized to the cell surface, serving as a functional marker for NK activity.37 We assessed surface expression of CD107a on NK cells in the presence of target MM1S cells and XmAb5592 or other control antibodies, with or without BMSCs. XmAb5592 induced ∼ 10-fold more NK degranulation than the IgG1 analog, regardless of the presence of BMSCs (Figure 2B). Calcein-AM release ADCC assays performed simultaneously with serial dilutions of XmAb5592 and the IgG1 analog show a similar lysis pattern, both in the presence or absence of BMSCs (Figure 2C). The enhanced CD107a-mediated NK degranulation triggered by XmAb5592 significantly correlated with increased ADCC against MM cells, regardless of the presence of BMSCs.
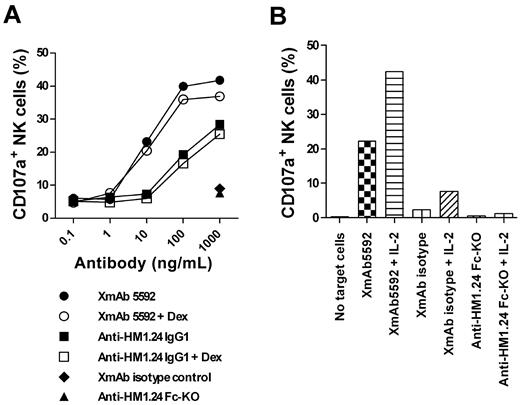
XmAb5592 also induced > 10-fold greater NK degranulation against primary CD138+ MM cells relative to the IgG1 analog, using NK cells from the same patient (Figure 3A). Pretreatment of MM cells with 0.1μM dexamethasone did not affect the NK degranulation, suggesting that the XmAb5592's therapeutic benefits would be combinable with conventional treatment regimens (Figure 3A). In addition, pretreatment of effector cells with IL-2 enhanced XmAb5592-induced cytotoxicity against MM patient cells (Figure 3B). IL-2 did not enhance NK degranulation in the presence of anti-HM1.24 Fc-KO, further confirming the importance of the engineered Fc-domain of XmAb5592 for improving therapeutic efficacy.
XmAb5592 induced NK degranulation activity is resistant to dexamethasone pretreatment of MM cells, and is enhanced by IL-2 pretreatment of effector cells. (A) CD138+ patient MM cells were pretreated with 0.1μM dexamethasone or PBS overnight, followed by incubation with purified NK cells and serial dilutions of indicated mAbs. The percentage of CD107a+ NK cells was determined by flow cytometry. Strong XmAb5592-induced NK degranulation is not affected by dexamethasone treatment of MM cells. (B) Patient NK cells were pretreated overnight with 100 units/mL IL-2 or PBS before incubation with autologous patient MM cells, in the presence of indicated mAbs (10 μg/mL). IL-2 augments CD107a+ expression on patient NK cells specifically induced by XmAb5592.
XmAb5592 induced NK degranulation activity is resistant to dexamethasone pretreatment of MM cells, and is enhanced by IL-2 pretreatment of effector cells. (A) CD138+ patient MM cells were pretreated with 0.1μM dexamethasone or PBS overnight, followed by incubation with purified NK cells and serial dilutions of indicated mAbs. The percentage of CD107a+ NK cells was determined by flow cytometry. Strong XmAb5592-induced NK degranulation is not affected by dexamethasone treatment of MM cells. (B) Patient NK cells were pretreated overnight with 100 units/mL IL-2 or PBS before incubation with autologous patient MM cells, in the presence of indicated mAbs (10 μg/mL). IL-2 augments CD107a+ expression on patient NK cells specifically induced by XmAb5592.
XmAb5592 strongly inhibits growth of established myeloma tumors in vivo
The in vivo activity of XmAb5592 was next examined in an established human MM tumor model. SCID mice bearing RPMI8226 subcutaneous tumors were treated with 0.9, 3.0, or 9.0 mg/kg of XmAb5592 twice a week to determine the optimal treatment dose. XmAb5592 inhibited RPMI8226 tumor growth in a dose-dependent manner (Figure 4A). On day 41, after 3 weeks of treatment, 0.9, 3.0, or 9.0 mg/kg of XmAb5592 decreased RPMI8226 tumor growth by 23%, 75%, and 80%, respectively, relative to vehicle control. The relative effectiveness of XmAb5592 was then evaluated in a similar RPMI8226 xenograft model, along with the anti-HM1.24 IgG1 and Fc-KO analog antibodies. All 3 antibodies were injected intraperitoneally at 9.0 mg/kg, twice per week for 4 weeks. XmAb5592 blocked tumor growth significantly better compared with its IgG1 analog (Figure 4B). On day 46 after 4 weeks of treatment, the mean tumor volume of the XmAb5592 treated group was 47 mm3 compared with 192 mm3 for the IgG1 group (P ≤ .001). Importantly, 7 of 15 mice (47%) were tumor-free in the XmAb5592 group compared with 1 of 15 (7%) tumor-free mice in the IgG1 group. Mice treated with anti-HM1.24 Fc-KO antibody showed no reduction in tumor growth, with mean tumor volumes similar to the vehicle treated control group. XmAb5592 also inhibited tumor growth to a greater extent than the IgG1 analog in another SCID mice xenograft study of established human OPM-2 MM tumors (supplemental Figure 4). Significantly improved in vivo activity of XmAb5592 relative to its IgG1 analog across multiple xenografts, coupled with the ineffectiveness of the anti-HM1.24 Fc-KO, indicates that the anti-tumor activity of XmAb5592 is mediated via FcγR-dependent mechanisms.
XmAb5592 strongly inhibits growth of established myeloma tumors in vivo and eradicates tumors in mice. The in vivo anti-myeloma activity of XmAb5592 was determined in a therapeutic setting using a xenograft model of human RPMI8226 MM cells. (A) SCID mice with palpable RPMI8226 tumors (30-92 mm3) were randomized into groups (n = 11), and then treated with vehicle (PBS) or 0.3, 3 or 9 mg/kg of XmAb5592, twice weekly for 7 doses (↓) by intraperitoneal injection. Tumor growth is presented as group mean volume ± SD. A dose dependent anti-tumor effect is evident (day 41 MTV = 484, 159 and 123 mm3 for 0.3, 3 and 9 mg/kg, respectively; P ≤ .001 for both 0.9 vs 3 mg/kg and 0.9 vs 9 mg/kg). (B) Antitumor activity of XmAb5592, anti-HM1.24 IgG1, or anti-HM1.24 Fc-KO was compared in vivo, at 9 mg/kg (n = 15). XmAb5592 is significantly more effective at blocking tumor growth compared with the IgG1 analog (day 46 MTV = 47 and 192 mm3, respectively, P ≤ .001). In addition, on day 46 there were 7 tumor-free mice in the XmAb5592-treated group and only 1 tumor-free mouse in the anti-HM1.24 IgG1-treated group. The anti-HM1.24 Fc-KO shows no antitumor activity, indicating the importance of FcγR engagement. Data are mean ± SD.
XmAb5592 strongly inhibits growth of established myeloma tumors in vivo and eradicates tumors in mice. The in vivo anti-myeloma activity of XmAb5592 was determined in a therapeutic setting using a xenograft model of human RPMI8226 MM cells. (A) SCID mice with palpable RPMI8226 tumors (30-92 mm3) were randomized into groups (n = 11), and then treated with vehicle (PBS) or 0.3, 3 or 9 mg/kg of XmAb5592, twice weekly for 7 doses (↓) by intraperitoneal injection. Tumor growth is presented as group mean volume ± SD. A dose dependent anti-tumor effect is evident (day 41 MTV = 484, 159 and 123 mm3 for 0.3, 3 and 9 mg/kg, respectively; P ≤ .001 for both 0.9 vs 3 mg/kg and 0.9 vs 9 mg/kg). (B) Antitumor activity of XmAb5592, anti-HM1.24 IgG1, or anti-HM1.24 Fc-KO was compared in vivo, at 9 mg/kg (n = 15). XmAb5592 is significantly more effective at blocking tumor growth compared with the IgG1 analog (day 46 MTV = 47 and 192 mm3, respectively, P ≤ .001). In addition, on day 46 there were 7 tumor-free mice in the XmAb5592-treated group and only 1 tumor-free mouse in the anti-HM1.24 IgG1-treated group. The anti-HM1.24 Fc-KO shows no antitumor activity, indicating the importance of FcγR engagement. Data are mean ± SD.
Lenalidomide further enhances XmAb5592 induced anti-myeloma activity both in vitro and in vivo
Lenalidomide is an immunomodulatory drug that has been used very effectively for treatment of MM,38 and is known to increase the activity of NK cells.4,5 To investigate the possible improvement of XmAb5592 induced, NK-mediated ADCC by lenalidomide, PBMCs from healthy donors were preincubated with 2μM lenalidomide for 48 hours before addition of antibody-opsonized MM cells in ADCC assays. PBMCs treated similarly with PBS served as control. Pretreatment of effector cells with lenalidomide enhanced the cytotoxic activity of XmAb5592 against the MM cell lines tested (Figure 5A), with activity against RPMI8226 and MM1R cells showing the most enhancement (P < .001). Lenalidomide pretreatment also increased the ADCC activity of the IgG1 analog against some of the cell lines, notably MM1R cells. The increased XmAb5592 ADCC with lenalidomide pretreated effector cells was further augmented with IL-2 pretreatment of NK cells (supplemental Figure 5).
Lenalidomide further enhances XmAb5592 induced anti-myeloma activity both in vitro and in vivo. (A) PBMCs were preincubated with or without lenalidomide (2μM) for 48 hours before LDH release-based ADCC assays against target MM cells. Percent specific lysis is shown, as mean ± SD from triplicate measurements. Lenalidomide pretreatment significantly enhanced improved ADCC activity of XmAb5592 than the IgG1 analog. (B) RPMI8226 tumor-bearing mice (n = 5) were treated with PBS, lenalidomide alone, XmAb5592 alone, or XmAb5592 plus lenalidomide. Lenalidomide (25 mg/kg) was injected intraperitoneally twice weekly for 4 doses (▽). XmAb5592 (6 mg/kg) was injected intraperitoneal twice weekly for a total of 8 doses (↓). Combination treatment with XmAb5592 and lenalidomide was more efficacious than either of the treatments alone (day 56 MTV = 46, 127 and 411 mm3 for the combination, XmAb5592 alone and lenalidomide alone, respectively).
Lenalidomide further enhances XmAb5592 induced anti-myeloma activity both in vitro and in vivo. (A) PBMCs were preincubated with or without lenalidomide (2μM) for 48 hours before LDH release-based ADCC assays against target MM cells. Percent specific lysis is shown, as mean ± SD from triplicate measurements. Lenalidomide pretreatment significantly enhanced improved ADCC activity of XmAb5592 than the IgG1 analog. (B) RPMI8226 tumor-bearing mice (n = 5) were treated with PBS, lenalidomide alone, XmAb5592 alone, or XmAb5592 plus lenalidomide. Lenalidomide (25 mg/kg) was injected intraperitoneally twice weekly for 4 doses (▽). XmAb5592 (6 mg/kg) was injected intraperitoneal twice weekly for a total of 8 doses (↓). Combination treatment with XmAb5592 and lenalidomide was more efficacious than either of the treatments alone (day 56 MTV = 46, 127 and 411 mm3 for the combination, XmAb5592 alone and lenalidomide alone, respectively).
The potential for enhancing the therapeutic activity of XmAb5592 in vivo through combination with lenalidomide treatment was evaluated in a murine xenograft model of RPMI8226 human MM cells. Tumor-bearing SCID mice were treated with 25 mg/kg of lenalidomide (by intraperitoneal injections twice a week for 2 weeks), alone or in combination with 6 mg/kg of XmAb5592 (by intraperitoneal injections twice a week for 4 weeks). The doses of both treatments were chosen to be suboptimal to see a synergistic effect. Combination treatment with XmAb5592 and lenalidomide was more efficacious than either of the treatments alone (Figure 5B). On day 56, the mean tumor volumes were 46, 127, and 411 mm3 for the combination, XmAb5592 alone and lenalidomide alone groups, respectively. The in vitro and in vivo therapeutic enhancement seen with lenalidomide and XmAb5592 support a combination strategy for their clinical evaluation.
XmAb5592 depletes blood/BM plasma cells in cynomolgus monkeys
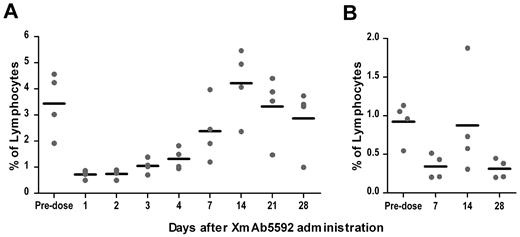
The murine anti-HM1.24 antibody, from which XmAb5592 was derived, cross-reacted with the HM1.24 antigen on lymphocytes from cynomolgus monkeys, but not with those from rats, mice, guinea pigs, rabbits, or dogs.39 In immunohistochemistry studies with normal tissues, XmAb5592 showed a similar cross-reactivity pattern with human and cynomolgus monkey tissues (data not shown), supporting the use of cynomolgus monkey for assessing the biologic activity of the antibody. To investigate the impact of XmAb5592 on depletion of plasma cells in blood and BM, cynomolgus monkeys (2 males and 2 females) were given a single intravenous infusion of the drug at 20 mg/kg, and followed for28 days. Based on the staining of predose blood and BM samples from monkeys for CD19, CD20, CD38, and HM1.24, a strong coincidence was seen for CD38high/CD19low/−/CD20− plasma cells with HM1.24 expression (supplemental Figure 6), and this population was quantified after XmAb5592 injection. XmAb5592 depleted blood plasma cells an average of 80% after 1 day of injection, with recovery to starting levels by day 14 (Figure 6A). In BM, XmAb5592 depleted an average of 60% of the initial plasma cells by day 7 postinjection, which did not recover fully by day 28 (Figure 6B). These data clearly indicate that a single dose of XmAb5592 depletes plasma cells in both blood and BM compartments in cynomolgus monkey. Moreover, the XmAb5592 administration was well tolerated, and no changes were identified in food consumption, body weight, serum chemistry, or in anatomical pathology parameters, suggesting a favorable therapeutic index for clinical application.
Single dose of XmAb5592 depletes blood and bone marrow plasma cells in cynomolgus monkeys. XmAb5592 was injected as a single intravenous dose at 20 mg/kg into cynomolgus monkeys (2 males, 2 females). Blood samples were collected predose and at indicated days after administration. Bone marrow aspirates were also collected predose and at 7, 14 and 28 days after XmAb5592 administration. Plasma cells (CD38highCD19low/−CD20−) were analyzed by flow cytometry and their numbers expressed as a percentage of total lymphocytes. XmAb5592 rapidly depleted blood plasma cells (A) an average of ∼ 80% after 1 day of injection which recovered to the initial level by day 14. In bone marrow (B), the plasma cells were reduced an average of ∼ 60% at day 7 after injection; however the levels did not recover completely by the study end on day 28.
Single dose of XmAb5592 depletes blood and bone marrow plasma cells in cynomolgus monkeys. XmAb5592 was injected as a single intravenous dose at 20 mg/kg into cynomolgus monkeys (2 males, 2 females). Blood samples were collected predose and at indicated days after administration. Bone marrow aspirates were also collected predose and at 7, 14 and 28 days after XmAb5592 administration. Plasma cells (CD38highCD19low/−CD20−) were analyzed by flow cytometry and their numbers expressed as a percentage of total lymphocytes. XmAb5592 rapidly depleted blood plasma cells (A) an average of ∼ 80% after 1 day of injection which recovered to the initial level by day 14. In bone marrow (B), the plasma cells were reduced an average of ∼ 60% at day 7 after injection; however the levels did not recover completely by the study end on day 28.
Discussion
A large number of antibody therapeutics target surface antigens on tumors cells while simultaneously recruiting immune effector cells to specifically destroy the malignant cells. This mode of action is dependent on the interaction between the Fc region of the antibody therapeutic and the Fcγ receptors found on cells of the immune system.40 In this report, we have demonstrated that XmAb5592, a humanized, Fc-engineered anti-HM1.24 antibody with enhanced binding to FcγRIIIa and FcγRIIa receptors, augments HM1.24-specific MM cell lysis in vitro via ADCC and ADCP. In comparison to the anti-HM1.24 IgG1 analog, the nearly 2 orders of magnitude increased affinity for FcγRIIIa translated into significantly enhanced NK cell-mediated XmAb5592 ADCC against numerous MM cell lines and primary tumor cells derived from MM patients. XmAb5592 also increased ADCP by monocyte derived macrophages, albeit to a smaller extent. This is consistent with the relatively modest enhancement of FcγRIIa binding (∼ 1 order of magnitude), and its prominent role in driving macrophage mediated cellular immunity. Recently, a defucosylated anti-HM1.24 antibody was also shown to enhance autologous ADCC activity against primary MM cells,41 consistent with the observation that removal of fucose moieties from the N-linked oligosaccharides within the Fc region significantly increases affinity for FcγRIIIa.42 Compared with the XmAb Fc mutations, defucosylated human IgG1 show a similar level of binding enhancement for the 158V allele, but much lower affinity improvement for 158F allele; and no significant improvements in binding to FcγRIIa.30 The ADCP activity of defucosylated anti-HM1.24 antibody was not evaluated, but would be expected to be similar to IgG1.
Studies have shown that IgG FcγR polymorphisms (FcγRIIa-131H/R and FcγRIIIa-158V/F) independently predict response to therapeutic mAbs, ie, rituximab in patients with follicular lymphoma,25,43 cetuximab in metastatic colorectal cancer patients,26 and trastuzumab in breast cancer patients.27 Patients with the higher affinity allele (158V) responded better to rituximab therapy. The 2 amino acid modifications to the Fc domain of XmAb5592 significantly increased the affinity to both FcγRIIIa-158V/F and FcγRIIa-131H/R, indicating a potential for XmAb5592 to overcome the polymorphism obstacles that have challenged other therapeutic mAbs. The surface expression of HM1.24 correlated with the ADCC activity of XmAb5592, with stronger activity against MM cells with higher HM1.24 expression. XmAb5592 was also able to induce significant ADCC against MM cells with low HM1.24 surface expression. MM cells from patients with advanced clinical disease show higher expression of HM1.24 compared with plasma cells from healthy donors,44 making them more susceptible to XmAb5592 directed cytotoxicity of effector cells. Therefore, XmAb5592 may impact a much larger and heterogeneous HM1.24-overexpressing cancer patient population.
The Fc mutations used in XmAb5592 increase the affinity for all of the murine FcγRs, especially the activating FcγRI (CD64) and FcγRIV, which have been implicated in the in vivo activity of antibodies in mice. In mouse xenograft studies, XmAb5592 demonstrated significantly greater potency to block human MM cell growth compared with the IgG1 analog, emphasizing the importance of increased FcγR engagement on effector cells for antitumor activity. XmAb5592 was also able to eradicate established tumors, evidenced by absence of any detectable tumors in almost half of the mice after 4 weeks of twice weekly treatment. A humanized anti-HM1.24 IgG1 antibody showed antitumor activity in both ectopic and orthotopic human MM xenograft models, which was dependent on the effector cell function.15 Based on the improved efficacy of XmAb5592 seen here in both subcutaneous MM models, it would be expected to have superior efficacy in disseminated disease settings too. It is worth pointing out however that extrapolation of mouse results to human clinical efficacy is not straightforward, because of the subtle differences between mouse and human immune systems. In a recent study in cynomolgus monkeys, with an immune system closely homologous to that of humans, we have shown that a similarly Fc-engineered anti-CD19 antibody caused an immediate and substantial B-cell depletion, whereas the IgG1 version showed no B-cell depletion.31 These observations point to the potential therapeutic benefits of effector function enhanced antibodies for treatment of human malignancies.
Lenalidomide has been used in combination with low concentrations of dexamethasone to effectively treat relapsed MM after one prior treatment.38 It has been shown to modulate the activity of NK cells and macrophages in vitro and in vivo,5,10,45 providing the scientific rationale to combine it with mAb based cancer therapies. Lenalidomide pretreatment of effector cells significantly augmented XmAb5592-induced ADCC against dexamethasone-resistant MM1R and RPMI8226 MM cells. The synergistic interaction of XmAb5592 with lenalidomide is likely because of the ability of the latter to activate effector cells. IL-2 treatment of NK cells increased the overall effectiveness of this combination. Synergy between XmAb5592 and lenalidomide also translated into better anti-tumor activity in vivo, underscoring a potential clinical development strategy for XmAb5592 combined with lenalidomide. Elotuzumab, an anti-CS1 IgG1 antibody, has recently shown promising clinical response in early stages of testing in MM patients when combined with lenalidomide and low dose dexamethasone.46,47 Lenalidomide also enhances tumor-specific CD8+ T cell responses of MM patients,48 potentially leading to maintenance of a stronger antigen-specific immune response in vivo. XmAb5592, with its enhanced effector cell interaction capability is expected to have superior anti-MM activity in combination with lenalidomide.
The strong ADCC activity of XmAb5592 in presence of BMSCs clearly indicates its ability to overcome the MM growth and survival advantages conferred by the BM microenvironment. Of special note is the strong ADCC of XmAb5592 against IL-6 dependent INA-6 MM cell line in the presence of BMSCs. INA-6 cells are particularly resistant to NK-mediated killing, even though target antigens are expressed on INA-6 cells. Compared with the minimal killing activity seen with other therapeutic antibodies targeting CS110 and CD3849 (from 2 ± 0.9% to 18 ± 1.3% lysis at 1-10 μg/mL of mAbs), XmAb5592 induced significant cell lysis against INA-6 cells (43 ± 1.5% lysis at 1μg/mL).
Cross-reactivity with cynomolgus monkey HM1.24 antigen39 allowed us to quickly evaluate the overall effectiveness of XmAb5592 at depleting the plasma cells in a species with a more closely related immune system. With an Fc-engineered anti-CD19 antibody, we have previously shown that S239D/I332E mutations increase the binding affinity to relevant cynomolgus FcγRs, analogous to that seen with human FcγRs.29 Plasma cells were depleted rapidly from blood and BM after a single dose of XmAb5592 at 20 mg/kg, suggesting that a clinically-relevant dose of this antibody therapeutic would be able to reduce the levels of malignant plasma cells in MM patients. Recently, an anti-HM1.24 antibody targeting the same epitope was shown to be cleared rapidly from the plasma in cynomolgus monkey.50 HM1.24-dependent internalization in target cells, followed by degradation in lysosomes was suggested as the possible mechanism for this elimination. In our study, the XmAb5592 also showed rapid clearance from plasma (mean half-life ∼ 33 hours, data not shown), with rapid recovery of plasma cells to normal levels in blood. Multiple doses of XmAb5592 would possibly overcome this antigen-sink and maintain the effectiveness of the drug.
Prior clinical experience with an anti-HM1.24 antibody (IgG1) indicated that the drug was safe, but possibly lacked efficacy.23 However, XmAb5592, an Fc-engineered anti-HM1.24 antibody with significantly enhanced in vitro and in vivo antitumor efficacy, appears to be a more promising next-generation immunotherapeutic for the treatment of MM. Moreover, lenalidomide potentiates XmAb5592-induced MM cell killing via NK-mediated ADCC, providing a rationale to combine these drugs to improve patient outcome in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank XianFeng Li, Araz Eivazi, Cristina Bautista, Sabikun Nahar, Heather Herman, Allison Harris, and Weihua Song for technical assistance; and David Szymkowski for help with coordinating the cynomolgus in vivo studies.
Authorship
Contribution: Y-T.T., S-Y.K., G.A.L., J.R.D., and U.S.M. designed the research; H.M.H. E.P., H.C., S-Y.K., S.C., M.J.B., D-H.T.N., S.K., S.Y.C., and U.S.M. performed the research, collected and analyzed data; N.C.M. and K.C.A provided patient samples and analyzed data; and Y-T.T., J.R.D, K.C.A., and U.S.M. wrote the manuscript.
Conflict-of-interest disclosure: H.M.H., E.P., H.C., S.C., M.J.B., D-H.T.N., S.K., S.Y.C., G.A.L., J.R.D., and U.S.M. are current or former employees of Xencor Inc. The remaining authors declare no competing financial interests.
Correspondence: Umesh S. Muchhal, Xencor Inc, 111 W Lemon Ave, Monrovia, CA 91016; e-mail: umuchhal@xencor.com.
References
Author notes
Y.-T.T., H.M.H. and S.-Y.K. contributed equally to this work.