Abstract
Personalized immunotherapy of lymphoma based on tumor idiotype (Id) has shown anti-idiotype humoral immune responses in 40%-50% and cellular immune responses in 50%-75% of follicular lymphoma patients, indicating that this therapy can be clinically successful. We have developed a novel vaccine against lymphoma consisting of an anti-CD40 Ab (ADX40) chemically conjugated to the tumor idiotype A20 and tested it in a murine lymphoma model. BALB/c mice were immunized with 2 doses of immunogen alone or in conjunction with additional adjuvants before tumor challenge. ADX40-Id vaccination resulted in significantly retarded tumor growth and reduced mouse morbidity. Moreover, similar mouse survival was obtained with 2 injections of ADX40-Id as with 8 injections using the standard therapy of keyhole limpet hemocyanin Id + GM-CSF. Co-administration of ADX40-Id with 3-O-deacyl-4′-monophosphoryl lipid A further significantly enhanced vaccine efficacy, resulting in an increased overall survival. Anti-Id–specific Abs were detected at elevated levels after ADX40-Id immunization; however, in vivo depletion of CD4 and/or CD8 T cells before challenge showed that CD8 effector T cells were the major mediators of tumor protection. The results of the present study show that the ADX40-Id conjugate vaccine is a potential candidate as a stand-alone vaccine or in combination with currently licensed adjuvants for lymphoma immunotherapy.
Introduction
Non-Hodgkin lymphoma (NHL) is a heterogenous group of malignancies representing approximately 4% of all cancers. Most NHLs (> 90%) are of a B-cell phenotype, and the current standard of care for these patients is a combination of chemotherapy and total B-cell ablation with an anti-CD20mAb.1 B-cell lymphomas express a unique Ig idiotype (Id) on their surface that can be targeted for active immune therapy.2 Id vaccines have been used in active immunization against lymphoma and have consisted of autologous Id or recombinant Id protein conjugated to keyhole limpet hemocyanin (KLH) and injected with the adjuvant GM-CSF. Vaccination with tumor-derived Id has been shown to elicit a polyclonal Ab response, as well as CD8 and CD4 T-cell recognition of Id-derived peptides presented by MHC class I and II at the tumor surface.3,4 Studies have shown that vaccination with KLH-Id conferred protection against tumor challenge in several animal models.5–7 Based on these animal studies, clinical trials in B-cell lymphoma patients were initiated.8,9 In the first clinical trial, patients who mounted an anti-Id immune response were found to have an increase in the median time to disease progression,8 and another study showed that patients could achieve complete molecular remission.9 After these encouraging immunologic and clinical outcomes, randomized, double-blind, placebo-controlled phase 3 clinical trials were performed to evaluate the clinical efficacy of the KLH-Id + GM-CSF vaccine.10,11 A phase 3 trial using the personalized BiovaxID vaccine showed a statistically significant improvement in disease-free survival in vaccine versus control arms in follicular lymphoma patients in first remission.12 The personalized MyVax vaccine (www.genitope.com) was successful in a phase 2 trial; however, a phase 3 trial showed no significant difference in the progression-free survival overall (the primary end point) of patients receiving MyVax compared with control, but did show a significant clinical effect in the immune responding group. Because the trial did not meet its primary end point, Genitope suspended the development of this vaccine. However, in a subsequent follow-up study of 91 recently diagnosed follicular lymphoma patients, induction of anti-idiotype Abs after vaccination was associated with superior overall survival.13 Ten years after treatment, the overall survival of patients with anti-Id Ab responses was 90% versus 69% in patients who did not mount an anti-Id Ab response. Roughly half of the patients mounted an anti-Id–specific Ab response. This response rate is fairly typical of the various clinical trials run in lymphoma vaccines. The utilization of novel adjuvants is an attractive option to boost response rates and the strength of immune responses and thus to improve the effectiveness of this immunotherapy in treatment of lymphoma.
CD40 is a member of the TNF receptor superfamily expressed on APCs, including B cells, macrophages, and dendritic cells (DCs), as well as on epithelial, endothelial, and some tumor cells.14–18 Ligation of CD40 on B cells by its ligand, CD154, is one of the key events required for T cell–mediated help to B cells, resulting in Ig class switching, affinity maturation, and germinal center formation.19–20 CD40 ligation also conditions DCs to efficiently prime CD8 T-cell responses.21–23 Many CD40mAbs mimic the natural ligand, CD154. We showed previously that immunization of mice with low doses of anti-CD40mAb conjugated to Ag results in enhanced primary24 and secondary Ab responses, increased IFN-γ production, and enhanced delayed hypersensitivity responses, indicating an initiation of a Th1-like cell response.25 It is widely known that Th1 immune responses are required for efficient tumor cell destruction; therefore, we considered the use of an anti-CD40mAb as an adjuvant might be beneficial in lymphoma Id vaccination.
In the present study, we assessed the effectiveness of an anti-CD40mAb as an adjuvant in a murine tumor model. We also investigated whether anti-CD40mAb had a synergistic effect with GM-CSF and the adjuvant 3-O-deacyl-4′-monophosphoryl lipid A (MPL). MPL has been evaluated as a cancer vaccine adjuvant in clinical trials,26 and is a component of the cervical cancer prophylactic vaccine Cervarix. MPL comprises the lipid A portion of Salmonella minnesota lipopolysaccharide (LPS) in an altered form. LPS and MPL induce similar cytokine profiles, however MPL is at least 100-fold less toxic.27,28 The murine A20 lymphoma model was used to assess the novel vaccine anti-CD40mAb conjugated to the A20-Id in prophylactic and therapeutic immunization schedules. The effectiveness of this novel conjugate vaccine alone and with additional adjuvants was directly assessed against the conventional therapy used in most clinical trials of KLH-Id conjugate with booster injections of the adjuvant GM-CSF. We show that after prophylactic vaccination with anti-CD40mAb conjugated to A20-Id, median and overall survival of mice was significantly enhanced. Moreover, anti-CD40mAb conjugated to A20-Id showed synergy with MPL resulting in further significant increases in survival. Although passive transfer of serum from protected to naive mice showed that anti-Id Abs were able to delay tumor progression, we demonstrate here using in vivo depletion studies that CD8 T cells were the major mediators of the tumor protection.
Methods
Mice
Female BALB/c mice (6-8 weeks of age) were obtained from Harlan UK. Experiments were performed under a United Kingdom Home Office–approved project license in compliance with strict Home Office regulations.
Tumor cells
The A20 cell line is a BALB/c B-cell lymphoma cell line originally derived from a spontaneous reticulum cell neoplasm (ATCC). Cells were cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated FCS (Bioclear), 2mM l-glutamine (Sigma-Aldrich), and 0.05mM β-mercaptoethanol (Invitrogen) in a humidified atmosphere at 37°C and 5% CO2. For tumor inoculation, 50 μL of A20 cells in PBS were given subcutaneously (SC) in the right flank.
Immunogens and adjuvants
A20 cells (ATCC) were fused with P3×63 (ATCC) myeloma cells using polyethylene glycol (Sigma-Aldrich), and a clone producing A20-Id was selected using limiting dilution screening. A20-Id was conjugated to the rat anti–mouse CD40mAb clone 10C8,29 hereafter referred to as ADX40, a rat isotype control IgG1 (anti–human IL-12, clone 20C2; ATCC), and KLH (Sigma-Aldrich). Adjuvants included 10 μg/dose of MPL (InvivoGen) and 50 ng/dose of recombinant murine GM-CSF (PeproTech).
Conjugate production
ADX40, its isotype control 20C2, and A20-Id were chemically treated with N-succinimidyl-S-acetylthioacetate (SATA; Pierce) and sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (Sulfo-SMCC; Pierce), respectively, following the manufacturer's instructions. Briefly, sulfo-SMCC was added to 2 mg/mL of A20-Id for 1 hour at room temperature (RT). Free sulfo-SMCC was removed by extensive filtration using a 30-kDa MWCO spin filter (Amicon Ultra-4; Millipore) following the manufacturer's instructions. Next, 120 μg/mL of SATA was added to 2 mg/mL of ADX40 or 20C2 for 30 minutes at RT and free SATA was removed by filtration. SATA-treated ADX40 was incubated in 0.05M hydroxylamine and 2.5mM EDTA in PBS (pH 7.2-7.5) for 2 hours at RT. Activated ADX40 and A20-Id Abs were conjugated overnight at 4°C. The reaction was stopped with 50mM L-cysteine (Sigma-Aldrich) and conjugate buffer exchanged into PBS. KLH-A20-Id conjugate was produced using glutaraldehyde. Briefly, KLH (Sigma-Aldrich) and A20-Id were mixed at an equal ratio and 800 μL of glutaraldehyde (Sigma-Aldrich) was added per 1 mL of protein mixture and rotated for 1 hour at RT and dialyzed against 2 changes of PBS. A BCA protein assay (Pierce) determined the conjugate concentration.
Production of FITC-labeled conjugate
A20-Id was labeled with FITC (Pierce) according to the manufacturer's instructions. One milligram of FITC-labeled A20-Id was reduced in 0.1mM DTT (Sigma-Aldrich) for 1 hour at 37°C and dialyzed into PBS with 0.1M EDTA as described previously,30 and further incubated with SATA-treated ADX40 or 20C2 as described in the preceding paragraph.
ELISA
Goat anti–human IgG (10 μg/mL, Fc specific; Sigma-Aldrich) was adsorbed to ELISA plates for 16 hours at 4°C. All subsequent incubation steps were for 1 hour at RT. Recombinant mouse CD40/Fc chimera (R&D Systems) was added at 0.1 μg/mL in PBS and plates blocked with 5% BSA (Sigma-Aldrich) in PBS. Conjugate samples were added at a starting concentration of 10 μg/mL and 4-fold serial dilutions were carried out. Peroxidase-conjugated polyclonal anti–mouse Ig (multiple adsorbed; BD Biosciences) was added. Absorbance at 450 nm was determined (ELX808; Bio-Tek Instruments) after the addition of o-phenylenediamine dihydrochloride (Sigma-Aldrich).
Verification of conjugation: flow cytometric analysis
CD40L929 cells are stably transfected murine L929 fibroblast cells expressing cell surface CD40.31 Cells were incubated with 5 μg of conjugate or control for 30 minutes on ice in PBS with 0.1% BSA and washed twice at 400g for 5 minutes. To detect staining, a secondary anti–mouse IgG2a-FITC mAb (BD Biosciences) was added for 30 minutes on ice in PBS with 0.1% BSA. After 2 centrifugation steps in PBS + 0.1% BSA, cells were analyzed on an LSRII (BD Biosciences) using FACSDiva Version 6.11 software (BD Biosciences). Immediately before analysis, 0.3μM TO-PRO-3-iodide (Invitrogen) was added to allow dead cell exclusion. FlowJo Version 9.3.1 software (TreeStar) was used for data analysis.
Immunization schedules
For prophylactic studies, groups of 10 mice were immunized SC in the left flank on day −28 and day −13 before challenge. Mice receiving the adjuvant GM-CSF had additional boosts on days −27, −26, −25, −12, −11, and −10 at the same injection site. Two weeks after boosting (day 0), mice received a lethal dose of 1 × 105 A20 cells. For therapeutic studies, groups of 10 mice were inoculated SC in the right flank with 105 A20 cells on day 0. Mice were immunized SC on the left flank on days 1 and 8. Early work was based on United Kingdom Co-ordinating Committee on Cancer Research guidelines allowing a maximum tumor size of 15 mm in diameter. New guidelines reduced this to 12 mm.32 Experiments shown in Figures 6 and 7 reflect this earlier cutoff point. Tumor size was measured using calipers from every 3 days to daily for a period of 120 days.
Passive serum transfer
Serum from mice immunized with ADX40-A20 + MPL and protected from a subsequent challenge of A20 tumor cells was collected at the experimental end point (day 60). The equivalent of serum from 1 mouse was transferred via IP injection into 1 naive mouse 3 days before A20 tumor cell challenge.
In vivo T cell–depletion studies
CD4+ and/or CD8+ T cells in immunized mice were depleted using 0.5 mg of anti-CD4mAb (YTS191.1) and/or 0.5 mg of anti-CD8mAb (YTS169.4.2.1) via IP injection 2 days before tumor challenge.33 Flow cytometry using fluorescently labeled anti–mouse CD4-FITC (clone YTS177.9; Serotec) and anti–mouse CD8-PE (clone 53-6.7; BD Biosciences) Abs 24 hours after injection verified cell depletion.
Anti–A20-Id Ab responses
B cell–mediated immunity was assessed by the measurement of anti–A20-Id specific Abs in the serum of immunized mice using ELISA. A20-Id Fab fragments (produced in-house using a Fab fragmentation kit; Pierce) at a concentration of 2.5 μg/mL in 0.1M sodium bicarbonate buffer, pH 9.4, were adsorbed to ELISA plates for 16 hours at 4°C. All subsequent incubations were for 1 hour at RT. Plates were blocked with 5% skim milk powder in PBS, serum samples were added at a 10-fold starting dilution, and further 2-fold serial dilutions of serum samples in blocking buffer were performed. Peroxidase-conjugated anti–mouse IgG (Fc specific; Sigma-Aldrich) was added, followed by o-phenylenediamine dihydrochloride substrate. Absorbance at 450 nm was determined.
Targeting and activation of APC by ADX40-A20 in vitro
To determine ADX40-A20 targeting and activation of APCs in vitro, splenocytes were cultured for 24 hours in the presence of 10 μg/mL of 20C2-A20FITC (isotype control), ADX40-A20FITC or 1μg/mL of concanavalin A (Sigma-Aldrich). Cells were prepared for flow cytometric analysis using the following anti–mouse Ab panel: CD19-Pacific Blue (BioLegend), CD11c-PE (BioLegend), F4/80-APC (BioLegend), and I-A/I-E-biotin (BD Biosciences), followed by streptavidin-Qdot (Invitrogen) or streptavidin-PerCP-Cy5.5 (BD Biosciences). A live/dead–discrimination UV dye (Invitrogen) enabled analysis of live cells.
Targeting and activation by APC by ADX40-A20 in vivo
Mice were immunized with 10 μg/mL of 20C2-A20FITC SC in the left groin and 10 μg/mL of ADX40-A20FITC SC in the right groin. Twenty-four hours after immunization, the draining inguinal lymph nodes were resected. Cytospins were used to transfer lymph node cells onto slides. Cells were fixed with ethanol and slides were mounted with 4′,6-diamidino-2-phenylindole. The presence of FITC in the cytoplasm of cells was determined by confocal microscopy using an Olympus ix81 microscope, oil 40×/1.3 objective on ethanol-fixed sections and an ultraview confocal imaging system (9100 EMCCD; Hamamatsu) with velocity acquisition software.
Statistical analysis
A 2-tailed Student t test or a 1-way ANOVA with a Tukey or Dunnett multiple comparison posttest was used to determine statistical significance between immunization groups when measuring immune responses. P < .05 was deemed statistically significant. A Kaplan-Meier survival curve determined the mean survival rate between groups using a log-rank test (GraphPad Prism 5.0c software).
Results
ADX40-A20 conjugate vaccine assessment
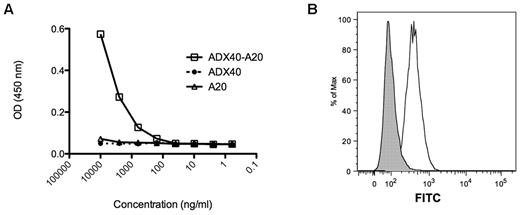
Formation of ADX40-A20-Id and control conjugates was routinely verified by sandwich ELISA and by flow cytometry. Figure 1A shows a representative plot of the specific binding of ADX40-A20 conjugate to recombinant mouse CD40/Fc chimera and anti–mouse IgG simultaneously. The ADX40 is specific for the CD40 and only the A20mAb will be recognized by anti–mouse IgG. To ensure that the ADX40-A20 conjugate recognized CD40 expressed in vitro, we assayed it on CD40-expressing L929 fibroblast cells. A representative plot of the ADX40-A20 binding to live CD40L929 cells is shown in Figure 1B.
ADX40-A20 conjugate vaccine batch testing. (A) ADX40-A20 conjugate binds efficiently down to a concentration of 0.5 μg/mL in the CD40/anti–mouse hybrid ELISA, whereas at a concentration of 10 μg/mL, parental A20 and ADX40mAbs do not bind. (B) Binding of ADX40-A20 to CD40-expressing L929 cells is shown as an increase in fluorescence and a shift to the right (white peak) compared with the negative control 20C2-A20 conjugate (gray peak).
ADX40-A20 conjugate vaccine batch testing. (A) ADX40-A20 conjugate binds efficiently down to a concentration of 0.5 μg/mL in the CD40/anti–mouse hybrid ELISA, whereas at a concentration of 10 μg/mL, parental A20 and ADX40mAbs do not bind. (B) Binding of ADX40-A20 to CD40-expressing L929 cells is shown as an increase in fluorescence and a shift to the right (white peak) compared with the negative control 20C2-A20 conjugate (gray peak).
ADX40-adjuvanted A20-Id vaccine improves mouse survival at low doses
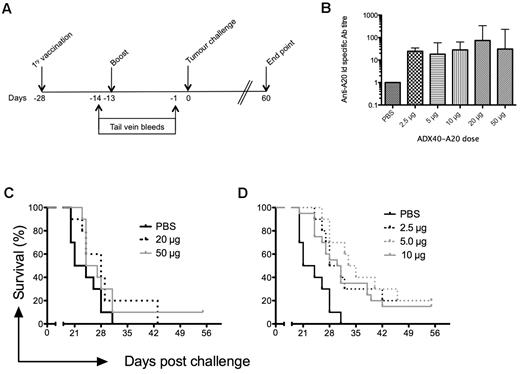
To determine the optimum dose of ADX40-A20 for efficacy studies, groups of mice were inoculated according to the schedule shown in Figure 2A, with doses ranging from 2.5-50 μg (of which half is idiotype protein). To assess anti–A20-Id specific Ab responses in vivo, mice were bled twice before challenge and the sera analyzed by ELISA. Generally, anti-Id–specific Ab levels after primary immunization were undetectable or very low (not shown). Anti-Id Ab levels were higher after boost, and these end-point titers are shown in Figure 2B. All mice immunized with ADX40-A20 generated anti-Id responses. Although there was no significant difference between the anti-Id Ab titers between doses (Figure 2C), immunization with the lower doses of 2.5, 5, and 10 μg significantly increased median survival by 7 (P = .0003), 11.5 (P < .004) and 8 (P < .008) days, respectively (Figure 2D). We opted to use the highest dose that showed significantly enhanced survival (10 μg of ADX40-A20) in further experiments because we found previously that ADX40 conjugated to other Ags enhances immune responses at a dose of 10 μg.24
Dose response of ADX40-A20 vaccine in BALB/c mice. (A) Mice were immunized on day −28 and boosted on day −13 before challenge with A20 cells (day 0), as depicted in the immunization schedule. Tail-vein bleeds were performed on day −14 and −1, and the experimental end point was 60 days after challenge. (B) Mean (± SD) end point anti-Id titers after boost show increased levels at all doses of ADX40-A20 compared with PBS, as determined by ELISA (P < .0005). (C) Kaplan-Meier survival curve shows there was no significant survival advantage after inoculation with 20 or 50 μg of ADX40-A20. (D) Median survival was significantly increased using the lower doses (2.5 μg dose, P < .0005; 5 μg dose, P < .005; and 10 μg dose, P < .05).
Dose response of ADX40-A20 vaccine in BALB/c mice. (A) Mice were immunized on day −28 and boosted on day −13 before challenge with A20 cells (day 0), as depicted in the immunization schedule. Tail-vein bleeds were performed on day −14 and −1, and the experimental end point was 60 days after challenge. (B) Mean (± SD) end point anti-Id titers after boost show increased levels at all doses of ADX40-A20 compared with PBS, as determined by ELISA (P < .0005). (C) Kaplan-Meier survival curve shows there was no significant survival advantage after inoculation with 20 or 50 μg of ADX40-A20. (D) Median survival was significantly increased using the lower doses (2.5 μg dose, P < .0005; 5 μg dose, P < .005; and 10 μg dose, P < .05).
ADX40-A20 vaccination is superior to KLH-A20 vaccination in the A20 mouse model
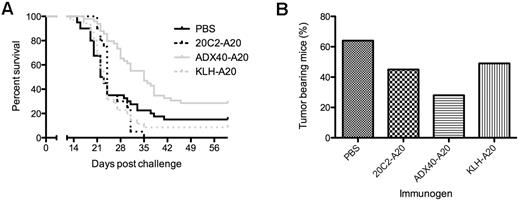
To demonstrate that the enhanced survival of ADX40-A20–immunized mice was specific to the binding of ADX40 to CD40, rather than to a carrier or an Fc-binding effect, we compared it with immunization with the isotype control 20C2 (IgG1) mAb conjugated to A20-Id (20C2-A20). 20C2 mAb reacts with the human IL12 p70 heterodimer and has no known Ag specificity in mice. Therefore, we investigated whether the enhanced mouse survival was equal to or superior to that seen after immunization with KLH-A20. Figure 3A shows pooled data from 6 independent experiments of the overall survival of mice immunized as follows: PBS vehicle control, 10 μg of 20C2-A20 control conjugate, 20 μg of KLH-A20 conjugate, and 10 μg of ADX40-A20 conjugate. Immunization with 20C2-A20 or KLH-A20 conjugate (no GM-CSF) showed no survival advantage over PBS-immunized mice. However, median survival after ADX40-A20 immunization was significantly increased to 35 days (P < .0003) from 22.5 for PBS, 24 days for 20C2-A20, and 23 days for KLH-A20, illustrating that ADX40-A20 vaccination was superior to KLH-A20 in this tumor model (P < .02). We assessed the median tumor burden (in cubic millimeters) and the percentage of tumor-bearing mice in all groups on day 15. This time point was chosen because this was the first day a mouse in the PBS group was culled because of its tumor size reaching the maximum limit. As shown in Figure 3B, the percentage of mice bearing visible tumors in the PBS control group (64%) was much greater than in mice vaccinated with ADX40-A20 (28%), whereas 45% of 20C2-A20 conjugate- and 49% of KLH-A20 conjugate–immunized mice had developed tumors by this day.
ADX40-A20 conjugate vaccine enhances tumor protection. (A) ADX40-A20 shows efficacy in the lymphoma tumor model, with significantly increased overall survival compared with the PBS, 20C2-A20, and KLH-A20 control groups (P < .0003). (B) Percentage of mice with tumors on day 15 after challenge showing a much lower percentage of ADX40-A20–immunized mice with tumors compared with the control groups. PBS, n = 39; 20C2-A20, n = 20; ADX40-A20, n = 50; and KLH-A20, n = 35.
ADX40-A20 conjugate vaccine enhances tumor protection. (A) ADX40-A20 shows efficacy in the lymphoma tumor model, with significantly increased overall survival compared with the PBS, 20C2-A20, and KLH-A20 control groups (P < .0003). (B) Percentage of mice with tumors on day 15 after challenge showing a much lower percentage of ADX40-A20–immunized mice with tumors compared with the control groups. PBS, n = 39; 20C2-A20, n = 20; ADX40-A20, n = 50; and KLH-A20, n = 35.
The effect of ADX40-A20 vaccination is not through a direct ligation of CD40 expressed by A20 tumor cells
To exclude the possibility that prophylactic immunization with ADX40 may directly affect the development of A20 tumors through the expression of CD40 on their surface, we included a control group of ADX40 conjugated to an irrelevant mouse IgG mAb (16.5H2; Developmental Studies Hybridoma Bank, Columbia University, New York, NY). Immunization with this immunogen did not protect against the development of A20 tumors (Figure 4A). Moreover, immunizing mice with 5 μg of ADX40 admixed with 5 μg of A20-Id did not improve mouse survival compared with PBS vehicle control, showing that for an antitumor protective immune response to occur, ADX40 must be chemically conjugated to A20-Id (Figure 4B).
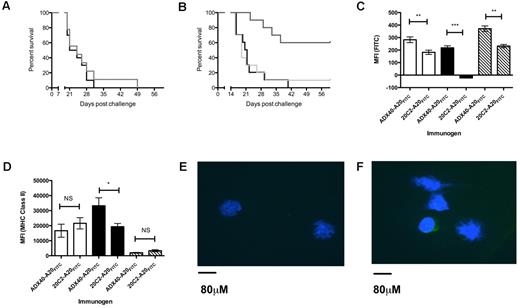
Chemical conjugation of ADX40 to A20-Id is required to achieve antitumor effects. (A) Immunization with ADX40 conjugated to the irrelevant mouse IgG 16.5H2 (gray line) did not enhance mouse survival compared with the vehicle control PBS (black line). (B) In addition, admixing ADX40 with A20-Id (light gray line) was not sufficient to induce antitumor immune responses leading to tumor protection. Chemical conjugation of ADX40 to A20-Id (dark gray line) resulted in significantly increased overall survival compared with admixed ADX40 and A20-Id and PBS (black line). (C) In vitro culture of splenocytes with ADX40-A20FITC and 20C2-A20FITC showed specific uptake of ADX40-A20 FITC through the CD40 receptor, with a significantly increased fluorescence on B cells (black bars), DCs (open bars), and macrophages (hashed bars) compared with isotype control. (D) MHC class II expression was significantly enhanced on B cells (black bars) in response to uptake of ADX40-A20FITC compared with the isotype control. Panels C and D represent 1 experiment with n = 3 of a total of 4 individual experiments carried out. Background fluorescence (cells cultured with concanavalin A) has been subtracted from plotted values in panels C and D. MFI indicates mean fluorescent intensity. *P < .05; **P < .005; and ***P < .0005. (E) Confocal image (40× magnification) showing cells from left draining lymph node (20C2-A20FITC), in which there is no green fluorescence. (F) Confocal image (40× magnification) of cells from the right draining lymph node (ADX40-A20 FITC) showing the presence of green fluorescence in the cytoplasm of lymph cells, indicating specific A20-Id uptake by these cells.
Chemical conjugation of ADX40 to A20-Id is required to achieve antitumor effects. (A) Immunization with ADX40 conjugated to the irrelevant mouse IgG 16.5H2 (gray line) did not enhance mouse survival compared with the vehicle control PBS (black line). (B) In addition, admixing ADX40 with A20-Id (light gray line) was not sufficient to induce antitumor immune responses leading to tumor protection. Chemical conjugation of ADX40 to A20-Id (dark gray line) resulted in significantly increased overall survival compared with admixed ADX40 and A20-Id and PBS (black line). (C) In vitro culture of splenocytes with ADX40-A20FITC and 20C2-A20FITC showed specific uptake of ADX40-A20 FITC through the CD40 receptor, with a significantly increased fluorescence on B cells (black bars), DCs (open bars), and macrophages (hashed bars) compared with isotype control. (D) MHC class II expression was significantly enhanced on B cells (black bars) in response to uptake of ADX40-A20FITC compared with the isotype control. Panels C and D represent 1 experiment with n = 3 of a total of 4 individual experiments carried out. Background fluorescence (cells cultured with concanavalin A) has been subtracted from plotted values in panels C and D. MFI indicates mean fluorescent intensity. *P < .05; **P < .005; and ***P < .0005. (E) Confocal image (40× magnification) showing cells from left draining lymph node (20C2-A20FITC), in which there is no green fluorescence. (F) Confocal image (40× magnification) of cells from the right draining lymph node (ADX40-A20 FITC) showing the presence of green fluorescence in the cytoplasm of lymph cells, indicating specific A20-Id uptake by these cells.
Durability of tumor protection
To test the durability of protection offered following inoculation of ADX40-A20 or the vehicle control PBS, mice were observed for a period of 120 days after challenge. Results showed that if mice were tumor free on day 40 after challenge they remained this way (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the outline article).
ADX40-A20 targets and activates professional APCs
To show specific cellular uptake (through CD40 targeting) of the A20-Id Ag, we labeled A20-Id with FITC before conjugation with ADX40 or the isotype control 20C2. In vitro culture of splenocytes with ADX40- A20FITC showed a specific uptake of A20-Id by professional CD40-expressing APC, including B cells, DCs, and macrophages, compared with isotype control conjugate (Figure 4C). Simultaneous staining for MHC class II showed that B cells, but not DCs or macrophages, had enhanced expression levels of this activation marker after culture with ADX40-A20FITC compared with 20C2-A20FITC (Figure 4D). To confirm in vivo targeting, and to ensure that the ADX40-A20FITC was internalized by APCs, we isolated draining lymph nodes from immunized mice and imaged these using confocal microscopy. Figure 4E shows an image of the left lymph node into which the 20C2-A20FITC drained. It can be seen that there was no FITC fluorescence from these cells. Figure 4F shows distinct cytoplasmic staining in 20%-25% of cells in the right lymph node, confirming lymphocyte uptake of ADX40-A20FITC.
Median survival after immunization with ADX40-A20 alone is comparable to KLH-A20 + GM-CSF vaccination using fewer injections
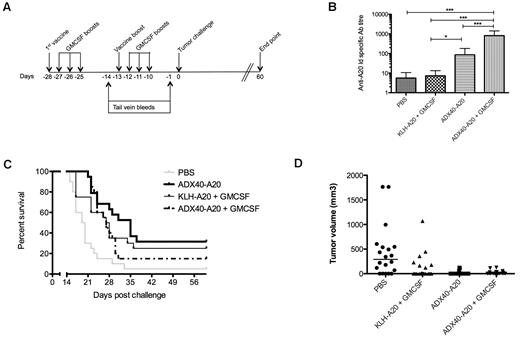
GM-CSF is commonly used to attract APCs to the injection site to boost immune responses. Current lymphoma immunotherapy strategies in trials use KLH-Id administered in conjunction with GM-CSF. To ascertain whether GM-CSF would have a synergistic effect with the CD40-targeting adjuvant, GM-CSF was included in the immunization schedule. The efficacy of ADX40-A20 in the presence and absence of GM-CSF was also compared with the current treatment strategy of KLH-Id + GM-CSF. PBS and ADX40-A20 groups were immunized according to the schedule shown in Figure 2A. Figure 5A shows the schedule used for mice receiving the additional GM-CSF boosts. Mice were inoculated with a dose of 50 ng of GM-CSF on the day of vaccination, followed by 3 daily boosts, resulting in a total of 8 GM-CSF injections per mouse.
Lack of synergy between ADX40 and GM-CSF. (A) Groups of mice requiring GM-CSF injections were immunized on days −28, −27, −26, −25, −13, −12, −11, and −10. (B) Anti-Id–specific titer after boost (day −1) was significantly increased in ADX40-A20 ± GM-CSF groups compared with the PBS and KLH-A20 + GM-CSF groups (mean ± SD; n = 20). *P < .05; *** P < .0005. (C-D) Kaplan-Meier survival curve shows significantly increased median survival of mice (C) and lower tumor burden (D) of all treatment groups compared with PBS (n = 20). ***P < .0001.
Lack of synergy between ADX40 and GM-CSF. (A) Groups of mice requiring GM-CSF injections were immunized on days −28, −27, −26, −25, −13, −12, −11, and −10. (B) Anti-Id–specific titer after boost (day −1) was significantly increased in ADX40-A20 ± GM-CSF groups compared with the PBS and KLH-A20 + GM-CSF groups (mean ± SD; n = 20). *P < .05; *** P < .0005. (C-D) Kaplan-Meier survival curve shows significantly increased median survival of mice (C) and lower tumor burden (D) of all treatment groups compared with PBS (n = 20). ***P < .0001.
Mice that received ADX40-A20 alone had significantly higher anti-Id–specific Ab titers than mice immunized with KLH-A20 + GM-CSF (P < .007, Figure 5B). Immunization with ADX40-A20 + GM-CSF induced significantly higher Ab titers than any of the other regimes (P < .0001); however, there was no improvement in overall survival (Figure 5C). Vaccination with ADX40-A20 alone or in combination with GM-CSF did show a significantly longer median survival compared with PBS control (P < .0001 and P < .002). The tumor burden on day 15 (Figure 5D) showed retarded growth of all immunized groups compared with the PBS control group (P < .0001).
ADX40 shows synergy with MPL and treatment combination improves mouse survival
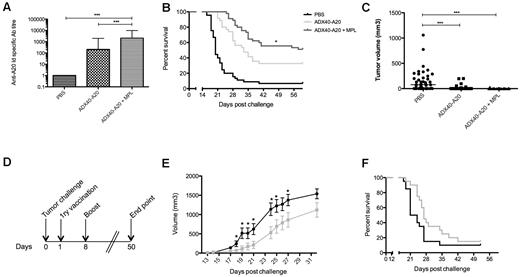
To investigate a possible synergy of ADX40-A20 with MPL, we injected mice with both materials according to the immunization schedule shown in Figure 2A. Figure 6A shows pooled end-point anti-Id titers after boost from 4 independent challenge experiments (n = 35). The end-point titers in ADX40-A20 + MPL–vaccinated mice were significantly higher than after ADX40-A20 immunization alone (P < .0001), and 51% of mice co-immunized with ADX40-A20 and MPL remained tumor free at day 60 after tumor injection (Figure 6B, n = 45); this was significantly higher than the ADX40-A20–vaccinated group, in which 32% of mice were tumor free (P = .024). The addition of MPL to the ADX40-A20 vaccine was superior to the standard therapy of KLH-A20 + GM-CSF, with greater than 30% more mice being tumor free compared with the therapy used in previous clinical trials (P = .0003, data not shown). The median tumor burden of mice on day 15 (Figure 6C) shows that ADX40-A20 vaccination alone and with MPL significantly retarded tumor growth compared with control mice (P < .0001). Control groups included PBS + MPL, A20-Id + MPL, and KLH-A20 + MPL. The addition of MPL to the control groups was not sufficient to increase the anti-Id–specific Ab titer or median or overall survival (data not shown).
MPL synergizes with ADX40 to increase immune responses against A20-Id and retards tumor progression in a therapeutic setting. (A) Anti-Id end-point titer after boost shows enhancement after immunization with ADX40-A20 and significant further enhancement when co-immunized with MPL (mean ± SD; n = 35). (B-C) Kaplan-Meier survival curve showing significantly increased median survival of mice given the combination treatment to ADX40-A20 vaccine alone (B; n = 45) and significantly lower tumor burden (C). * P < .05; *** P < .0005. (D) In a therapeutic setting, mice were challenged with A20 cells on day 0, followed by a primary vaccination on day 1 and a booster vaccination on day 8. (E) ADX40-A20 + MPL vaccination after challenge resulted in significantly slower tumor growth (gray line) compared with PBS (black line). *P < .05 by Student t test. (F) Graph showing the 4.5-day increase in median survival of ADX40-A20–vaccinated mice (gray line) compared with PBS–injected mice (black line); however, this was not significant. Figure shows pooled data from 2 independent experiments (n = 20).
MPL synergizes with ADX40 to increase immune responses against A20-Id and retards tumor progression in a therapeutic setting. (A) Anti-Id end-point titer after boost shows enhancement after immunization with ADX40-A20 and significant further enhancement when co-immunized with MPL (mean ± SD; n = 35). (B-C) Kaplan-Meier survival curve showing significantly increased median survival of mice given the combination treatment to ADX40-A20 vaccine alone (B; n = 45) and significantly lower tumor burden (C). * P < .05; *** P < .0005. (D) In a therapeutic setting, mice were challenged with A20 cells on day 0, followed by a primary vaccination on day 1 and a booster vaccination on day 8. (E) ADX40-A20 + MPL vaccination after challenge resulted in significantly slower tumor growth (gray line) compared with PBS (black line). *P < .05 by Student t test. (F) Graph showing the 4.5-day increase in median survival of ADX40-A20–vaccinated mice (gray line) compared with PBS–injected mice (black line); however, this was not significant. Figure shows pooled data from 2 independent experiments (n = 20).
Therapeutic vaccination with ADX40-A20 retards tumor growth
Vaccination with ADX40-A20 + MPL after tumor inoculation according to the immunization schedule shown in Figure 6D resulted in a significantly slower growth of tumors (P < .05) compared with mice immunized with PBS (Figure 6E) from day 18 up to day 27 after challenge; however, median survival was not significantly increased (Figure 6F).
The involvement of anti-idiotype Abs in tumor clearance
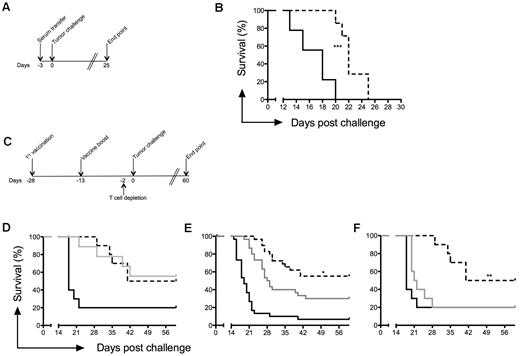
To investigate the importance of anti-Id Abs in the prevention of tumor growth, serum from protected mice (after immunization with ADX40-A20 + MPL) was transferred into naive mice 3 days before tumor challenge (Figure 7A). Mice injected intraperitoneally with PBS served as a control. Transfer of immune sera to naive mice resulted in retarded tumor growth and consequently a significantly increased median survival (22 days vs 18 days for control, P = .0003; Figure 7B).
CD8 effector T cell–mediated tumor protection. (A) Serum from A20 tumor cell–protected mice was transferred into naive mice 3 days before tumor challenge. (B) Passive transfer of serum from protected mice resulted in a 4-day increase in median survival of naive mice. (C) T-cell depletion using anti-CD4 (n = 10) and anti-CD8 (n = 30) mAbs was carried out 2 days before tumor challenge. Mice immunized with PBS (black line), ADX40-A20+MPC (dashed line), or ADX40-A20+MPC with cell depletion (gray line). (D) CD4 depletion did not affect median or overall survival. (E) CD8 depletion significantly reduced median and overall survival. Graph shows pooled data from 3 independent challenge experiments (n = 30). (F) Depletion of CD4 and CD8 cells significantly reduced median and overall survival (n = 10). *P < .05; **P < .005.
CD8 effector T cell–mediated tumor protection. (A) Serum from A20 tumor cell–protected mice was transferred into naive mice 3 days before tumor challenge. (B) Passive transfer of serum from protected mice resulted in a 4-day increase in median survival of naive mice. (C) T-cell depletion using anti-CD4 (n = 10) and anti-CD8 (n = 30) mAbs was carried out 2 days before tumor challenge. Mice immunized with PBS (black line), ADX40-A20+MPC (dashed line), or ADX40-A20+MPC with cell depletion (gray line). (D) CD4 depletion did not affect median or overall survival. (E) CD8 depletion significantly reduced median and overall survival. Graph shows pooled data from 3 independent challenge experiments (n = 30). (F) Depletion of CD4 and CD8 cells significantly reduced median and overall survival (n = 10). *P < .05; **P < .005.
CD8+ effector T cells as inhibitors of tumor growth
To determine the importance of T cells in inhibiting tumor growth, CD4 lymphocytes, CD8 lymphocytes, or both were depleted using the appropriate Abs 2 days before tumor challenge according to the immunization schedule shown in Figure 7C. Twenty-four hours after depletion, < 1% CD4 and < 3% CD8 cells were detected in the blood of mice (data not shown). Four groups of mice were vaccinated with ADX40-A20 + MPL, of which 1 group was depleted of CD4, 1 of CD8, and 1 of CD4 and CD8 cells before tumor challenge. Depletion of CD4 cells alone did not affect the tumor growth or the overall survival of the ADX40-A20 + MPL–immunized mice (Figure 7D). Depletion of the CD8 T-cell subset resulted in significantly reduced (P < .02) median and overall survival (Figure 7E). Depletion of CD4 and CD8 T-cell subsets significantly reduced overall survival, as shown in Figure 7F (P < .0011), and mice succumbed to their tumors as quickly as PBS controls (P = .1863).
Discussion
A personalized immunization strategy using lymphoma Id conjugated to the immunostimulatory carrier KLH and adjuvanted with GM-CSF has been used in clinical trials in conjunction with conventional treatments, including chemotherapy and anti-CD20 Ab therapy, to improve outcome for NHL patients. Clinical benefit has been demonstrated in patients with follicular lymphoma who received idiotype vaccination after standard chemotherapy without anti-CD20 Ab therapy. Twenty of 25 patients in that study had a vaccine-induced immune response that resulted in a statistically significantly longer median duration of second complete response compared with their first complete response.34 Although at first sight, the results from 2 phase 3 clinical trials were not overwhelmingly encouraging, further analyses of the data showed that for the approximately 55% of patients who mounted an anti-Id Ab response, overall survival was significantly improved.13 We hypothesized that the use of adjuvants more potent than GM-CSF may be able to enhance the immune responses seen and thereby improve patient outcome on an individual basis, in addition to the number of patients aided by the treatment.
We have over the last decade been investigating the use of the novel adjuvant ADX40 (reviewed by Carlring et al35 ). We first showed that immunization with anti-CD40mAb mixed with Ag in large doses leads to enhanced Ab responses against T cell–independent type 2 and type 1 Ags.36,37 To reduce the side effects induced by large doses, anti-CD40mAb was chemically conjugated to Ag and a low dose was injected into mice. This regimen resulted in specific Ab titers up to 1000-fold higher compared with a control Ab conjugate and without any associated side effects.24 Conjugated anti-CD40mAb is also able to enhance delayed hypersensitivity responses, T-cell proliferation, and type 1 cytokine production in response to Ag in vitro.25 Enhanced Ab responses are also more rapid than those induced by the currently licensed adjuvants alum or MPL.38 In the present study, we show for the first time that the ADX40-A20 conjugate mediates its effect by targeting APCs, including B cells, CD11c+ DCs, and macrophages. The high levels of MHC class II seen on the B cells after ADX40-A20 exposure indicates that these cells became activated after uptake.
To test the efficacy of the ADX40 adjuvant in a tumor setting, we used the established A20 lymphoma mouse model. After chemical conjugation of A20-Id to ADX40, mice were immunized along with controls in a prophylactic or therapeutic immunization schedule and anti-Id–specific Ab titer, tumor growth, and median and overall survival were determined.
The initial dose-response experiments showed that a dose range of 2.5-10 μg of ADX40-A20 conjugate could effectively enhance mouse survival. These low doses were in agreement with previous studies in our laboratory using ADX40 as an adjuvant.23 Immunization with an isotype control Ab conjugated to the A20-Id had no effect in vivo, demonstrating that the effect seen with the ADX40-A20-Id conjugate was mediated through CD40. Moreover, immunization with ADX40 conjugated to an irrelevant mouse IgG Ab or ADX40 admixed with A20-Id had no effect on tumor progression, showing that the effect was not because of direct targeting to CD40 expressed by A20 lymphoma cells. In addition to showing efficacy in a prophylactic model, ADX40-A20 vaccination resulted in a significant retardation in tumor growth in a therapeutic setting.
Based on findings in preclinical mouse studies using the A20 model,7 current immunotherapy treatments in trials use GM-CSF as an additional adjuvant to further attract APCs to the injection site.8,9 For maximum effect, GM-CSF is given on 4 consecutive days, including the day of KLH-Id injection (reviewed by Bendandi39 ). We included GM-CSF in our immunization schedule to determine whether the ADX40 adjuvant alone would have a similar adjuvant effect to GM-CSF (with KLH vaccine) and also whether there was a synergistic effect between CD40 signaling and GM-CSF. Although serum anti-Id Abs were significantly higher in mice immunized with ADX40-A20 ± GM-CSF than mice injected with KLH-A20 + GM-CSF, this did not result in significant differences in the median or overall survival. Interestingly, 2 injections of ADX40-A20 resulted in similar mouse survival to 2 injections of KLH-A20 plus 8 injections of GM-CSF, indicating the potency of ADX40-Id as an adjuvant. There was no synergy between ADX40 and GM-CSF in this model; in fact, animals immunized with ADX40-A20 in the absence of GM-CSF had a superior survival, possibly due to endogenous release of GM-CSF induced by CD40 ligation (A.W.H., J.C., and S. Clark, unpublished data, February-September 2010).
The synergy between ADX40 and MPL resulted in an enhanced immune response and greater tumor protection. Interestingly, co-injection of A20-Id with MPL, or indeed KLH-A20 with MPL, had no effect on tumor progression or survival, indicating a real synergy between CD40 and TLR4 signaling. Because CD40 and CD154 interactions exert their action independently of the TLR pathway, the ligation of CD40 by ADX40 and TLR4 by MPL may result in a magnified pro-inflammatory immune response because of the activation of these 2 pathways simultaneously. In clinical trials, patients have shown various immune responses, from CD8 T-cell activity to enhanced anti-Id Ab titer8,9 ; however, to date, there has not been a clear mechanism elucidated as to how protection is mediated, and there may be a need for both humoral and cell-mediated immunity to be mounted for maximum clinical benefit. Studies using the A20 mouse model have produced conflicting data with regard to the involvement of humoral and cell-mediated responses in protection. Anti-Id Ab, CD4 T cells, and CD8 T cells have all been implicated or credited with protection after various immunization regimes.40–45 Currently, the standard therapy of care for follicular lymphoma is a combination of chemotherapy and anti-CD20 Ab therapy.1,46 After injection with the anti-CD20 Ab (Rituxan or rituximab; Genentech) all B cells are depleted; therefore, if a vaccine mediates its effect via Ab, it will be ineffective after anti-CD20. For this reason, there is usually a recovery period required between cessation of rituximab treatment and idiotype vaccination. If Id-vaccination could induce a T cell–mediated antitumor effect, it could be given much sooner after rituximab therapy, possibly even concurrently, and could be more effective at eliminating minimal residual disease. The major mediator of tumor protection seen in the present study was likely CD8 T cells, because depletion of these cells before tumor challenge removed a significant effect of the vaccine. Conversely, depletion of CD4 cells did not alter the tumor protection offered by vaccination with ADX40-A20 in the presence of MPL. Depletion of CD4 and CD8 effector cells before challenge in our system totally abolished the vaccine effect, further showing T-cell involvement in tumor protection. We show significantly enhanced anti-Id Ab titers after immunization with ADX40-A20 in the absence or presence of MPL, and this might suggest that these Abs provide protection from tumor progression in these mice. However, across our studies, anti-Id titers were not correlated with protection, and passive transfer of serum from protected mice, while delaying growth, did not enhance overall survival. These data show that co-immunization of ADX40-A20 with MPL generates both a humoral and a Th1/CD8 response, with the latter being primarily responsible for protection.
We conclude that the use of the ADX40-Id conjugate requires substantially fewer injections and may prove to be a more potent vaccine strategy for the treatment of NHL patients than the currently used KLH-Id + GM-CSF immunization schedule.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Susan Clark and Kay Hopkinson for assistance with flow cytometric analysis and Fiona Morrow and Simon Tazzyman for assistance with cytospins and confocal microscopy, respectively.
This work was supported by a grant from Yorkshire Cancer Research (S286 to J.C. and R.D.) and by Adjuvantix (to J.C., M.J.S, and E.D.L.).
Authorship
Contribution: J.C., M.J.S., R.D., and E.D.L. performed the experiments; J.C. analyzed the results and produced the figures; J.C. and A.W.H. designed the research; and J.C. wrote the manuscript.
Conflict-of-interest disclosure: J.C. and A.W.H. are listed as inventors for patent number I3101P/WOI. A.W.H. owns stock in and is a director of Adjuvantix, which has commercial interest in lymphoma vaccines. The remaining authors declare no competing financial interests.
Correspondence: Andrew Heath, Department of Infection and Immunity, L floor Medical School, University of Sheffield, Beech Hill Road, Sheffield, S10 2RX, United Kingdom; e-mail: a.w.heath@shef.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal