Abstract
Histone methylation is thought to be important for regulating Ag-driven T-cell responses. However, little is known about the effect of modulating histone methylation on inflammatory T-cell responses. We demonstrate that in vivo administration of the histone methylation inhibitor 3-deazaneplanocin A (DZNep) arrests ongoing GVHD in mice after allogeneic BM transplantation. DZNep caused selective apoptosis in alloantigen-activated T cells mediating host tissue injury. This effect was associated with the ability of DZNep to selectively reduce trimethylation of histone H3 lysine 27, deplete the histone methyltransferase Ezh2 specific to trimethylation of histone H3 lysine 27, and activate proapoptotic gene Bim repressed by Ezh2 in antigenic-activated T cells. In contrast, DZNep did not affect the survival of alloantigen-unresponsive T cells in vivo and naive T cells stimulated by IL-2 or IL-7 in vitro. Importantly, inhibition of histone methylation by DZNep treatment in vivo preserved the antileukemia activity of donor T cells and did not impair the recovery of hematopoiesis and lymphocytes, leading to significantly improved survival of recipients after allogeneic BM transplantation. Our findings indicate that modulation of histone methylation may have significant implications in the development of novel approaches to treat ongoing GVHD and other T cell–mediated inflammatory disorders in a broad context.
Introduction
Pathogenic T-cell responses can be detrimental to the host. For example, GVHD is a life-threatening complication after allogeneic BM transplantation (BMT).1-3 GVHD is caused by donor T cells that attack normal tissues of the recipient.1-3 Standard immunosuppressive therapy for GVHD lacks efficacy and impairs the antitumor activity.1,2,4 New approaches are needed to control GVHD.
Epigenetic modifications are thought to be important for T-cell immune responses.5,6 These modifications include histone methylation, DNA methylation, and histone acetylation.7-10 Histone methylation is the modification of certain amino acids of a histone by adding methyl groups.8-10 Depending on the site and degree of methylation, histone methylation can be activating or repressive. For example, trimethylation of histone H3 at lysine 4 (H3K4me3), H3K36me3, and H3K79me3 are associated with transcriptional activation, whereas H3K9me3, H3K27me3, and H4K20me3 are related to gene repression.8,9 Recent studies have reported that histone methylation may play important roles in regulating the expression of genes associated with survival, proliferation, and differentiation of Ag-activated T cells.11,12 Unlike histone methylation, DNA methylation results in global silencing of gene expression,10 whereas histone acetylation is associated with a relaxing chromatin structure that facilitates transcription.10 It has been shown that the DNA methylation inhibitor 5-Aza-2-deoxycytidine (5-AzaC) and the histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA) may prevent GVHD in mice through a mechanism of modulating regulatory T cells (Tregs)13,14 and APCs,15-17 respectively. However, global modifications of DNA and chromatin structures are found to be associated with toxicities and adverse effects.10 Thus, novel epigenetic approaches capable of targeting a specific set of genes in alloreactive T cells are desirable for controlling GVHD while minimizing adverse effects.
3-Deazaneplanocin A (DZNep) possesses the potent ability to selectively inhibit some histone methylation, such as H3K27me3, H3K4me3, and H4K20me3.18,19 DZNep is an inhibitor of S-adenosyl-L-homocysteine (AdoHcy) hydrolase. AdoHcy hydrolase catalyzes the reversible hydrolysis of AdoHcy to adenosine and homocysteine.20,21 When this enzyme is inhibited, AdoHcy accumulates in cells, leading to inhibition of the histone methyltransferease (HMT) activity and the subsequent histone methylation inhibition.20 DZNep acts through a different pathway than DNA methylation inhibitors and HDAC inhibitors.10,18,19 For example, DZNep activates different sets of genes that are regulated by 5-AzaC and by a HDAC inhibitor.18,19 Furthermore, DZNep did not affect histone acetylation.18,19 Inhibition of histone methylation by DZNep causes selective apoptosis in cancer but not in normal cells.18,19,22 This effect may result from DZNep-mediated depletion of Ezh2,19 which is a HMT that catalyzes H3K27me3.23 Because data from our recent studies indicate that alloreactive effector T cells expressed high levels of Ezh2 during the GVHD process,24 we hypothesized that inhibition of histone methylation by DZNep may modulate allogeneic T-cell responses and control established GVHD.
In this report, we demonstrate that inhibition of histone methylation by DZNep arrested ongoing GVHD in mouse models of human allogeneic BMT. Importantly, DZNep therapy preserved the antileukemia activity and had no impairment on the hematopoietic reconstitution, leading to significantly improved survival of mice after allogeneic BMT and leukemia. Our findings suggest that pharmacologic inhibition of histone methylation should be explored as a novel strategy for controlling established GVHD.
Methods
Mice
C57BL/6 (B6, H-2b, Thy1.2+, CD45.2+), B6/SJL (H-2b, CD45.1+), BALB/c, Bim−/−(H-2b), and transgenic OT-II and Pmel-1 mice were purchased from The Jackson Laboratories. C3H.SW and B6xDBA/2 F1 mice (BDF1, H-2b/d) mice were obtained from Taconic. Experimental protocols were approved by the University of Michigan's Committee on Use and Care of Animals.
Antibodies, flow cytometric analysis, and cell lines
The Abs used for flow cytometric analyses were purchased from eBioscience, BioLegend, or BD Biosciences. Flow cytometric analyses were performed with FACScan and Canto cytometer (BD Biosciences) as described.25 P815 cells were obtained from American Type Culture Collection. A20 (BALB/c, H-2d) lymphoma/leukemia cells expressing luciferase were kindly provided by Marcel van den Brink (Memorial Sloan-Kettering Cancer Center, New York).26 MBL-2 (H-2b) cells were kindly provided by Pavan Reddy (University of Michigan).
Cell preparation and culture
T cell–depleted (TCD) BM, CD4+ T cells, and CD8+ T cells were prepared as described.25 T cells and spleen cells were labeled with CFSE (Invitrogen). B6-derived CD4+ or CD8+ T cells were stimulated with anti-CD3 and anti-CD28 Ab-conjugated microbeads (Miltenyi Biotech) in the presence of IL-2 (5 ng/mL).
Induction of GVHD and GVL
Mice underwent BMT as described.25 The GVHD score and severity were assessed as described.27 GVHD severity was also assessed by histopathologic analysis.28 In GVL experiments, the leukemic cells A20 (1 × 106) were administered on the day when transplantation was performed. In vivo bioluminescence was used to monitor leukemia growth by intraperitoneal injection of 4.5 mg of Firefly D-Luciferin (Biosynth) and was assessed with the IVIS 200 system (Xenogen).
RT-PCR
Total RNA was extracted from sorted T-cell subsets with TRIzol (Invitrogen Life Technologies). Real-time RT-PCR was performed with a SYBR green PCR mix (ABI Biosystems) in the Realplex2 Eppendorf Real-time PCR instrument (Eppendorf AG). Gene expression levels were calculated relative to the Gapdh gene. Data were collected and quantitatively analyzed on a Realplex sequence detection system (Eppendorf AG). The primer sequences are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Ex vivo and in vivo cytotoxicity assays
CFSE-based ex vivo and in vivo cytotoxicity assays were as described.29
Statistical analysis
Survival in different groups was compared with the log-rank test. Comparison of 2 means was analyzed with the 2-sided 2-sample t test.
Results
DZNep treatment reduces CD8+ T cell–mediated GVHD
We first determined the effect of DZNep on allogeneic T cells in vivo. Spleen cells from C57BL/6 mice (B6, H-2b) were labeled by CFSE and transplanted into unirradiated BDF1 mice (H-2b/d; supplemental Figure 1A). In this model, dividing (CFSElo) T cells represent mostly alloantigen-activated cells, whereas nondividing (CFSEhi) T cells contain alloantigen-unresponsive cells.30 Four days after transplantation, infused donor T cells had undergone extensive cell division (supplemental Figure 1B). We observed that 3 injections of DZNep (1.0 or 3.0 mg/kg/d) significantly reduced the number of dividing CD8+ (3- to 4-fold) and CD4+ (1.6- to 2-fold) T cells but not nondividing donor T cells (supplemental Figure 1C-D). This suggests that in vivo administration of DZNep may cause selective reduction of alloantigen-activated dividing donor T cells.
Of note, DZNep treatment had no effect on the number of host T cells that are known not to respond to infused donor cells (supplemental Figure 2A). Furthermore, DZNep treatment led to a reduction in frequency of FoxP3+CD4+ Tregs (supplemental Figure 2B). Thus, DZNep-mediated reduction of alloantigen-reactive T cells may not be associated with Tregs, which is in sharp contrast to the effect of DNA methylation inhibitor 5-AzaC on FoxP3+CD4+ Tregs.13,14
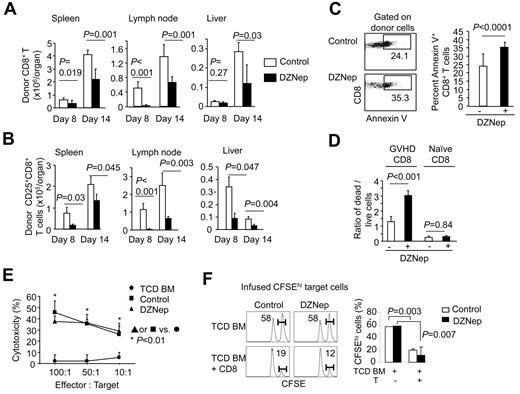
To assess the effect of DZNep treatment on GVHD, we transplanted TCD BM from donor C3H.SW mice (H-2b), with and without CD44loCD8+ naive T cells (TN), into lethally irradiated B6 mice (Figure 1A). This is a MHC-identical but minor histocompatibility Ag–mismatched mouse model of GVHD. We reasoned that administration of DZNep from day 3 to day 17 after transplantation would permit initial activation of donor T cells. To minimize possible adverse actions of DZNep therapy, we administered DZNep (1 mg/kg/d) every other day (named DZNep), with vehicle treatment as control (named control). As expected, B6 mice receiving donor TCD BM alone developed no signs of GVHD, whereas ∼ 90% of control B6 mice receiving donor TCD BM + CD8+ TN cells died of GVHD (Figure 1B). In contrast, DZNep treatment inhibited GVHD in T-cell recipients, with 90% of them surviving without severe GVHD (Figure 1B). Histologic examination showed a significant reduction of inflammation in the skin and liver of DZNep-treated recipients (Figure 1C; supplemental Figure 3). In this model, there was no significant inflammation in the intestine (Figure 1C; supplemental Figure 3).
DZNep inhibits GVHD mediated by donor CD8+ T cells. (A-C) The diagram shows the experimental design (A). Lethally irradiated B6 recipients (10 Gy [1000 rad]) were transplanted with TCD BM (5 × 106) derived from C3H.SW mice (n = 10; ●), or with TCD BM + CD44loCD8+ TN cells (5 × 105). Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 22) from day 3 to day 17 after transplantation, with vehicle treatment as control (Control; n = 22). The survival and clinical signs were monitored over time (B). Tissues were collected between day 25 and day 35 after transplantation for histologic examination. The graphs (mean ± SD) show the histologic scores of GVHD in B6 recipients (C). (D-E) Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 10) from day 14 to day 28 after transplantation, with vehicle treatment as control (Control; n = 10). The survival and clinical signs were monitored over time (D). (E) Tissues were collected on day 14 (before DZNep treatment) and day 81 (after DZNep treatment) after transplantation, respectively, and graphs show the histologic score of GVHD (mean ± SD).
DZNep inhibits GVHD mediated by donor CD8+ T cells. (A-C) The diagram shows the experimental design (A). Lethally irradiated B6 recipients (10 Gy [1000 rad]) were transplanted with TCD BM (5 × 106) derived from C3H.SW mice (n = 10; ●), or with TCD BM + CD44loCD8+ TN cells (5 × 105). Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 22) from day 3 to day 17 after transplantation, with vehicle treatment as control (Control; n = 22). The survival and clinical signs were monitored over time (B). Tissues were collected between day 25 and day 35 after transplantation for histologic examination. The graphs (mean ± SD) show the histologic scores of GVHD in B6 recipients (C). (D-E) Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 10) from day 14 to day 28 after transplantation, with vehicle treatment as control (Control; n = 10). The survival and clinical signs were monitored over time (D). (E) Tissues were collected on day 14 (before DZNep treatment) and day 81 (after DZNep treatment) after transplantation, respectively, and graphs show the histologic score of GVHD (mean ± SD).
We next determined the therapeutic effect of DZNep on established GVHD. Fourteen days after transplantation, significant clinical signs of GVHD and tissue injury had occurred in CD8+ T-cell recipients (Figure 1D-E), suggesting the production of GVHD. Eight doses of DZNep treatment from day 14 to day 28 after transplantation inhibited GVHD (Figure 1D) and reduced inflammation in the liver and skin (Figure 1E). These data indicate that DZNep treatment has both prophylactic and therapeutic effects on controlling CD8+ T cell–mediated GVHD.
DZNep causes increased apoptosis in alloreactive CD8+ effector T cells in vivo
To understand the mechanism by which DZNep treatment reduced GVHD, we administered DZNep to B6 mice receiving C3H.SW CD8+ TN cells and TCD BM from 3 to 13 days after transplantation every other day, with vehicle treatment as control. Donor T cells were recovered from these B6 recipients at day 8 and day 14 after transplantation. Compared with control, DZNep treatment markedly decreased the number of donor CD8+ T cells and CD25+CD8+ T cells in the spleen, lymph node, and liver at day 8 and day 14 after transplantation (Figure 2A-B). A significant increase was observed in the fraction of annexin V–positive donor CD8+ T cells in DZNep-treated B6 recipients (35.3% ± 3.2%) compared with controls (24.1% ± 7.2%; Figure 2C), suggesting that DZNep treatment reduced the number of alloreactive effector T cells via a mechanism of increasing cell apoptosis. To further confirm the direct effect of DZNep on donor T cells, we isolated donor CD8+ T cells from control B6 recipients with GVHD at day 14 after transplantation (named GVHD CD8+ T cells) and cultured in vitro with IL-2 for 24 hours. The addition of DZNep significantly increased (2.5-fold) cell death in GVHD CD8+ T cells compared with the culture without DZNep (Figure 2D). In contrast, DZNep did not increase cell death in CD8+ TN cells cultured in the presence of IL-2 (Figure 2D).
DZNep increases apoptosis in alloreactive CD8+ T cells in vivo. Donor C3H.SW mouse-derived TCD BM + CD44loCD8+ TN cells (Ly9.1) were transplanted into lethally irradiated B6 mice (Ly9.2). DZNep was administered subcutaneously to these recipients from day 3 to day 13, with vehicle treatment as control. (A-B) Graphs show (mean ± SD) the absolute number of donor-derived CD8+ T cells in spleen, lymph node, and liver on day 8 (n = 3 for each group) and day 14 (n = 5 for each group) after transplantation (A) and the percentage and absolute number of donor-derived CD25+CD8+ cells (B). (C) Dot plots and graphs show (mean ± SD) the fraction of annexin V–positive donor-derived CD8+ T cells isolated from B6 recipients on day 14 after transplantation (n = 5 for each group). (D) Donor CD8+ T cells were isolated from control recipients of T cells at day 14 after transplantation and cultured with IL-2 (5 ng/mL), with or without addition of DZNep (200nM) for 24 hours. Graphs (mean ± SD) show the ratio of dead cells versus live cells. (E) Donor T cells were isolated from the spleen at day 14 for CTL assay against MBL-2 cells. (F) In vivo CTL assay against infused host and donor-type B220 cells. B6 allogeneic target splenocytes were labeled with 3.0μM CFSE (CFSEhi). Control targets were C3H.SW splenocytes labeled at a low CFSE concentration (0.3μM; CFSElo). On day 21, a 1:1 mixture of CFSE-labeled B6 and C3H.SW splenocytes (107) were infused into B6 recipients of TCD BM or B6 recipients of TCD BM and CD8+ T cells, which had been treated with or without DZNep from day 3 to day 17. Spleens were harvested 18 hours later to assess killing of B6-derived CFSEhi-target B cells. CD8+ TN cells were cultured as controls. Representative results from 2 separate experiments are shown.
DZNep increases apoptosis in alloreactive CD8+ T cells in vivo. Donor C3H.SW mouse-derived TCD BM + CD44loCD8+ TN cells (Ly9.1) were transplanted into lethally irradiated B6 mice (Ly9.2). DZNep was administered subcutaneously to these recipients from day 3 to day 13, with vehicle treatment as control. (A-B) Graphs show (mean ± SD) the absolute number of donor-derived CD8+ T cells in spleen, lymph node, and liver on day 8 (n = 3 for each group) and day 14 (n = 5 for each group) after transplantation (A) and the percentage and absolute number of donor-derived CD25+CD8+ cells (B). (C) Dot plots and graphs show (mean ± SD) the fraction of annexin V–positive donor-derived CD8+ T cells isolated from B6 recipients on day 14 after transplantation (n = 5 for each group). (D) Donor CD8+ T cells were isolated from control recipients of T cells at day 14 after transplantation and cultured with IL-2 (5 ng/mL), with or without addition of DZNep (200nM) for 24 hours. Graphs (mean ± SD) show the ratio of dead cells versus live cells. (E) Donor T cells were isolated from the spleen at day 14 for CTL assay against MBL-2 cells. (F) In vivo CTL assay against infused host and donor-type B220 cells. B6 allogeneic target splenocytes were labeled with 3.0μM CFSE (CFSEhi). Control targets were C3H.SW splenocytes labeled at a low CFSE concentration (0.3μM; CFSElo). On day 21, a 1:1 mixture of CFSE-labeled B6 and C3H.SW splenocytes (107) were infused into B6 recipients of TCD BM or B6 recipients of TCD BM and CD8+ T cells, which had been treated with or without DZNep from day 3 to day 17. Spleens were harvested 18 hours later to assess killing of B6-derived CFSEhi-target B cells. CD8+ TN cells were cultured as controls. Representative results from 2 separate experiments are shown.
Interestingly, DZNep treatment in vivo resulted in a significant decrease in absolute number but not the frequency of donor CD8+ T cells producing IFN-γ, granzyme B, and Fas ligand 14 days after transplantation compared with the control (supplemental Figure 4). In vitro cytotoxic assays showed that donor T cells derived from DZNep-treated B6 recipients of C3H.SW CD8+ T cells retained the ability to kill MBL-2 leukemic cells (Figure 2E). In vivo CTL assay further confirmed that DZNep treatment did not impair the cytolytic activity of donor CD8+ T cells against host hematopoietic cells (Figure 2F).
Taken altogether, these data suggest that increased apoptosis in alloreactive CD8+ effector T cells may play a predominant role in DZNep-mediated inhibition of ongoing GVHD.
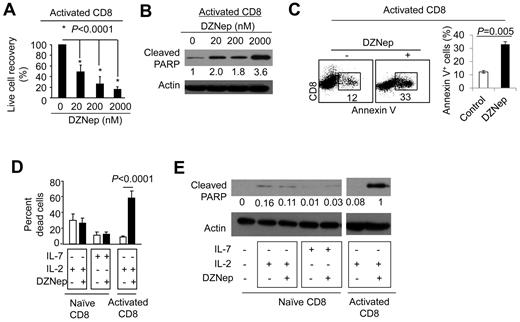
DZNep induces apoptosis in TCR-activated T cells in vitro
To determine the mechanism by which DZNep treatment causes increased apoptosis in alloreactive CD8+ effector T cells, we purified CD8+ TN cells from B6 mice and cultured in the presence of anti-CD3 Ab + anti-CD28 Ab. Five days later, these activated CD8+ T cells were recovered from the culture and seeded in secondary cultures in the presence of various concentrations of DZNep (ranging from 0 to 2000nM). This allowed assessing the direct effect of DZNep on T cells activated by TCR/CD28 stimulation. We found that the addition of DZNep resulted in dose-dependent reduction of activated CD8+ T cells (Figure 3A). This DZNep-mediated reduction of activated CD8+ T cells was associated with an increase in the amount of cleaved poly (ADP-ribose) polymerase (PARP; Figure 3B), an early marker of cell apoptosis.31 Of note, DZNep treatment for 24 hours induced 2.8-fold more annexin V+CD8+ T cells than control (Figure 3C). In contrast, DZNep treatment of CD8+ TN cells that were cultured in the presence of IL-7 or IL-2 had no effect on their survival (Figure 3D). Western blot analysis confirmed that DZNep treatment did not induce cleavage of PARP in these CD8+ T cells (Figure 3E). This may explain why administration of DZNep caused a selective decrease of alloreactive effector T cells but not alloantigen unresponsive T cells in vivo (supplemental Figure 1).
DZNep causes selective apoptosis in activated CD8+ T cells in vitro. (A-C) CD8+ TN cells derived from B6 mice were stimulated with anti-CD3 and anti-CD28 Abs. Five days later, these activated T cells were further treated with different doses of DZNep for 24 hours. Live cell number was counted, and the recovery rate (output/input) was calculated (A). T-cell lysates were prepared for Western blot analysis to determine the amount of cleaved PARP (B). Dot plots and graphs (mean ± SD) show the fraction of annexin V+ cells in activated T cells treated with or without DZNep (200nM; C). (D-E) CD8+ TN cells derived from B6 mice were cultured in the presence of IL-7 or IL-2 with or without the addition of DZNep (200nM) for 24 hours. CD8+ T cells that had been stimulated with anti-CD3 + anti-CD28 Abs for 5 days were collected and cultured with or without DZNep (200nM) for an additional 24 hours as controls. (D) Graphs (mean ± SD) show the percentage of dead cells after culture. (E) Western blots show the expressions of cleaved PARP. Representative results from ≥ 3 separate experiments are shown.
DZNep causes selective apoptosis in activated CD8+ T cells in vitro. (A-C) CD8+ TN cells derived from B6 mice were stimulated with anti-CD3 and anti-CD28 Abs. Five days later, these activated T cells were further treated with different doses of DZNep for 24 hours. Live cell number was counted, and the recovery rate (output/input) was calculated (A). T-cell lysates were prepared for Western blot analysis to determine the amount of cleaved PARP (B). Dot plots and graphs (mean ± SD) show the fraction of annexin V+ cells in activated T cells treated with or without DZNep (200nM; C). (D-E) CD8+ TN cells derived from B6 mice were cultured in the presence of IL-7 or IL-2 with or without the addition of DZNep (200nM) for 24 hours. CD8+ T cells that had been stimulated with anti-CD3 + anti-CD28 Abs for 5 days were collected and cultured with or without DZNep (200nM) for an additional 24 hours as controls. (D) Graphs (mean ± SD) show the percentage of dead cells after culture. (E) Western blots show the expressions of cleaved PARP. Representative results from ≥ 3 separate experiments are shown.
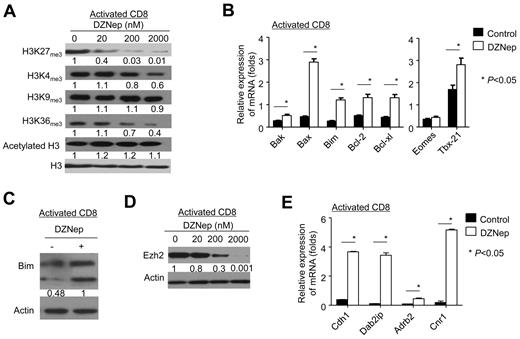
Previous studies suggested that DZNep inhibits histone methylation and depletes Ezh2, leading to selective cell death in cancer but not normal cells via a mechanism of activating genes associated with apoptosis.18,19,22 We then tested the effect of DZNep treatment on histone methylation and gene expression in activated T cells. Compared with control, DZNep treatment reduced H3K27me3, H3K4me3, and H3K36me3 in activated CD8+ T cells, without affecting H3K9me3 and acetylated H3 (Figure 4A). DZNep treatment resulted in a significant elevation of genes associated with apoptosis (eg, Bim, Bak, Bax, Bcl-2, Bcl-xl, and Mcl-1; Figure 4B). Western blot analysis validated the elevation of Bim protein in activated CD8+ T cells after DZNep treatment (Figure 4C), which is a proapoptotic molecule critical to the survival of activated T cells.32
DZNep inhibits histone methylation, reduces cellular Ezh2, and alters gene expression in activated CD8+ T cells. Naive CD8+ T cells were isolated from B6 mice and then stimulated with anti-CD3 and anti-CD28 Abs. Five days later, those activated T cells were further treated with or without DZNep. Cell lysates and RNA were prepared for measuring the amount of histone methylation, Ezh2 expression, and gene expression. (A) Western blot analysis shows the amount of histone methylation and acetylation in activated T cells treated with or without DZNep for 72 hours. (B) Graphs show (mean ± SD) the expression of genes associated with apoptosis and effector differentiation in activated T cells. (C) Western blot analyses show the expression of Bim protein in activated T cells treated with or without DZNep (200nM). (D) Western blot analysis shows the expression of Ezh2 in activated T cells treated with various doses of DZNep. (E) Graphs (mean ± SD) show the expression of genes repressed by Ezh2 in activated T cells treated with or without DZNep for 24 hours. Data are representative of ≥ 3 independent experiments.
DZNep inhibits histone methylation, reduces cellular Ezh2, and alters gene expression in activated CD8+ T cells. Naive CD8+ T cells were isolated from B6 mice and then stimulated with anti-CD3 and anti-CD28 Abs. Five days later, those activated T cells were further treated with or without DZNep. Cell lysates and RNA were prepared for measuring the amount of histone methylation, Ezh2 expression, and gene expression. (A) Western blot analysis shows the amount of histone methylation and acetylation in activated T cells treated with or without DZNep for 72 hours. (B) Graphs show (mean ± SD) the expression of genes associated with apoptosis and effector differentiation in activated T cells. (C) Western blot analyses show the expression of Bim protein in activated T cells treated with or without DZNep (200nM). (D) Western blot analysis shows the expression of Ezh2 in activated T cells treated with various doses of DZNep. (E) Graphs (mean ± SD) show the expression of genes repressed by Ezh2 in activated T cells treated with or without DZNep for 24 hours. Data are representative of ≥ 3 independent experiments.
T-bet and Eomes are 2 transcription factors important for effector differentiation of activated CD8+ T cells.33,34 DZNep treatment increased the expression of Tbx21 but had no effect on Eomes expression in these activated CD8+ T cells (Figure 4B). This coincides with our observations that DZNep did not affect the generation of effector T cells in vivo (supplemental Figure 4).
Interestingly, DZNep effectively reduced cellular Ezh2 in these activated CD8+ T cells (Figure 4D). This was accompanied with elevated expression of genes specifically repressed by Ezh2 (eg, Adrb2, Cdh1, Dab2ip, and Cnr135-37 ; Figure 4E). These results suggest that alteration of apoptotic genes and depletion of Ezh2 may account for, at least in part, DZNep-induced apoptosis in activated T cells.
DZNep inhibits CD4+ T cell–mediated GVHD
We next determined the effect of DZNep on CD4+ T cell–mediated GVHD. TCD BM derived from B6 mice were transplanted, with or without B6 CD4+ T cells, into lethally irradiated BALB/c mice (supplemental Figure 5A). Twelve doses of DZNep were given to BALB/c recipients of B6 CD4+ T cells from day 0 to day 27 after transplantation. This scheme was chosen because GVHD directed against allogeneic MHC Ags occurs early after transplantation and is much more virulent than GVHD directed against minor histocompatibility Ags. BALB/c recipients of TCD BM did not develop clinical signs of GVHD, whereas all BALB/c recipients of CD4+ T cells died of severe GVHD within 35 days after transplantation (supplemental Figure 5B). DZNep treatment inhibited the development of severe GVHD in BALB/c mice receiving CD4+ T cells, with 90% of them surviving > 70 days after transplantation (supplemental Figure 5B). Histologic examination showed a significant reduction of inflammation in the intestine in DZNep-treated recipients compared with controls (supplemental Figures 5C and 6). These results suggest that DZNep treatment can effectively prevent the development of acute GVHD mediated by MHC-mismatched allogeneic CD4+ T cells.
We further evaluated the therapeutic effect of DZNep treatment on established GVHD mediated by allogeneic CD4+ T cells (Figure 5A). Seven days after transplantation, BALB/c mice receiving B6 CD4+ T cells developed severe inflammation in the intestine (Figure 5B) and clinical signs of GVHD by day 7 after transplantation (Figure 5C right), with some dying of the disease (Figure 5C left). This suggests that GVHD has been established in these mice. Twelve doses of DZNep treatment from day 7 to day 29 after transplantation inhibited ongoing GVHD in these recipients, with ∼ 70% of them surviving > 60 days (Figure 5C). Histologic examination showed a significant reduction of inflammation in the intestine in these DZNep-treated recipients compared with controls (Figure 5B). Thus, DZNep treatment arrests ongoing GVHD mediated by MHC-mismatched allogeneic CD4+ T cells.
Control of established GVHD by DZNep. (A) Diagram shows the experimental design. Lethally irradiated BALB/C recipients (8 Gy [800 rad]) were transplanted with B6 mice–derived TCD BM (5 × 106; n = 8) or with TCD BM + CD4+ TN cells (1 × 106). CD4+ T-cell recipients were treated with 12 doses of DZNep (n = 20) or vehicle (■; n = 10) from day 7 to day 29 after transplantation. (B) Tissues were collected on day 7 and day 60 after transplantation for histologic examination. Graphs (mean ± SD) show the histologic score of GVHD. (C) The survival and clinical signs were monitored over time.
Control of established GVHD by DZNep. (A) Diagram shows the experimental design. Lethally irradiated BALB/C recipients (8 Gy [800 rad]) were transplanted with B6 mice–derived TCD BM (5 × 106; n = 8) or with TCD BM + CD4+ TN cells (1 × 106). CD4+ T-cell recipients were treated with 12 doses of DZNep (n = 20) or vehicle (■; n = 10) from day 7 to day 29 after transplantation. (B) Tissues were collected on day 7 and day 60 after transplantation for histologic examination. Graphs (mean ± SD) show the histologic score of GVHD. (C) The survival and clinical signs were monitored over time.
DZNep increases apoptosis in alloreactive CD4+ effector T cells
To understand the mechanism by which DZNep treatment reduced donor CD4+ T cell–mediated GVHD, we transplanted B6 T cells plus TCD BM into lethally irradiated BALB/c mice, gave 6 doses of DZNep (1 mg/kg/d) from day 0 to day 15 after transplantation, and isolated donor T cells from DZNep-treated BALB/c recipients at day 10 and day 17 after transplantation, respectively. We observed that DZNep treatment significantly decreased the number of donor CD4+ T cells in the liver and BM at day 10 and day 17 after transplantation (supplemental Figure 7A). Histologic examination showed that DZNep treatment reduced lymphocyte infiltration in the intestine of T-cell recipients compared with control (supplemental Figure 6). There were also markedly reduced donor T cells in the spleen and lymph node of DZNep-treated recipients at day 10 after transplantation (supplemental Figure 7A), which was accompanied with increased annexin V+ apoptotic donor CD4+ T cells (supplemental Figure 7B). Intriguingly, DZNep treatment did not affect the frequency of donor CD4+ T cells that produced high levels of effector cytokines compared with the control, including IFN-γ, TNF-α, IL-2, IL-4, and IL-17 (supplemental Figure 7C-D). Thus, this scheme of DZNep administration may not impair the effector differentiation of alloantigen-activated CD4+ T cells but can effectively reduce the survival of differentiated effector cells through a mechanism of enhancing apoptosis, leading to GVHD inhibition.
To further test this possibility, we purified CD44loCD4+ TN cells from B6 mice and cultured in the presence of anti-CD3 Ab + anti-CD28 Ab. Five days after TCR/CD28 stimulation, these activated CD4+ T cells were cultured in the presence of various concentrations of DZNep. DZNep dose dependently reduced the recovery of activated CD4+ T cells 3 days after reculture (Figure 6A). Importantly, DZNep treatment for 24 hours induced 1.8-fold more annexin V+CD4+ T cells than control (Figure 6B). In contrast, DZNep treatment of CD4+ TN cells that were cultured in the presence of IL-7 or IL-2 alone did not affect their survival (Figure 6C). Like its effects on activated CD8+ T cells, DZNep treatment markedly reduced H3K27me3, H3K4me3, and H3K36me in activated CD4+ T cells (Figure 6D); increased the expression of Bim, Bak, and Bax (Figure 6E); reduced cellular Ezh2 (Figure 6F); and activated genes specifically repressed by Ezh2 (Figure 6G). Notably, we observed that DZNep treatment failed to inhibit GVHD in BALB/c mice receiving allogeneic donor T cells lacking Bim (Bim−/− T cells), with all of them dying of the disease (Figure 6H). Thus, DZNep-induced GVHD inhibition may be accounted for, at least in part, by up-regulating T-cell expression of the apoptotic gene Bim.
DZNep inhibits histone methylation, reduces cellular Ezh2, and alters gene expression in activated CD4+ T cells. (A-B) Naive CD4+T cells were isolated from B6 mice and then stimulated with anti-CD3 and anti-CD28 Abs. Five days later, those activated T cells were further treated with different doses of DZNep for 24 hours. Live cell number was counted, and the recovery rate (output/input) was calculated (A). (B) Dot plots show the expression of annexin V, and the accompanying graph shows the percentage of annexin V+ cells. (C) CD4+ TN cells were stimulated with IL-7 or IL-2 in the presence or absence of DZNep for 24 hours. Graphs show the percentage of dead cells after culture (mean ± SD). (D-G) CD4+ TN cells were stimulated with anti-CD3 and anti-CD28 Abs for 5 days later and then treated with DZNep. Western blots show the amount of trimethylated histones (D) and Ezh2 protein (F) in activated T cells treated with DZNep for 72 hours. Real-time RT-PCR analyses show the expression genes in T cells treated with or without DZNep (200nM) for 24 hours. (H) Lethally irradiated BALB/C recipients [8 Gy (800 rads)] were transplanted with B6 mice–derived TCD BM (5 × 106) or with TCD BM + B6 wild-type (WT) or Bim−/− mice–derived CD4+ TN cells (1 × 106) + CD8+ TN cells. T-cell recipients were treated with vehicle (Control) or 12 doses of DZNep (DZNep) from day 0 to day 27 after transplantation. Survival was monitored.
DZNep inhibits histone methylation, reduces cellular Ezh2, and alters gene expression in activated CD4+ T cells. (A-B) Naive CD4+T cells were isolated from B6 mice and then stimulated with anti-CD3 and anti-CD28 Abs. Five days later, those activated T cells were further treated with different doses of DZNep for 24 hours. Live cell number was counted, and the recovery rate (output/input) was calculated (A). (B) Dot plots show the expression of annexin V, and the accompanying graph shows the percentage of annexin V+ cells. (C) CD4+ TN cells were stimulated with IL-7 or IL-2 in the presence or absence of DZNep for 24 hours. Graphs show the percentage of dead cells after culture (mean ± SD). (D-G) CD4+ TN cells were stimulated with anti-CD3 and anti-CD28 Abs for 5 days later and then treated with DZNep. Western blots show the amount of trimethylated histones (D) and Ezh2 protein (F) in activated T cells treated with DZNep for 72 hours. Real-time RT-PCR analyses show the expression genes in T cells treated with or without DZNep (200nM) for 24 hours. (H) Lethally irradiated BALB/C recipients [8 Gy (800 rads)] were transplanted with B6 mice–derived TCD BM (5 × 106) or with TCD BM + B6 wild-type (WT) or Bim−/− mice–derived CD4+ TN cells (1 × 106) + CD8+ TN cells. T-cell recipients were treated with vehicle (Control) or 12 doses of DZNep (DZNep) from day 0 to day 27 after transplantation. Survival was monitored.
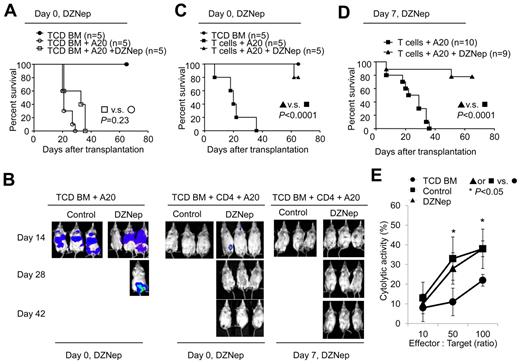
DZNep preserves donor T cell–mediated GVL effects
To evaluate whether DZNep treatment retained GVL activity, we challenged BALB/c recipients with host-type A20 leukemia/lymphoma cells (H-2d), mimicking residual leukemia in patients receiving allogeneic BMT. We tracked luciferase-expressing A20 cells through bioluminescence imaging.26 Mice transplanted with TCD BM and challenged with A20 cells died of leukemia (Figure 7A-B). In vivo administration of DZNep did not control leukemia growth in mice transplanted with TCD BM and challenged with A20 cells (Figure 7A-B). No leukemia growth was observed in mice receiving donor TCD BM + CD4+ T cells, indicating potent GVL effect. However, all these mice succumbed to GVHD within 40 days after transplantation (Figure 7B-D). In contrast, treatment of mice receiving TCD BM + CD4+ T cells + A20 cells with DZNep from day 0 to day 27 after transplantation controlled leukemia without GVHD (Figure 7C). As a result, > 80% of DZNep-treated CD4+ T-cell recipients survived free of leukemia (Figure 7B-C).
DZNep preserves CD4+ T cell-mediated GVL while inhibiting GVHD. (A-D) Lethally irradiated BALB/C recipients were transplanted with donor B6 TCD BM, with or without CD4+ TN cells, and challenged with or without A20TGL cells (1 × 106/mouse). Twelve doses of DZNep were administered from day 0 to day 27 after transplantation (A-C) or from day 7 to day 29 after transplantation (B,D). The survival was monitored over time (A,C-D). In vivo detection of luciferase activity at days 14, 28, and 42 after transplantation (B). (E) Donor T cells were isolated from the spleen at day 14 for CTL assay against A20 cells.
DZNep preserves CD4+ T cell-mediated GVL while inhibiting GVHD. (A-D) Lethally irradiated BALB/C recipients were transplanted with donor B6 TCD BM, with or without CD4+ TN cells, and challenged with or without A20TGL cells (1 × 106/mouse). Twelve doses of DZNep were administered from day 0 to day 27 after transplantation (A-C) or from day 7 to day 29 after transplantation (B,D). The survival was monitored over time (A,C-D). In vivo detection of luciferase activity at days 14, 28, and 42 after transplantation (B). (E) Donor T cells were isolated from the spleen at day 14 for CTL assay against A20 cells.
We further assessed the effect of delayed DZNep treatment on controlling leukemia and GVHD in BALB/c recipients challenged with A20 cells. Twelve doses of DZNep were given to mice receiving TCD BM + CD4+ T cells + A20 cells 7-29 days after transplantation. These DZNep-treated T-cell recipients challenged with A20 cells completely eliminated leukemic cells by day 14 after transplantation and showed inhibition of GVHD (Figure 7B). Consequently, ∼ 80% of these recipients survived without leukemia and severe GVHD (Figure 7B,D). In a separate experiment, we observed that donor T cells recovered from DZNep-treated T-cell recipients had a similar cytolytic activity against A20 cells to that from controls (Figure 7E). Taken altogether, these data suggest that DZNep treatment can control CD4+ T cell–mediated GVHD while preserving beneficial GVL, leading to significantly improved survival of allogeneic BM transplant recipients.
We then used a second mouse model of allogeneic BMT and leukemia to validate these observations. Lethally irradiated BDF1 mice received parent B6 TCD BM, with or without CD4+ plus CD8+ T cells, and were challenged with or without P815 leukemic cells (supplemental Figure 8A). We found that 12 doses of DZNep treatment from 7 to 29 days after transplantation reduced GVHD in BDF1 mice receiving donor T cells, with all of them surviving after transplantation (supplemental Figure 8B). In contrast, ∼ 90% of control mice receiving donor T cells died of GVHD (supplemental Figure 8B). In this model, DZNep treatment did not affect leukemia growth in TCD BM recipients challenged with P815 leukemic cells (supplemental Figure 8C). Addition of donor T cells to the BM graft significantly prolonged the survival time of recipients (supplemental Figure 8C) with 25% (2 of 8) of them dying of leukemia and the rest dying of GVHD (supplemental Figure 8D). The leukemia death was determined on necropsy and histologic examination. This suggests that donor T cells are able to control leukemia growth but cause lethal GVHD in these mice. Notably, DZNep therapy preserved GVL and inhibited GVHD in these T-cell recipients, with 75% (6 of 8) of them surviving > 70 days and 25% dying of leukemia (supplemental Figure 8C-D).
To understand the mechanisms by which DZNep reduced GVHD while preserving GVL, we first determined the effect of DZNep on donor T cells stimulated by allogeneic leukemic cells. Donor B6 T cells (H2b) were cultured in vitro in the presence or absence of host-type A20 leukemic cells (H2d), with or without the addition of BALB/c dendritic cells (DCs; H2d). As shown in supplemental Figure 9A and B, allogeneic DCs rather than A20 leukemic cells activated donor T cells to proliferate and produce IFN-γ. The addition of leukemic cells to allogeneic DC-stimulated cultures did not affect the proliferation and IFN-γ production of activated B6 T cells. Interestingly, despite the presence of leukemic cells DZNep markedly reduced the number of allogeneic DC-activated donor T cells but not the frequency of IFN-γ–producing T cells. These results suggest that DZNep-mediated reduction of alloreactive effector T cells may bring their pathogenicity below the threshold required for GVHD, whereas GVL activity could be less sensitive to this effect.
To further examine the effect of DZNep on T cells activated by specific Ags, we isolated TCR transgenic CD4+ and CD8+ T cells from OT-II mice and Pmel-1 mice38 and stimulated them in vitro with syngeneic DCs pulsed with different concentrations of the specific peptide OT-II and gp100, respectively. Three days later, DZNep was added into the cultures for an additional 24 hours. We observed that DZNep only caused the reduction of T cells activated by high concentration of specific peptide but not those stimulated with low concentration of peptide or without peptide (supplemental Figure 9C). Thus, the activation status may differentiate the apoptotic effect of histone methylation inhibition on T cells.
DZNep has no adverse effect on hematopoiesis after BMT
To evaluate the effect of DZNep on hematopoietic reconstitution, we first performed syngeneic BMT by transplanting B6 TCD BM (CD45.2+) into lethally irradiated B6/SJL (CD45.1+) mice. Twelve doses of DZNep were given to these recipients from 0 to 27 days after transplantation, with vehicle treatment as control. This mimicked the scheme of DZNep treatment in the B6 anti-BALB/c mouse model. We harvested BM cells, splenocytes, and thymocytes 35 days after transplantation when thymopoiesis was fully recovered. Despite DZNep treatment, all mice showed complete donor BM engraftment (supplemental Figure 10A) and similar reconstitution of immune cells in either the thymus or spleen after syngeneic BMT (supplemental Table 2). Compared with untreated controls, mice treated with DZNep had a marginal reduction of lineage-negative Sca-1+C-kit+ hematopoietic stem/progenitor cells without statistical significance (supplemental Figure 10B). Furthermore, DZNep did not impede the reconstitution of intestinal mucosa by rapidly proliferating enterocytes in these mice (data not shown).
Discussion
To date, strategies to treat ongoing GVHD have been limited.2 Many studies have reported that continual generation of pathogenic host-reactive effector T cells is associated with the persistence and progression of GVHD.39,40 Thus, new strategies should target these host-reactive effector T cells. We found that DZNep inhibition of histone methylation drastically reduced the accumulation of alloreactive effector T cells and infiltration of inflammatory cells in GVHD target organs of mice after allogeneic BMT. Interestingly, this blockade of alloreactive effector T cells inhibited GVHD in mice even when the disease had been fully established. Most importantly, DZNep therapy preserved the potent GVL effect and had no impairment on the recovery of hematopoiesis and immune cells, resulting in significantly improved survival of mice after allogeneic BMT and leukemia challenge. Epigenetic therapy is of keen interest in the treatment of cancer.10,41 Most recent studies have reported the efficacy of modulating histone methylation by DNZep in the treatment of both hematologic and nonhematologic malignancies.22,42,43 These observations suggest that pharmacologic modulation of histone methylation could be explored as novel approaches to improve the efficacy of allogeneic BMT.
The mechanism that DZNep controls GVHD may differ from that mediated by either DNA methylation inhibitors13,14 or HDAC inhibitors.15,17,44 For example, treatment with the DNA methylation inhibitor 5-AzaC led to up-regulation of FoxP3, increased expansion of Tregs, and amelioration of GVHD in mice,13,14 whereas in vivo administration of the pan HDAC inhibitor SAHA prevented the production of GVHD by modulating APC functions.15,17,44 However, whether 5-AzaC and SAHA can control ongoing GVHD has not been reported.15,17,44 In contrast, we found that DZNep resulted in a decrease in both frequency and number of FoxP3+CD4+ Tregs during GVH reaction in allogeneic recipients. The major effect of DZNep-mediated inhibition of histone methylation was the selective induction of apoptosis in activated T cells. In addition, DZNep did not affect histone acetylation, which differs from the effect of SAHA.15,17,44 Thus, modulation of histone methylation may represent a novel and effective strategy to control ongoing GVHD.
Several lines of evidence indicate that DZNep-induced apoptosis in activated T cells may result from the activation of proapoptotic gene Bim and depletion of Ezh2. Bim is a major regulator of apoptosis in T-cell homeostasis and responses.32 Bim-deficient mice accumulate mature T cells and develop severe autoimmunity.32 We found that DZNep treatment resulted in a dramatic increase in expression of both Bim mRNA and protein in activated T cells. Thus, Bim appears to be a major target of DZNep in activated T cells. This was further confirmed by our observation that DZNep treatment failed to control GVHD induced by donor T cells lacking Bim. Most importantly, it has been shown that Bim is a target gene specifically repressed by Ezh2. In agreement with previous studies of others,18,19,22,45 we found that DZNep depleted Ezh2 protein, reduced Ezh2-catalyzed H3K27me3, and activated genes repressed by Ezh2, which was accompanied with increased expression of Bim. In a separate study we have recently shown that alloreactive CD8+ effector T cells increased the expression of Ezh2.24 Reducing Ezh2 by small interference RNA selectively inhibited the proliferation of T cells stimulated with allogeneic APCs but not by IL-7 in vitro.24 Therefore, it is highly probable that H3K27me3 and its catalyzing enzyme Ezh2 may account for DZNep-mediated effects on alloreactive effector T cells. Future studies will use genetic approaches to investigate the precise role of Ezh2 in T-cell responses and the selectivity of the effect of DZNep on Ezh2.
Many studies have reported that alloreactive effector T cells mediating GVHD are also important for GVL activity, although not completely overlapping.1,46 Therefore, inhibition of GVHD without impairing GVL effect has proven to be a difficult task.1 We found that allogeneic DCs rather than leukemic cells activated allogeneic T cells to become effector T cells. DZNep treatment resulted in a reduction but not complete depletion of alloreactive effector T cells. These residual alloreactive T cells were sufficient to eliminating leukemic cells but not causing GVHD. Thus, it appears that the strength of GVH reaction required for controlling leukemia could be lower than that mediating GVHD. This is supported by our recent observations and others that eradication of leukemic cells after allogeneic BMT could be mediated by a lower threshold of alloreactivity than induction of severe GVHD.29,47 However, we found that in vivo administration of DZNep did not impair the induction of effector T cells during GVH reaction and the survival of T cells stimulated by cytokines. Because the expression of genes in DZNep-treated cells could return to the original levels within 24 hours after removal of the inhibitor,18 and because the time-dependent disappearance of DZNep occurred between 30 minutes and 24 hours after in vivo administration,48 it is possible that the treatment scheme of DZNep in mice every other day may allow surviving donor T cells to be reactivated to become effector T cells. This could be another explanation that the administration of DZNep at an interval of ≥ 48 hours in this study did not impair the induction of alloreactive effector T cells and their mediated GVL activity. These data suggest that the potency of DZNep in regulating inflammatory T-cell responses could also be adjusted on the basis of the time intervals of administering the inhibitor.
A major concern with pharmacologic inhibition of histone methylation is its potential effects on the recovery of hematopoiesis and thymopoiesis after allogeneic BMT. We found that systemic administration of DZNep did not impair the engraftment of donor hematopoietic stem cells and reconstitution of donor thymocytes, lymphocytes, granulocytes, and erythrocytes after either syngeneic or allogeneic BMT. This coincides with previous observations that DZNep only marginally reduces the survival of normal human CD34+ hematopoietic progenitor cells.22 In contrast, DZNep treatment selectively induced cell death of acute myeloid leukemia cells and CD34+ leukemic hematopoietic cells.22 Furthermore, DZNep has broad and potent antiviral activity, including against vesicular stomatitis virus, rotavirus, and vaccinia virus.21,49 In particular, this antiviral spectrum of DZNep extends to human CMV,49 which causes serious infection in patients with reduced immune functions.1,2 These observations suggest that modulation of histone methylation by pharmacologic compounds such as DZNep could have translational potential for treating pathogenic T cell–mediated inflammatory diseases.
In summary, we have identified that inhibition of histone methylation causes selective apoptosis of alloreactive effector T cells and arrest of ongoing GVHD. This effect is associated with depletion of the HMT Ezh2 and activation of proapoptotic genes such as Bim. Our findings may provide a new experimental method of treating acute GVHD after allogeneic BMT by modulating histone methylation. Given its powerful effects on reducing allogeneic T-cell responses, inhibition of histone methylation by DZNep may lead to the control of other T cell–mediated inflammatory conditions such as autoimmune diseases and graft rejection after organ transplantation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James Ferrara, Max Wicha, Phillip King, Bruce Richardson, Eric Fearon, Stephen Emerson, and Pavan Reddy for their thoughtful discussion and critical reading of the manuscript.
This work was supported by a Damon Runyon–Rachleff Innovation Award (Y.Z.) and the American Cancer Society (Y.Z.), and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Authorship
Contribution: S.H. and Y.Z. conceived and designed the project and wrote the manuscript; S.H., J.W., K.K., F.X., S.V., S.M., Y.L., K.M., E.N., R.-S.M., and Y.Z. performed experiments and analyzed the data; R.K. analyzed the gene expression profile data; and A.M.C. and V.E.M. designed experiments and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zhang, Department of Internal Medicine, University of Michigan, 6219 Cancer Center, 1500 E Medical Dr, Ann Arbor, MI 48109-5942; e-mail: yizha@med.umich.edu.
References
Author notes
S.H., J.W., and K.K. contributed equally to this study.

![Figure 1. DZNep inhibits GVHD mediated by donor CD8+ T cells. (A-C) The diagram shows the experimental design (A). Lethally irradiated B6 recipients (10 Gy [1000 rad]) were transplanted with TCD BM (5 × 106) derived from C3H.SW mice (n = 10; ●), or with TCD BM + CD44loCD8+ TN cells (5 × 105). Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 22) from day 3 to day 17 after transplantation, with vehicle treatment as control (Control; n = 22). The survival and clinical signs were monitored over time (B). Tissues were collected between day 25 and day 35 after transplantation for histologic examination. The graphs (mean ± SD) show the histologic scores of GVHD in B6 recipients (C). (D-E) Eight doses of DZNep were injected subcutaneously to CD8+ T-cell recipients (DZNep; n = 10) from day 14 to day 28 after transplantation, with vehicle treatment as control (Control; n = 10). The survival and clinical signs were monitored over time (D). (E) Tissues were collected on day 14 (before DZNep treatment) and day 81 (after DZNep treatment) after transplantation, respectively, and graphs show the histologic score of GVHD (mean ± SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/5/10.1182_blood-2011-06-364422/4/m_zh89991184740001.jpeg?Expires=1770983721&Signature=n6Gfj8GSUGL61cCS0HCgoBenyP83qzkMQDtGEVmbg2FCkqACuUnEsOTVstin9UycxXiRCRM-lAcc0X3L4ti19S8sGMO9LyqmBhgaVYQpL6CBuZ8p-hUrXqSpKeW-TyyWHizdF2HR3gusnxot~duP8F7rX06KAFfj7Y85-3nDlaxWCsV1AbfXISFJgXvNnQonxnvruCgsPyN337INEIq7FWwfCbvzTs3Mppx4AGJ-tCCiLIig0luTjWKOZHou-geBOGt~9n-IF0bQ-juBbE3wL0jFOy3YwMbxfTZSz5LUkW0IcB6bNTd7EB2uqRpzUxE0GA9PfNlyceXfgnU97LdpHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Control of established GVHD by DZNep. (A) Diagram shows the experimental design. Lethally irradiated BALB/C recipients (8 Gy [800 rad]) were transplanted with B6 mice–derived TCD BM (5 × 106; n = 8) or with TCD BM + CD4+ TN cells (1 × 106). CD4+ T-cell recipients were treated with 12 doses of DZNep (n = 20) or vehicle (■; n = 10) from day 7 to day 29 after transplantation. (B) Tissues were collected on day 7 and day 60 after transplantation for histologic examination. Graphs (mean ± SD) show the histologic score of GVHD. (C) The survival and clinical signs were monitored over time.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/5/10.1182_blood-2011-06-364422/4/m_zh89991184740005.jpeg?Expires=1770983721&Signature=J4U2WderxvURZ9AvuLYt8DUFyFPTfDF2q7nGBBFUb4mIucNp-gXx0iQtCi~amzDZnSP8aH7ODY5GvB5qeEqlK7W097oxV9C7jOxV6nZaQV~m6CiXAYwhyjMqt9ginemKHEa-VKP2fWWHCHFcak3j7liZzndGkmV2SuHqFtyP9S7r~8ZTTvWqfKZbmiqT3~x6~KVCUfciVgT3vXJV2G0HoL9uhuTzfZ7zoKo56hKvyP-HwruMCH1wcLYunnnElk4b9ysjm3UVwDbg71ey2YMT0RQghbSoO7Odv69bMb6hbcpTlUNgLQC8VuLkOxAVCuvKfPl~Q~DC1lvPYUYMxXYCfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. DZNep inhibits histone methylation, reduces cellular Ezh2, and alters gene expression in activated CD4+ T cells. (A-B) Naive CD4+T cells were isolated from B6 mice and then stimulated with anti-CD3 and anti-CD28 Abs. Five days later, those activated T cells were further treated with different doses of DZNep for 24 hours. Live cell number was counted, and the recovery rate (output/input) was calculated (A). (B) Dot plots show the expression of annexin V, and the accompanying graph shows the percentage of annexin V+ cells. (C) CD4+ TN cells were stimulated with IL-7 or IL-2 in the presence or absence of DZNep for 24 hours. Graphs show the percentage of dead cells after culture (mean ± SD). (D-G) CD4+ TN cells were stimulated with anti-CD3 and anti-CD28 Abs for 5 days later and then treated with DZNep. Western blots show the amount of trimethylated histones (D) and Ezh2 protein (F) in activated T cells treated with DZNep for 72 hours. Real-time RT-PCR analyses show the expression genes in T cells treated with or without DZNep (200nM) for 24 hours. (H) Lethally irradiated BALB/C recipients [8 Gy (800 rads)] were transplanted with B6 mice–derived TCD BM (5 × 106) or with TCD BM + B6 wild-type (WT) or Bim−/− mice–derived CD4+ TN cells (1 × 106) + CD8+ TN cells. T-cell recipients were treated with vehicle (Control) or 12 doses of DZNep (DZNep) from day 0 to day 27 after transplantation. Survival was monitored.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/5/10.1182_blood-2011-06-364422/4/m_zh89991184740006.jpeg?Expires=1770983721&Signature=TeRD9S4db~Vrkm5qGdtKs1CY1SjHwGuqRkPSB4Y4thQlkMR8xrWr48LpUeA5KBWIGRaCMe9XkpuoTZj~cO4e9bRD3P2~rbwgURiTwO3oQk-Hg6ypIkdfJDy3XrurOyorKu0AHWtCRxgZKqOvVtamJ1-BquUJP6NWbfici-P6LeV6iNNmhFNM0tw-yG4uBloiGFXMoSR3s~UdqJhUwq-6AzdDdH2GJIglJ9J5hU5jkge~z8gmuvM5aNXx8qg4uWSEfzI6rpwQcMtblFTlVz~s-QIcqSgQ0dHILxgZC8Bdt8AWWXir8E7ftWyRBZpoNyjVzT10ZU5S7OiGCs3KRPq8ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal