Selective depletion of the alloreactive T cells causing graft-versus-host disease (GVHD) without loss of the graft-versus-leukemia (GVL) effect is the holy grail of hematopoietic cell transplantation (HCT). In this issue of Blood, He et al demonstrate that inhibition of histone methylation leads to selective apoptosis of the alloreactive effector cells.1 Moreover, they demonstrate that this inhibition of histone methylation remarkably stops ongoing GVHD.
Because our whole being is mostly dictated by our genetic code, its regulation is rightfully tightly controlled. This process at the DNA level resides with the addition or removal of methyl or acetyl groups to specific amino acids found on histones or on the DNA nucleotides. In the cell nucleus, DNA is wound around these histone proteins. Histone methylation is the modification of certain amino acids in a histone protein by the addition of 1, 2, or 3 methyl groups. In general, methylation and demethylation are the “off” and “on” switches, respectively. This control is accomplished either by loosening the tails of the histone, thereby allowing transcription factors and other proteins to bind the DNA, or by winding their tails around the DNA, thereby restricting access to the DNA.2 Histone methylation is normally associated with transcriptional repression. However, methylation of some lysine and arginine residues of histones results in transcriptional activation.
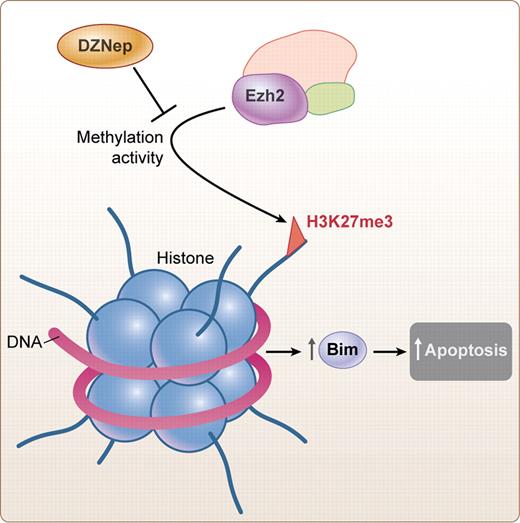
Proposed mechanism of action of DZNep. Inhibition of methylation by Ezh2 leads to increase in Bim and increase in apoptosis. Professional illustration by Kenneth X. Probst.
Proposed mechanism of action of DZNep. Inhibition of methylation by Ezh2 leads to increase in Bim and increase in apoptosis. Professional illustration by Kenneth X. Probst.
PolycombGroup (PcG) proteins maintain gene repression through histone modifications and are involved in cell regulation including cancers. Ezh2 is part of Polycomb Repressive Complex 2 (PRC2) and trimethylates the histone H3 at lysine 27 (H3K27me3). Among other functions, this histone silences Bim, a proapoptotic gene. Recent studies have shown that 3-Deazaneplanocin A (DZNep), a histone methyltransferase inhibitor, disrupts polycomb-repressive complex 2 (PRC2), and preferentially induces apoptosis in cancer cells, including acute myeloid leukemia (see figure).3
He and colleagues sought to study the impact of modulating histone methylation in GVHD specifically with DZNep. They demonstrate that inhibition of histone methylation with this molecule is associated with depletion of the Ezh2, decrease in the methylation of H3K27 leading to the increase in the mRNA and protein of the proapoptotic protein Bim, a major regulator of apoptosis in T-cell homeostasis and responses. He et al demonstrate that this effect is lost in Bim knockout mice. One of the most remarkable findings was that they were able to show that the effect on GVHD was present even after GVHD had occurred. The use of DZNep turned off ongoing GVHD with a decrease in the accumulation of allo-reactive effector T cells and inflammatory cells in the target organs of GVHD. In contrast to previous data in GVHD mediated by either DNA methylation inhibitors or histone deacetylase (HDAC) inhibitors, DZNep did not increase regulatory T cells or affect antigen-presenting cells.4-7
So, does this mean that GVHD versus GVL is solved? He and colleagues have taken us in an important direction but as with all good papers, more questions arise. They do demonstrate that DZNep did not lead to a complete elimination of the alloreactive T cells. Thus the positive data reflect a balance between the stimulation needed to promote GVL versus GVHD. In other words, it may take either fewer T cells or less stimulation of T cells to effect GVL compared with GVHD. Our own data suggest that by simply changing the numbers of T cells without any further intervention, we can find a sweet spot where there is no clinical GVHD but with persistent GVL (Chen et al, Prevention of GVHD and preservation of GVL based on T-cell numbers, manuscript in preparation). Moreover, the hurdles of translation from murine models to humans are high. Ezh2 is involved in multiple signaling pathways such as Wnt/β catenin, Ras, NFkB, Notch, and so on, so off-target effects will need to be carefully understood. On the positive side, DZNep has activity against tumor cells and antiviral effects that could be beneficial. It will be exciting to see if this approach can be extended to other murine models of T cell–mediated inflammatory or autoimmune responses and then on to clinical studies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal