Abstract
The role of CD4+ T cells in the pathogenesis of aplastic anemia (AA) is not well characterized. We investigate CD4+ T-cell subsets in AA. Sixty-three patients with acquired AA were studied. Th1 and Th2 cells were significantly higher in AA patients than in healthy donors (HDs; P = .03 and P = .006). Tregs were significantly lower in patients with severe AA than in HDs (P < .001) and patients with non-severe AA (P = .01). Th17 cells were increased in severe AA (P = .02) but normal in non-severe AA. Activated and resting Tregs were reduced in AA (P = .004; P = .01), whereas cytokine-secreting non-Tregs were increased (P = .003). Tregs from AA patients were unable to suppress normal effector T cells. In contrast, AA effector T cells were suppressible by Tregs from HDs. Th1 clonality in AA, investigated by high-throughput sequencing, was greater than in HDs (P = .03). Our results confirm that Th1 and Th2 cells are expanded and Tregs are functionally abnormal in AA. The clonally restricted expansion of Th1 cells is most likely to be antigen-driven, and induces an inflammatory environment, that exacerbate the functional impairment of Tregs, which are reduced in number.
Introduction
Aplastic anemia (AA) is a BM failure syndrome characterized by apoptosis of CD34+ cells that leads to a reduction or absence of primitive long-term culture-initiating cells and mature hemopoietic progenitors of all cell lineages, which results in a hypocellular marrow and pancytopenia. Most cases of AA are acquired and idiopathic and are associated with an aberrant immune response.1,2 CD8+ cytotoxic T cells with restricted TCR diversity (oligoclonal T cells) are expanded in AA and secrete proinflammatory cytokines such as IFN-γ and TNF-α, which induce apoptosis of CD34+ cells, in part through the Fas-dependent pathway.3 The detection of similar clonotypes, as shown by significantly skewed CDR3 size distribution, suggests the presence of common antigen-driven T-cell expansion.4,5 The size of the expanded CD8 T-cell clone ranges from 25%-100% of a given Vβ family. Although there are comparable clonotypes, a unique clonotype is not present in all patients. In AA, there is overrepresentation of HLA-DR2 and its split HLA-DR15 and DRB1*1501 and 1502 alleles, as well as class I HLA-B*4002 and HLA-A*0206.6–8 Hematologic recovery after immunosuppressive therapy (IST) with anti-thymocyte globulin (ATG) and cyclosporin occurs in 60%-75% of patients with AA9,10 and may be associated with diminution or disappearance of the expanded T-cell clones. Relapse of AA may be associated with reemergence of the original oligoclonal T cells, sometimes along with new clones.4
Although CD8+ T cells contribute to the pathogenesis of autoimmune responses, recent studies have confirmed the importance of CD4+ T cells in autoimmunity, which may be even more important than CD8+ cells.11,12 CD4+ cells include IFN-γ–producing CD4+ T cells (Th1 cells), IL-4–producing CD4+ T cells (Th2 cells), regulatory T cells (Tregs), and IL-17–producing CD4+ T cells (Th17 cells), all of which play a pivotal role in autoimmunity.13,14 In AA, Tregs are reduced and express low levels of transcription factor Foxp3.15,16 However, the Treg population is not uniform, and distinct subpopulations of human Tregs, demonstrating functional heterogeneity,17 have been identified recently. On the basis of nuclear expression of Foxp3 and CD45RA surface expression, Tregs can be defined as resting Tregs (CD45RA+Foxp3low), activated Tregs (CD45RA−Foxp3high), or cytokine-secreting CD4+ T cells (CD45RA−Foxp3low). Some Tregs suppress proliferation, whereas other subpopulations, for example, CD4+ CD25+ CD45RA−Foxp3low cells, secrete proinflammatory cytokines such as IL-17, IL-2, and IFNγ and have minimal inhibitory function. The proportions and functions of these Treg subpopulations vary in different autoimmune disorders.17,18
In the present study, we sought to assess the number and function of Treg subsets and to identify correlations with disease severity and evaluate changes in T-helper cell subsets. Furthermore, we examined the clonality of CD4+ Th1 subsets and correlated the changes with abnormalities of the other CD4+ T cells.
We show that AA is characterized by a marked expansion of Th1 and Th2 cells and an abnormal phenotype and function of Tregs and that abnormalities of the Tregs and Th17 cells correlate with the severity of AA. Spectratyping and high-throughput deep sequencing showed that CD4+ Th1 cells are clonally restricted, which can serve as a potential tool for the follow-up of patients with this disease and also help to identify potential antigen(s) that drive this clonality.
Methods
Patient cohort
Blood and BM samples were obtained from 63 patients with AA and 10 age-matched healthy donors. Of 63 patients, 48 were analyzed at diagnosis, and an additional 15 patients were analyzed after immunosuppressive therapy. Thirty-one patients had severe (n = 17) or very severe (n = 14) AA, and 32 had nonsevere AA. Standard criteria were used for diagnosis of AA and for disease severity.19 Serial samples before and after ATG were collected in 4 patients. Each patient donated peripheral blood on 1 occasion before therapy and/or after ATG therapy. The King's College Hospital Local Research Ethics Committee approved the clinical study of IST and sample collection. The median age of the patients was 42 years (range 18-76 years). All patients who were < 35 years old and up to < 50 years of age and who were potential transplantation candidates were screened for Fanconi anemia. All patients were screened for paroxysmal nocturnal hemoglobinuria (PNH) by flow cytometry for glycosylphosphatidylinositol-anchored protein expression on red cells, granulocytes, and monocytes, with or without FLAER (fluorescent-labeled inactive toxin aerolysin). A small to moderate PNH clone was detected in 24 (38%) of 63 patients at the time of this study (Table 1). There were no cases of hemolytic PNH. Abnormal BM cytogenetics [−7 and del(10q)] were detected in 2 patients whose BM was consistent with AA and showed no morphologic evidence of myelodysplastic syndrome.
Characteristics of patients and type and response to treatment
| Characteristic . | Value . |
|---|---|
| Number of patients | 63 |
| Median age, y (range) | 42 (18-76) |
| Disease severity at diagnosis (SAA:NSAA), n | 31:32 |
| Median disease duration, mo (range) | 41 (0.4-300) |
| Sex (M:F), n | 28:35 |
| PNH clone at time of study, n (%) | 24 (38) |
| Size of PNH clone, median % (range) | |
| Red cells | 0.9 (0-27.7) |
| Granulocytes | 2.9 (0-97.0) |
| Monocytes | 5.7 (0-54.4) |
| Etiology | |
| Idiopathic | 59 |
| Pregnancy | 3 |
| Thymoma | 1 |
| Treatment and response | |
| ATG + CSA (1 course) | 23 |
| Responders | 14 (3 CR, 11 PR) |
| ATG + CSA (2 courses) | 5 |
| Responders | 2 (1 CR, 1 PR) |
| ATG + CSA (3 or 4 courses) | 3 |
| Responders | 2 (2 PR) |
| ATG alone | 3 |
| Responders | 2 (1 CR, 1 PR) |
| ATG + oxymetholone (1 or 2 courses) | 3 |
| Responders | 3 (1 CR, 2 PR) |
| ATG + CSA + oxymetholone | 1 |
| Responders | 1 (1 PR) |
| CSA alone | 9 |
| Responders | 3 (3 PR) |
| Characteristic . | Value . |
|---|---|
| Number of patients | 63 |
| Median age, y (range) | 42 (18-76) |
| Disease severity at diagnosis (SAA:NSAA), n | 31:32 |
| Median disease duration, mo (range) | 41 (0.4-300) |
| Sex (M:F), n | 28:35 |
| PNH clone at time of study, n (%) | 24 (38) |
| Size of PNH clone, median % (range) | |
| Red cells | 0.9 (0-27.7) |
| Granulocytes | 2.9 (0-97.0) |
| Monocytes | 5.7 (0-54.4) |
| Etiology | |
| Idiopathic | 59 |
| Pregnancy | 3 |
| Thymoma | 1 |
| Treatment and response | |
| ATG + CSA (1 course) | 23 |
| Responders | 14 (3 CR, 11 PR) |
| ATG + CSA (2 courses) | 5 |
| Responders | 2 (1 CR, 1 PR) |
| ATG + CSA (3 or 4 courses) | 3 |
| Responders | 2 (2 PR) |
| ATG alone | 3 |
| Responders | 2 (1 CR, 1 PR) |
| ATG + oxymetholone (1 or 2 courses) | 3 |
| Responders | 3 (1 CR, 2 PR) |
| ATG + CSA + oxymetholone | 1 |
| Responders | 1 (1 PR) |
| CSA alone | 9 |
| Responders | 3 (3 PR) |
SAA indicates severe aplastic anemia; NSAA, nonsevere aplastic anemia; M, male; F, female; PNH, paroxysmal nocturnal hemoglobinuria; CSA, cyclosporin A; CR, complete response; and PR, partial response.
Treatment details
There were 16 untreated patients, and 47 received IST, mostly ATG-based therapy, as outlined in Table 1. Of the 16 untreated patients, 6 had transfusion-independent nonsevere AA, 8 were analyzed only before HSCT, 1 was analyzed only before IST, and 1 patient died before receiving treatment. Response was defined as red cell and platelet transfusion independence; complete response and partial response were defined by standard criteria.20,21 Before 2007, horse ATG (Lymphoglobulin; Genzyme) was used for the first course of IST and rabbit ATG (Thymoglobulin; Genzyme) for the second course. After the withdrawal of Lymphoglobulin from the market in 2007, rabbit ATG (Thymoglobulin) was used for the first course as part of the European Blood and Marrow Transplant Severe Aplastic Anemia Working Party phase 2 pilot study (EudraCT number = 2007-000902-55). Response to IST is summarized in Table 1. HSCT was performed in 18 patients, either as first-line treatment (n = 6) or for nonresponse (n = 10) or relapse (n = 2) after IST. Of 63 patients, 56 are alive and 7 have died (3 after HSCT, 3 after IST, and 1 untreated). Serial samples before and after IST were collected in 4 patients. Each patient donated blood on 1 occasion before or after IST. No samples were analyzed after HSCT.
Mononuclear cell separation
Peripheral blood mononuclear cells (PBMCs) were separated by density gradient sedimentation of whole blood on Histopaque (Sigma-Aldrich). PBMCs were frozen in serum-free freezing media (CryoMaxx I; PAA Laboratories GmbH) and stored in liquid nitrogen. A total of 1 × 106 PBMCs were stained for flow cytometric analysis.
Flow cytometry
Anti-CD3–Pacific Orange and anti-CD4–Pacific Blue (BD Biosciences) and anti-CD25 conjugated to APC (eBioscience) were used for surface staining. PE-conjugated anti–human IL-17 and IL-4, anti–human IFN-γ conjugated with FITC, and APC-conjugated anti–human TNF-α (with appropriate isotype controls; eBioscience) were used for intracellular cytokine staining according to the manufacturers' instructions, after a 4-hour stimulation with phorbol myristate acetate (PMA) and ionomycin in the presence of brefeldin.22 Anti–human Foxp3 conjugated with PE was used for intracellular staining after fixation and permeabilization according to the manufacturer's instructions (eBioscience).
Analysis was performed on a FACSCantoII (BD Biosciences) with FACSDiva Version 6 software (BD Biosciences). The absolute numbers of CD4+IL-17+ and CD4+IFN-γ+ cells within gated CD3 populations were determined. Data were analyzed with FlowJo Version 9.4.6 software.
Cell purification and functional assays
Treg isolation.
To obtain CD4+CD25+ Tregs and CD4+CD25− effector T cells (TE), PBMCs were first enriched for CD4+ T cells by use of a negative isolation kit (Miltenyi Biotec). CD4+-enriched PBMCs were then stained by anti–human CD3, CD4, CD25, and CD27. Tregs as CD3+CD4+CD25high CD27+ were sorted on a FACSAria (BD Biosciences) as described previously23 (sorted cells were consistently > 90% Foxp3+; data not shown).
Primary cells were cultured in RPMI 1640 (Invitrogen) supplemented with 50 IU/mL penicillin, 50 μg/mL streptomycin, and 2mM l-glutamine (PSG; PAA Laboratories GmbH) that contained 10% human AB serum (Biosera). A total of 104 TEs were additionally cultured with 0.05 μL of anti-CD3/CD28 expander beads (Dynal Biotech) in the presence or absence of a 1:1 ratio of autologous Tregs. To investigate the functionality of Tregs in AA patients, a 1:1 coculture of Tregs and effector T cells (Te; CD3+CD4+CD25low) was set up after overnight stimulation with CD3/CD28 beads (Dynal). Supernatants were analyzed by Luminex and ELISA for different cytokines. Each experiment was repeated at least twice for validation.
Cytokine measurement
Serum cytokine assays were performed with a cytokine 30-plex antibody bead kit (Invitrogen) according to the manufacturer's instructions, and data were acquired on a Luminex 200 (Luminex Corp). Sandwich ELISAs for human IFN-γ were performed in duplicate with ELISA kits from R&D Systems, and optical density was measured at 450 nm on a BioTek EL800X automatic plate reader (Wolf Laboratories). The level of each cytokine was calculated from a standard curve derived from known controls.
Th1 isolation and spectratyping
A hybrid method was used to isolate IFN-γ–secreting CD4+ T cells. At first, all dead cells were removed with a dead cell removal kit (Miltenyi Biotec), and then viable PBMCs were stimulated with Cytostim (Miltenyi Biotec) for 3 hours. After stimulation, IFN-γ–secreting cells were enriched with an IFN-γ secretion cell enrichment kit (Miltenyi Biotec). To increase the purity of the cells, FACS sorting was also used to further enrich CD3+ CD4+ IFN-γ–secreting cells. Sorted cells were consistently > 90% IFN-γ+ (data not shown). TRIzol (Invitrogen) was used for RNA extraction, and first-strand cDNA was generated with a Superscript III kit (Invitrogen). CDR3 region products of the TCR Vβ chain were amplified with Vβ-specific forward and Cβ reverse primers.24 CDR3 lengths were analyzed with an ABI 3130xl capillary sequencer (Applied Biosystems). The overall complexity of Vβ subfamilies was calculated, and the cloning and sequencing of any skewed spectratype were performed as described previously.25,26
CDR3 cloning and sequencing
The CDR3 PCR products, from 5 pretreatment AA patients and 4 healthy age-matched donors, were subjected to 454 sequencing (Roche GS FLX titanium; 454 Life Sciences Corp) after a second round of PCR (10-12 cycles), which allowed incorporation of a sample-specific 10-bp “barcode” sequence and additional tags used in the sequencing process. Sequencing was performed to yield an average “depth” in excess of 1000 clonal reads (1000×) per sample-specific CDR3 PCR amplicon. Reads were primarily processed with Roche Amplicon Variant Analyzer Version 2.3 software (AVA). For this, a “model” consensus sequence for each CDR3 region product of TCR Vβ chain was used to initiate the sorting of all sequences into consensus groups within the amplicon population. This software essentially groups identical reads together, with compensation for known sequencing artifacts. Selected sample sequence reads were also exported in fasta format with barcodes used to separate sample reads and were processed in CD-HIT (http://www.bioinformatics.org/cd-hit/), which clustered similar reads together on the basis of custom sequence similarity. Where indicated in Results, the resulting sequence clusters were then processed by ImMunoGeneTics software (http://www.imgt.org), and clusters that had the same amino acid sequence (> 99% identity) were grouped together to give a final number of “clonal” variants. For selected samples, the final outcome after translation was essentially the same for the 2 informatics routes.
RNA isolation and quantitative real-time RT-PCR
Total RNA was isolated from 5 pretreatment AA patients' and 4 healthy age-matched donors' Tregs and Te cells by use of TRI reagents (Sigma-Aldrich) and quantified by absorbance at 260 nm. cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen). Primers were designed with Primer 3 online software (http://frodo.wi.mit.edu/primer3) and synthesized by Eurofins MWG Operon. Quantitative PCR was performed in an ABI 7900 HT sequence detection system with the SYBR Green PCR Master Mix and according to the protocol of the manufacturer (Applied Biosystems). The data were only accepted to determine gene expression when the quantitative PCR run had an efficiency ranging from 90% to 110%. To quantify gene expression, a relative standard method was used. The quantities of targets (Stat 1, 3, 5, 6; Rorc 2; T-bet; and Foxp3) and of the endogenous GAPDH were determined from the appropriate standard curves. The target amount was then divided by the GAPDH amount to obtain a normalized value. All data were normalized with the endogenous GAPDH control. One of the experimental samples (GAPDH normalized) was designated as the calibrator and given a relative value of 1.0. All quantities (GAPDH normalized) were expressed as n-fold relative to the calibrator. An unpaired t test with Welch correction was used to derive the significance of the difference between the median values.
Statistical analysis
Statistical analysis was performed with SPSS version 14.0. The unpaired t test and Mann-Whitney U test were used to compare parametric and nonparametric data, respectively. Pearson test was used for bivariate correlation. Significance was set at P < .05.
Results
Lymphocyte immunophenotyping and T cell subsets
The percentage and absolute numbers of the peripheral blood CD4+ and CD8+ T-cell subsets and of B cells in peripheral blood were investigated by flow cytometry. CD3+ CD4+ T-cell subsets were defined as CD45RO−CD27+ naive, CD45RO+ CD27+ CD62L+ central memory, CD45RO+ CD27+ CD62L− effector memory, CD45RO+CD27− effectors, and CD45RO− CD27− terminal effectors. CD4 Tregs were defined as CD3+CD4+ CD25high CD27+Foxp3+.27–29 After initial gating, Treg subsets were defined as (1) CD45RA+CD25lo resting Tregs, (2) CD45RA− CD25hi activated Tregs, and (3) cytokine-secreting CD45RA−CD25lo non-Tregs, as described previously.17 T cells were first stimulated and then stained intracellularly for IFN-γ, TNF-α (Th1), IL-4 (Th2), and IL-17 (Th17).
There were no significant differences in the number or percentage of total CD4+ T cells in AA patients compared with healthy donors; however, the frequencies of central memory (P = .01) and terminal effector (P = .001) CD4+ T cells were statistically lower in AA patients than in healthy age-matched donors, whereas the effector memory subset was increased (P = .04) Among AA patients, the percentage of B cells (CD3−CD19+) was not significantly different from that of healthy donors (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
T-helper and regulatory T cells
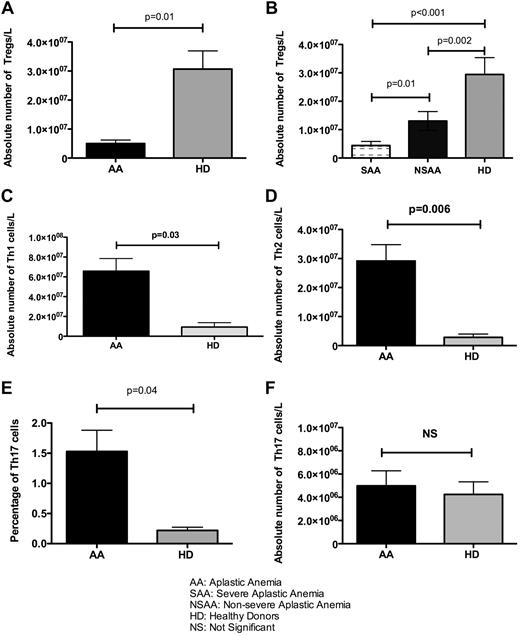
The absolute Treg number was significantly lower in pretreatment AA patients than in healthy donors (5.5 × 106 vs 3 × 107; P = .01; Figure 1A). In patients with severe and very severe AA at diagnosis, the absolute number and frequency of Tregs were significantly lower than in those with nonsevere disease (4.4 × 106/L vs 1 × 107/L, P = .01) and 10 age-matched healthy donors (4.4 × 106/L vs 3 × 107/L, P < .001; Figure 1B).
The number of T-helpers in 48 pretreatment AA patients and 10 healthy donors. (A) The absolute number of CD3+CD4+CD25high CD27+ Foxp3+ Tregs was significantly lower in the present study cohort of AA patients than in healthy age-matched donors (HD; P = .01). (B) The absolute number of Tregs was also correlated with the severity of disease, and this number was significantly lower in severe and very severe disease (SAA) than in nonsevere disease (NSAA; P = .01) and HDs (P < .001). (C-D) The absolute number of both Th1 and Th2 cells was significantly lower in HDs than in AA patients (P = .03 and P = .006, respectively). (E-F) Although the frequency of Th17 cells was higher in AA patients (P = .04), there was no significant difference between HDs and AA patients in terms of absolute numbers of Th17.
The number of T-helpers in 48 pretreatment AA patients and 10 healthy donors. (A) The absolute number of CD3+CD4+CD25high CD27+ Foxp3+ Tregs was significantly lower in the present study cohort of AA patients than in healthy age-matched donors (HD; P = .01). (B) The absolute number of Tregs was also correlated with the severity of disease, and this number was significantly lower in severe and very severe disease (SAA) than in nonsevere disease (NSAA; P = .01) and HDs (P < .001). (C-D) The absolute number of both Th1 and Th2 cells was significantly lower in HDs than in AA patients (P = .03 and P = .006, respectively). (E-F) Although the frequency of Th17 cells was higher in AA patients (P = .04), there was no significant difference between HDs and AA patients in terms of absolute numbers of Th17.
The absolute numbers of Th1 and Th2 cells in all 48 patients before treatment were significantly higher than in healthy donors (6.4 × 107/L vs 1.8 × 107/L, P = .03 for Th1 cells and 1.9 × 107/L vs 2.4 × 106/L, P = .006 for Th2 cells; Figure 1C-D). Although the mean percentages of Th17 cells were higher than in healthy donors (1.5% vs 0.15%, P = .04), the differences in absolute numbers were not significant (Figure 1E-F).
T helpers, Tregs, and disease severity and duration
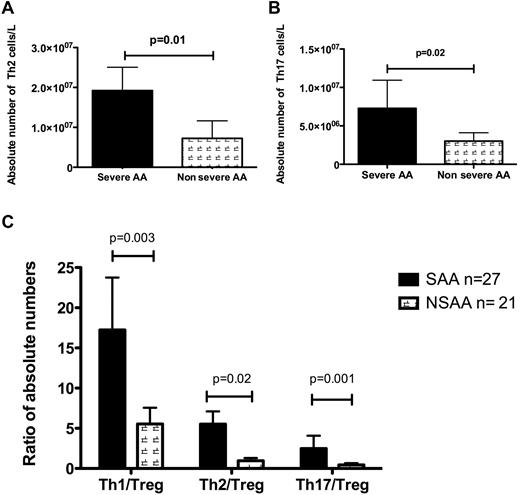
Absolute numbers of Th2 and Th17 cell numbers were increased in severe disease compared with nonsevere AA (1.92 × 107/L vs 7.4 × 106/L for Th2, P = .01 and 5.7 × 106/L vs 2.15 × 106/L for Th17, P = .02; Figure 2A-B). Although the number of Th1 cells was higher in severe than in nonsevere disease (7.1 × 107/L vs 3.5 × 107/L), this difference was not significant. However, the ratios of Th1/Tregs (P = .003), Th2/Tregs (P = .02), and Th17/Tregs (P = .001) were significantly higher in severe and very severe disease than in nonsevere disease (Figure 2C). There was also a significant correlation between Th2/Treg ratio and duration of disease (P = .007). Disease duration was not correlated with any other investigated parameters. There was no correlation between Th1/Th2 ratio and disease severity and response to treatment.
Number and ratio of T-helpers and disease severity. (A-B) The absolute numbers of Th2 and Th17 cells were significantly higher in pretreatment patients with severe (n = 15) and very severe (n = 12) AA (SAA) than in healthy donors. (C) The ratios of all T-helpers to Tregs were correlated with disease severity, and as shown, all ratios were significantly higher in patients with pretreatment severe and very severe disease than in those with nonsevere AA (NSAA).
Number and ratio of T-helpers and disease severity. (A-B) The absolute numbers of Th2 and Th17 cells were significantly higher in pretreatment patients with severe (n = 15) and very severe (n = 12) AA (SAA) than in healthy donors. (C) The ratios of all T-helpers to Tregs were correlated with disease severity, and as shown, all ratios were significantly higher in patients with pretreatment severe and very severe disease than in those with nonsevere AA (NSAA).
Treg subsets
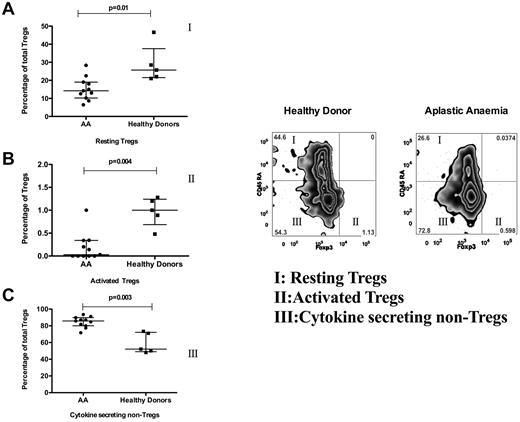
The percentage of both activated Tregs (CD4+ CD25high Foxp3+ CD45RA−) and resting Tregs (CD4+ CD25high Foxp3low CD45RA+) was decreased markedly in AA patients compared with healthy donors (P = .004 and P = .01, respectively). In contrast, cytokine-secreting non-Tregs (CD45RA−CD25lo Foxp3−) were increased in AA patients (P = .003; Figure 3).
Treg subsets in AA. Treg subsets were studied in 11 AA patients (pretreatment with nonsevere disease) and 6 healthy donors. This figure shows the frequency of CD45RA+/− and Foxp3+/− subsets of Tregs. The frequency of Foxp3+ CD45RA− (active Tregs; population II and graph in panel B) and Foxp3−CD45RA+ (resting Tregs; population I and graph in panel A) was decreased in AA (P = .004, P = .01), whereas the frequency of Foxp3−CD45RA− subset (cytokine-secreting non-Tregs; population III and graph in panel C) was increased in AA compared with healthy donors (P = .003).
Treg subsets in AA. Treg subsets were studied in 11 AA patients (pretreatment with nonsevere disease) and 6 healthy donors. This figure shows the frequency of CD45RA+/− and Foxp3+/− subsets of Tregs. The frequency of Foxp3+ CD45RA− (active Tregs; population II and graph in panel B) and Foxp3−CD45RA+ (resting Tregs; population I and graph in panel A) was decreased in AA (P = .004, P = .01), whereas the frequency of Foxp3−CD45RA− subset (cytokine-secreting non-Tregs; population III and graph in panel C) was increased in AA compared with healthy donors (P = .003).
Function of Tregs
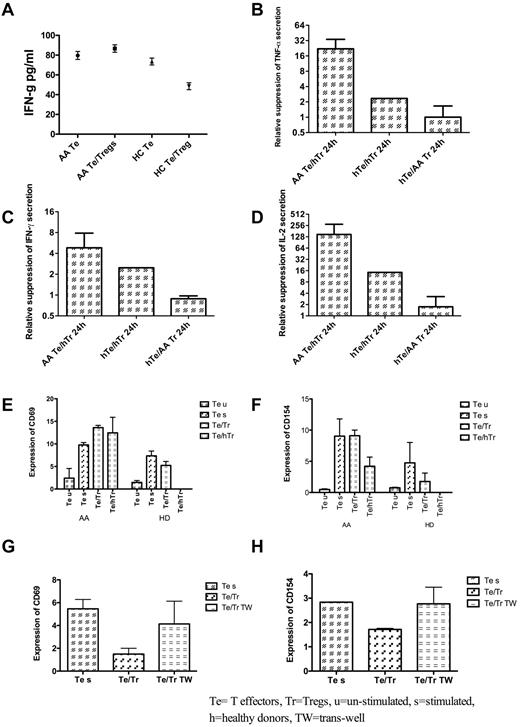
Sorted Tregs (CD4+CD25highCD127low) from AA patients (3 different pretreatment patients with nonsevere disease) were unable to suppress cytokine secretion by autologous Te cells in a 1:1 coculture; however, both IL-2 and IFN-γ secretion by AA Te was suppressible by allogeneic Tregs from healthy donors (P = .019 and P = .020, respectively), whereas Tregs from AA patients were unable to suppress healthy Te cells, which confirmed the dysfunction of Tregs from these patients (Figure 4).
Treg function and expression of CD69 and CD154 on T effector cells. (A) In contrast to healthy donors, IFN-γ secretion was not suppressed by the addition of autologous Tregs from AA patients to syngeneic T effector cells (Te). HC indicates healthy controls. (B) The relative suppression of Te cells from AA patients (measured by IFN-γ secretion after 2 hours of coculture with Tregs) was highest with healthy Tregs, whereas the Tregs from AA patients were almost unable to suppress IFN-γ secretion. hTr indicates Tregs from health donors; hTe, Te from healthy donors. (C) Similar pattern of suppression was noticed for both TNF-a and IL-2 secretion by Te cells in the presence of healthy Tregs. All tests were repeated with 3 different patients' samples. (E) Coculture of Tregs and Te (in 1:1 ratio), whether from AA patient (autologous Tregs) or from healthy donors (hTr), had no effect on Te expression level of CD69. In healthy donor Te cells, healthy Tregs reduced the level of CD69, whereas the AA Tregs were unable to suppress this marker. (F) Although Tregs from AA patients had minimal or no effect on Te CD154, healthy Tregs were able to suppress this marker both in patients and healthy donors. (G-H) To investigate the effect of cell contact on suppression of Te CD154 and CD69, a transwell coculture of Tregs and Te was set up, and as shown, prevention of cell contact between Te and Tregs by a transwell insert prevented the suppressor effect of Tregs on CD154 and CD69 expression by Te. All tests were repeated on 3 different pretreatment AA samples. u indicates unstimulated; s, stimulated; h, healthy donors; and TW, transwell.
Treg function and expression of CD69 and CD154 on T effector cells. (A) In contrast to healthy donors, IFN-γ secretion was not suppressed by the addition of autologous Tregs from AA patients to syngeneic T effector cells (Te). HC indicates healthy controls. (B) The relative suppression of Te cells from AA patients (measured by IFN-γ secretion after 2 hours of coculture with Tregs) was highest with healthy Tregs, whereas the Tregs from AA patients were almost unable to suppress IFN-γ secretion. hTr indicates Tregs from health donors; hTe, Te from healthy donors. (C) Similar pattern of suppression was noticed for both TNF-a and IL-2 secretion by Te cells in the presence of healthy Tregs. All tests were repeated with 3 different patients' samples. (E) Coculture of Tregs and Te (in 1:1 ratio), whether from AA patient (autologous Tregs) or from healthy donors (hTr), had no effect on Te expression level of CD69. In healthy donor Te cells, healthy Tregs reduced the level of CD69, whereas the AA Tregs were unable to suppress this marker. (F) Although Tregs from AA patients had minimal or no effect on Te CD154, healthy Tregs were able to suppress this marker both in patients and healthy donors. (G-H) To investigate the effect of cell contact on suppression of Te CD154 and CD69, a transwell coculture of Tregs and Te was set up, and as shown, prevention of cell contact between Te and Tregs by a transwell insert prevented the suppressor effect of Tregs on CD154 and CD69 expression by Te. All tests were repeated on 3 different pretreatment AA samples. u indicates unstimulated; s, stimulated; h, healthy donors; and TW, transwell.
To further investigate the activation status of Te cells in the presence and absence of autologous and allogeneic Tregs, the above experiments were repeated, and CD69 and CD154 expression was assessed by flow cytometry after overnight culture in the presence of CD3/CD28 beads. Tregs (CD4+CD25high CD127low) were unable to inhibit either CD154 or CD69 expression on Te cells. In contrast, Tregs from healthy donors suppressed CD154 expression but not CD69 expression (Figure 4). This effect was mainly dependent on cell contact, because in a transwell culture condition, the healthy Tregs were unable to suppress CD154 and CD69 (Figure 4).
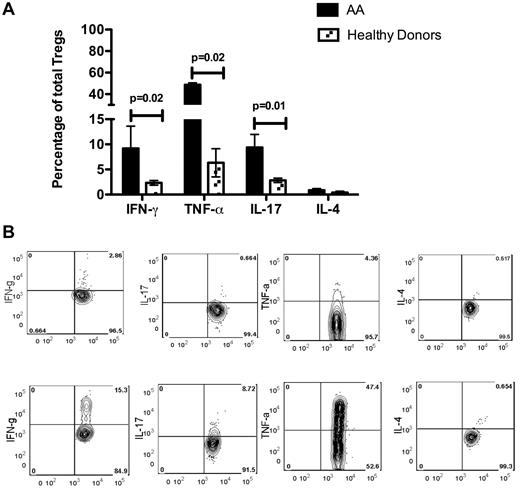
The level of IL-10 secretion by AA Tregs, stimulated overnight with CD3/CD28 beads, was not significantly different from that of healthy donors (data not shown); however, when the Tregs were stimulated by phorbol myristate acetate and ionomycin for 4 hours, the amount of IFN-γ, TNF-α, and IL-17 secretion was significantly higher (P = .02, P = .02, and P = .01, respectively) in 5 AA patients than in Tregs from 3 healthy donors (Figure 5).
Cytokine secretion by Tregs in 5 AA patients and 3 healthy donors. (A) Amounts of IFN-γ, TNF-α, and IL-17 secreted by Tregs were significantly higher (P = .02, P = .02 and P = .01, respectively) in 5 AA patients than in 4 healthy donors, whereas the amount of secreted IL-4 was not significantly different between the 2. (B) An example of intracellular staining of AA and healthy donor Tregs for cytokines after 4 hours of stimulation with phorbol myristate acetate and ionomycin in the presence of brefeldin A. The amount of intracellular IFN-γ (IFN-g), TNF-α (TNF-a), and IL-17 was markedly higher in patient Tregs than in healthy donor Tregs.
Cytokine secretion by Tregs in 5 AA patients and 3 healthy donors. (A) Amounts of IFN-γ, TNF-α, and IL-17 secreted by Tregs were significantly higher (P = .02, P = .02 and P = .01, respectively) in 5 AA patients than in 4 healthy donors, whereas the amount of secreted IL-4 was not significantly different between the 2. (B) An example of intracellular staining of AA and healthy donor Tregs for cytokines after 4 hours of stimulation with phorbol myristate acetate and ionomycin in the presence of brefeldin A. The amount of intracellular IFN-γ (IFN-g), TNF-α (TNF-a), and IL-17 was markedly higher in patient Tregs than in healthy donor Tregs.
Because the function of Tregs in AA patients was severely impaired, we next investigated the expression level of different transcription factors and STATs in Tregs and Te cells of AA patients. We observed normal levels of expression of FoxP3, RORγc, and T-bet in AA patients compared with age-matched normal donors, although the levels of STAT1, 3, 5, and 6 were lower than threshold because of the low number of cells (supplemental Figures 2-3).
TCR diversity of Th1 cells
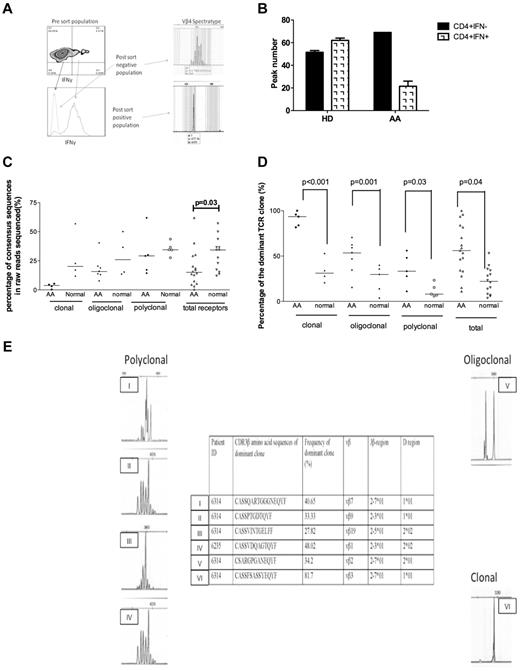
The clonality of Th1 cells was investigated in 5 AA patients before treatment (4 with nonsevere and 1 with severe disease) and 4 healthy age-matched donors. The PCR product of those samples with clonal and high-throughput deep sequencing sequenced oligoclonal patterns. The diversity of TCR receptors in AA Th1 samples was significantly lower than in healthy donors (P = .037), as shown by the percentage of consensus clusters in total sequence reads (Figure 6C). Any consensus cluster larger than 1% was analyzed with the ImMunoGeneTics information system sequence-alignment tool (http://www.imgt.org). Sequences that were not sufficiently long to encompass a CDR3 or that were out of frame, contained unassigned “N” nucleotides, or lacked complete identity with conserved Vβ and Jβ regions external to the CDR3 were discarded. Clusters of the consensus read with the same CDR3 amino acid sequence were combined, and frequencies of each CDR3 clone were recalculated. CDR3 amino acid sequences from major clones (> 1%) are presented in supplemental Table 4. Interestingly, percentages of the top dominant CDR3 clones revealed by high-throughput sequencing were significantly higher in AA samples than in healthy donors, regardless of the spectratyping pattern (Figure 6), which indicates clonally expanded Th1 cells in AA patients. In analyses of the amino acid sequences deduced from the rearranged TCR-β receptors, no striking similarities or dominant common CDR3 motifs could be identified among the 4 AA patients analyzed.
The TCR diversity of Th1 cells was evaluated in 5 pretreatment AA patients (4 with nonsevere disease and 1 with severe disease) and 4 healthy age-matched donors. (A) A hybrid method was used to purify Th1 cells on the basis of their IFN-γ secretion. In the first step, purified CD4+ cells (using negative bead selection) were stimulated in the presence of antibody-coated beads to capture secreted IFN-γ, and the CD4+ IFN-γ–secreting cells were enriched by use of magnetic columns (see Methods). The enriched cells were then further sorted by FACS sorter based on positivity for PE-conjugated anti-IFN-γ. The purity of cells was constantly > 95%. Extracted RNA from these cells was used for cDNA synthesis and PCR for 24 Vβ subfamilies (see “Methods”). The spectratypes of Th1 cells were clonal or oligoclonal in the majority of subfamilies. An example of the spectratypes of IFN-γ+ and IFN-γ− cells are shown. (B) To calculate the diversity of TCR, CDR3 region, the spectratypes of 24 Vβ subfamilies were scored between 1 and 5, based on the number of peaks. The total value was then calculated and used for comparison between samples. As shown, the CD4+ IFN-γ+ cells were less diverse (more clonal) than CD4+ IFN-γ− cells in AA patients, whereas there was no difference between these types in healthy donors (HD). (C) TCR-β diversity determined by percentages of consensus deep-sequencing clusters in total raw reads without data filtering. In general, there was less diversity in AA patients than in normal healthy donors with regard to spectratyping. The clonal diversity was shown to be statistically significant compared with all deep-sequencing data between the 2 groups (P = .03). (D) Comparison of percentages of dominant TCR-β clones between AA patients and normal healthy donors after removal of sequencing errors and combined clones with the same CDR3 amino acid sequences. Significantly higher percentages of TCR-β dominant clones were seen in AA patients (P values shown), which indicates the clonal expansion of TCRs. (E) CD4+ IFN-γ T-cell spectratypes from 2 patients are shown that are either polyclonal (I, II, III, IV), oligoclonal (V), or clonal (VI). Interestingly, in polyclonal spectratypes, large dominant clones (always larger than 25%) were detectable by high-throughput sequencing, which was not the case in healthy donors (C). However, in oligoclonal and clonal spectratypes, the size of the dominant clone was larger in AA patients than in healthy controls (see Figure 6C and supplemental Table 4). Deep sequencing in 2 patients (patient Nos. 6314 and 6235) shown in the figure illustrated immunodominant clones (Vβ7, 9, 2, and 3 in patient 6314 and Vβ1 in patient 6235). The clone size varied from 27% to 81.7% of all reads. Polyclonal spectratypes in AA were clearly associated with immunodominant clones by deep sequencing. Oligoclonal Vβ2 (V) in patient No. 6314 was associated with a clone size of 34.2%, and clonal (VI) spectratype depicted a Vβ3 clone of 81.7%. All dominant clones and the clonal expansions obtained from deep sequencing are shown in the table in panel E. Vβ indicates TCR Vβ chain; CDR3, complementary determining region 3; Jβ, joining region of the TCR-β chain; D region, D region of the TCR-β chain; and UPN, unique patient number.
The TCR diversity of Th1 cells was evaluated in 5 pretreatment AA patients (4 with nonsevere disease and 1 with severe disease) and 4 healthy age-matched donors. (A) A hybrid method was used to purify Th1 cells on the basis of their IFN-γ secretion. In the first step, purified CD4+ cells (using negative bead selection) were stimulated in the presence of antibody-coated beads to capture secreted IFN-γ, and the CD4+ IFN-γ–secreting cells were enriched by use of magnetic columns (see Methods). The enriched cells were then further sorted by FACS sorter based on positivity for PE-conjugated anti-IFN-γ. The purity of cells was constantly > 95%. Extracted RNA from these cells was used for cDNA synthesis and PCR for 24 Vβ subfamilies (see “Methods”). The spectratypes of Th1 cells were clonal or oligoclonal in the majority of subfamilies. An example of the spectratypes of IFN-γ+ and IFN-γ− cells are shown. (B) To calculate the diversity of TCR, CDR3 region, the spectratypes of 24 Vβ subfamilies were scored between 1 and 5, based on the number of peaks. The total value was then calculated and used for comparison between samples. As shown, the CD4+ IFN-γ+ cells were less diverse (more clonal) than CD4+ IFN-γ− cells in AA patients, whereas there was no difference between these types in healthy donors (HD). (C) TCR-β diversity determined by percentages of consensus deep-sequencing clusters in total raw reads without data filtering. In general, there was less diversity in AA patients than in normal healthy donors with regard to spectratyping. The clonal diversity was shown to be statistically significant compared with all deep-sequencing data between the 2 groups (P = .03). (D) Comparison of percentages of dominant TCR-β clones between AA patients and normal healthy donors after removal of sequencing errors and combined clones with the same CDR3 amino acid sequences. Significantly higher percentages of TCR-β dominant clones were seen in AA patients (P values shown), which indicates the clonal expansion of TCRs. (E) CD4+ IFN-γ T-cell spectratypes from 2 patients are shown that are either polyclonal (I, II, III, IV), oligoclonal (V), or clonal (VI). Interestingly, in polyclonal spectratypes, large dominant clones (always larger than 25%) were detectable by high-throughput sequencing, which was not the case in healthy donors (C). However, in oligoclonal and clonal spectratypes, the size of the dominant clone was larger in AA patients than in healthy controls (see Figure 6C and supplemental Table 4). Deep sequencing in 2 patients (patient Nos. 6314 and 6235) shown in the figure illustrated immunodominant clones (Vβ7, 9, 2, and 3 in patient 6314 and Vβ1 in patient 6235). The clone size varied from 27% to 81.7% of all reads. Polyclonal spectratypes in AA were clearly associated with immunodominant clones by deep sequencing. Oligoclonal Vβ2 (V) in patient No. 6314 was associated with a clone size of 34.2%, and clonal (VI) spectratype depicted a Vβ3 clone of 81.7%. All dominant clones and the clonal expansions obtained from deep sequencing are shown in the table in panel E. Vβ indicates TCR Vβ chain; CDR3, complementary determining region 3; Jβ, joining region of the TCR-β chain; D region, D region of the TCR-β chain; and UPN, unique patient number.
Serum level of cytokines
Serum levels of endothelial growth factor (EGF; P = .01), hepatocyte growth factor (HGF; P = .04), vascular endothelial growth factor (VEGF; P = .03), MCP-1 (P = .001), and the proinflammatory cytokines IL-13 (P = .02) and IL-8 (P = .001) were significantly higher in AA patients than in healthy donors (supplemental Figure 5).
Gene expression analysis
Because of the low number of Tregs in AA patients, it was extremely challenging to sort enough Tregs for any gene expression analysis. We managed, however, to collect enough Tregs from 3 pretreatment patients and 5 healthy control subjects to analyze global gene expression differences. A unique gene signature consisting of 86 genes that were significant was identified. There were only 8 down-regulated genes (fold change) in the pretreatment group: PIN4 (−4.1), OR2T12 (−3.3), AMAC1 (−2.73), PERP (−2.69), UTS2 (−2.27), RNF139 (−2.13), COMMD9 (−2.09), and LOC100128356 (−2.01). Of the 78 up-regulated genes in the pretreatment group, the top 10 genes were HBB (19.5), PSME2 (13.8), CSDA (13.07), FAM127A (7.78), EXOSC1 (7.73), BPGM (7.43), CYSLTR1 (7.17), CHPT1 (6.96), CHMPS (6.80), and PLAC8 (6.71). Quantitative PCR analysis for CSDA, HBB, PSMiE2, PERP, PIN4, and UTS2 confirmed a similar trend as seen in the microarray results (see supplemental Figure 6).
Effect of treatment and correlation with CD4+ T cells
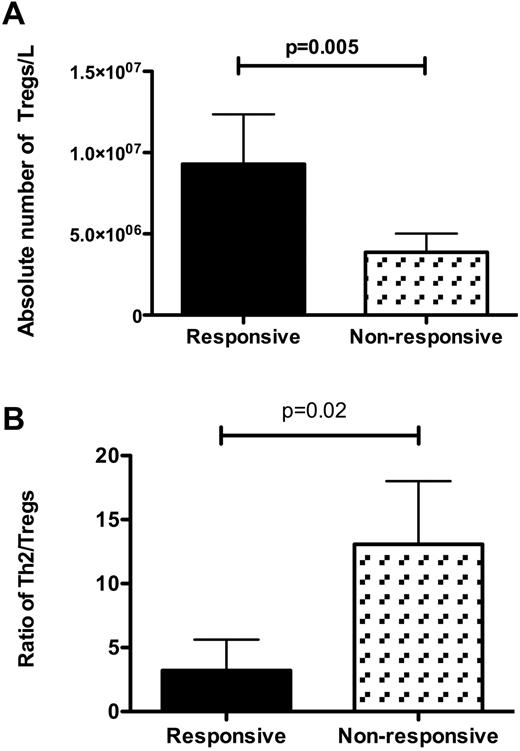
Among 15 patients (including 4 with serial samples) who received IST, the absolute number and frequency of Tregs were significantly lower than for pretreatment patients (5.4 × 106/L vs 1.1 × 107/L, P = .004); however, the absolute number of Tregs was significantly higher in 10 responsive patients than in 5 nonresponsive patients (1 × 107/L vs 3.3 × 106/L, P = .005; Figure 7). The ratio of Th2 to Tregs was also significantly higher in nonresponsive than responsive patients (P = .02; Figure 7). The median time from diagnosis to sampling in responsive patients was longer than in nonresponsive patients (260 and 52 months, respectively).
Correlation between Tregs and T-helpers and response to treatment. (A) Absolute numbers of Tregs were significantly higher in patients who responded to immunosuppressive therapy (n = 10 and P = .005) than in nonresponsive patients (n = 5). (B) Ratio of Th2 cells to Tregs was significantly higher in nonresponsive patients (P = .02).
Correlation between Tregs and T-helpers and response to treatment. (A) Absolute numbers of Tregs were significantly higher in patients who responded to immunosuppressive therapy (n = 10 and P = .005) than in nonresponsive patients (n = 5). (B) Ratio of Th2 cells to Tregs was significantly higher in nonresponsive patients (P = .02).
Discussion
There is mounting evidence that autoimmunity plays an important role in the pathogenesis of AA.1–5 This is particularly true in most patients with idiopathic AA, which constitutes up to 80% of cases. Furthermore, autoimmune responses also contribute to the development of AA in approximately 20% of patients who have had a proceeding viral infection, hepatitis, or drug or chemical exposure.30 The mechanism for the suppression of CD34+ cells is unknown. The aberrant immune response with oligoclonal CD8+ T-cell expansion that results in release of inhibitory cytokines and induction of apoptosis is unlikely to represent the primary immunologic event in the induction of BM failure. As with other autoimmune conditions, CD4+ T cells have been recognized recently as important drivers of the immune response to an as yet unidentified agent. The potential role of CD+ T helpers and regulatory T cells is less clear. To further our understanding of the potential role(s) of CD4+ T cells in this disease, we studied the functional characteristics of different T helpers and Treg subsets.
Tregs, T helpers, and AA
Since the rediscovery of Tregs by Sakaguchi et al,31,32 regulatory T cells have been considered crucial in the pathophysiology of human autoimmune diseases.33–35 The function of Tregs and their interaction with other members of the immune system are considered to be more of an “environmental” change than a binary function of switching off the function of 1 or a few effector cells.36 The complex functions of Tregs in combination with the enormous plasticity of CD4+ T cells, as well as their ability to switch their identity, make the study of autoimmunity challenging. However, any results in this field have a significant impact on therapies for autoimmune diseases.
In the present study, we analyzed the number of CD4+ and CD8+ T cells, natural killer cells, and B cells and the number and function of CD4+ subsets (Th1, Th2, Tregs, and Th17 cells) in 48 AA patients at the time of diagnosis and before any treatment. The absolute number and frequency of Tregs were significantly lower in AA patients than in healthy age-matched donors, which is in agreement with previously published studies.15 Nonetheless, we have shown for the first time that the reduction in Treg numbers correlates with disease severity, and the defect is most prominent in severe and very severe AA.
The present data also show that among different subsets of T helpers, IFN-γ/TNF-α–producing CD4+ T cells (Th1 cells) and IL-4–producing CD4+ T cells (Th2 cells), but not Th17 cells, are significantly expanded in all AA patients compared with healthy age-matched donors. Interestingly, there was no correlation between the expansion of Th1 cells and disease severity, whereas Th2 and Th17 cells were expanded only in severe and very severe disease, which is in contrast to the findings of de Latour et al,16 who showed that Th17 cells were expanded in all cases of AA. However, this difference is explained by the number of patients with very severe/severe and nonsevere disease in the present study cohort. Despite this, the ratios of all T-helper cells to Tregs were increased in AA compared with healthy donors, which suggests a general proinflammatory response in these patients.
It is interesting to note that the number and frequency of Th2 cells were increased in AA and correlated with disease severity. Although the interplay and balance between Th1, Th17, and Tregs is a well-established concept in immunology, the interplay between Th2 cells and Tregs is not fully established. Nonetheless, it has been shown recently that there is indeed a reverse correlation between Th2 cells and Tregs, and in the presence of dysfunctional Tregs, Th2 cells can expand and maintain an autoimmune response.37 Another study has shown that in a murine model, Foxp3+ Tregs have tighter control over Th2 cells than Th1 cells.38 Tregs are no longer considered as indiscriminate suppressors and instead use different suppressive mechanisms for the suppression of different T-cell subpopulations.38–41 Furthermore, these suppressive effects are not simply “frequency proportional,” and although Th1 suppression is very much dependent on the number of Tregs, Th2 suppression is not.38
The increased number of Th2 cells in AA patients, which is related to disease severity, may be the result of an inability of Tregs to induce apoptosis in Th2 cells by vasoactive intestinal peptide (VIP)–regulated granzyme B activity,42–44 whereas the expansion of Th1 cells may be because of the decreased number of Tregs.38 The inverse correlation of Th17 and Tregs in these patients may suggest a conversion of Tregs to Th17, as has been shown in other diseases.45,46 Although we did not test this hypothesis, the present data support this speculation and suggest a complex interaction between Th1, Th2, Th17, and Tregs in AA.
Interestingly, although the number of Th1 cells was increased in AA patients, the expansion was not significantly higher in severe disease. We speculate that in more severe disease, Th1 cells go through apoptosis quicker than other subsets because they have a more rapid activation-induced cell death,47 and therefore, their frequency is not increased in severe disease.
We found a significant difference in Tregs' subsets compared with healthy donors. The numbers of resting and activated Tregs were significantly reduced in AA patients, whereas levels of cytokine-secreting non-Tregs were normal.17 These data suggest that even in those patients with a “normal” number of Tregs, the Treg subsets are skewed. As expected, the coculture of patients' Tregs and T effectors showed very little suppression of IFN-γ and TNF-α compared with healthy donors. It was important to assess whether this lack of suppression was because of Treg dysfunction or unresponsive effector cells. To address this question, healthy donor T cells were used in a crisscross assay, and we showed that the main defect was in the ability of Tregs to suppress cytokine secretion. Tregs from AA patients were also unable to suppress the activation markers CD154 and CD69 on Te cells. Although normal donor Tregs were able to suppress both cytokine secretion and CD154 expression on AA Te cells, they were unable to suppress CD69, which suggests a higher level of activation of AA effector T cells. We have also shown that all of these effects require cell-to-cell contact and can be prevented by contact interference. Quantitative PCR analysis of transcription factors (FOXP3, RORγc, and T-bet) showed there was no significant difference between patients and healthy age-matched donors, which suggests that mechanisms other than the level of key transcription factors account for Treg dysfunction.
Th1 cell clonality and potential antigen specificity
Oligoclonal expansion of T cells is a known phenomenon in AA. Risitano et al,5 in a study of 23 AA patients, have shown the expansion of Vβ families among both CD4+ and CD8+ T cells; however, the degree of Vβ skewing among CD4+CD28dim T cells was significantly lower (less diverse) than that of CD8+CD28dim T cells. They concluded that Vβ expansions in CD4+ T cells were more likely to be polyclonal. In the present study, we focused on a specific subset of CD4+ T cells (Th1) for the clonality study. Using a combined method for the isolation of IFN-γ–producing CD4+ T cells, we enriched Th1 cells with > 90% purity and extracted a sufficient quantity and quality of RNA for a TCR diversity study.
On spectratyping, most of the Vβ subfamilies of TCRs were either clonal or oligoclonal, and the diversity of CDR3 was significantly lower in AA patients than in healthy donors. The spectratyping results were validated by high-throughput sequencing (supplemental Table 5).
In both AA patients and healthy donors, a dominant clone was detected by high-throughput sequencing in those Vβ subfamilies with clonal or oligoclonal spectratypes; however, the detected clones were markedly larger in AA samples regardless of the spectratype pattern (Figure 6). Importantly, the dominant clones were detectable even in polyclonal spectratypes of AA patients, which was not the case in healthy donors. This indicates that TCRs of Th1 cells were less diverse in almost all AA patients than in healthy age-matched donors. These data also suggest that in AA, individual subsets of CD4+ T cells must be isolated for clonality studies. It also advocates the idea that the traditional definition of polyclonality, based on the number of peaks in spectratypes, is not accurate enough for the detection of clonal expansion of CD4+ T cells.
The present data suggest that a combination of expansion of Th1, Th2, and Th17 and a decreased or skewed Treg immunophenotype and function constitutes a consistent and defining feature of severe and very severe AA. We also found an inverse correlation between the number of Th2, Th17, and Tregs that was associated with disease severity. It is difficult to identify the precise sequence of events that lead to aplastic BM in this disease, but the present data suggest that expansion of Th1 cells is most likely to be antigen driven and that the presence of dysfunctional Tregs would aggravate this autoimmune response. The postimmunosuppressive therapy data indicate the importance of Th2 and Treg balance and its correlation with the response to this treatment. Patients with higher number of Tregs and a lower ratio of Th2/Tregs are more likely to respond to IST and restore a less inflammatory environment. Further studies that focus on the identification and generation of antigen-specific immune response will shed more light on the immunopathogenesis of this rare but life-threatening disorder.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the nurses and clinical fellows of the haematology department at King's College Hospital for providing clinical samples and the tissue bank personnel for processing samples. They also thank Dr Behdad Afzali and Dr Linda Barber for their comments.
This work was supported by a program grant from Leukaemia & Lymphoma Research, London, United Kingdom.
Authorship
Contribution: S.K. designed and performed the research, analyzed data, and wrote the paper; J.M. collected clinical data, designed research, and wrote the paper; S.A.-K. performed research; J.J., A.S., A.M., P.P.A., C.V., B.C., A.G.K., T.S., and S.A.M. performed research and analyzed data; N.B.-Q. collected patient samples and clinical data; F.F. designed the research, reviewed data, and reviewed the paper; and G.J.M. designed the research, reviewed data, and wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Professor G. J. Mufti, Department of Haematological Medicine, Kings College London, Rayne Institute, 123 Coldharbour Lane, London, United Kingdom SE5 9NU; e-mail: ghulam.mufti@kcl.ac.uk.
References
Author notes
S.K. and J.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal