Abstract
Plasminogen activator inhibitor-1 (PAI-1), an endogenous inhibitor of a major fibrinolytic factor, tissue-type plasminogen activator, can both promote and inhibit angiogenesis. However, the physiologic role and the precise mechanisms underlying the angiogenic effects of PAI-1 remain unclear. In the present study, we report that pharmacologic inhibition of PAI-1 promoted angiogenesis and prevented tissue necrosis in a mouse model of hind-limb ischemia. Improved tissue regeneration was due to an expansion of circulating and tissue-resident granulocyte-1 marker (Gr-1+) neutrophils and to increased release of the angiogenic factor VEGF-A, the hematopoietic growth factor kit ligand, and G-CSF. Immunohistochemical analysis indicated increased amounts of fibroblast growth factor-2 (FGF-2) in ischemic gastrocnemius muscle tissues of PAI-1 inhibitor-treated animals. Ab neutralization and genetic knockout studies indicated that both the improved tissue regeneration and the increase in circulating and ischemic tissue-resident Gr-1+ neutrophils depended on the activation of tissue-type plasminogen activator and matrix metalloproteinase-9 and on VEGF-A and FGF-2. These results suggest that pharmacologic PAI-1 inhibition activates the proangiogenic FGF-2 and VEGF-A pathways, which orchestrates neutrophil-driven angiogenesis and induces cell-driven revascularization and is therefore a potential therapy for ischemic diseases.
Introduction
Approximately 500 to 1000 people per million per year are diagnosed with critical ischemia of the limb, which in most cases results in serious morbidity and mortality. Therapeutic restoration of blood flow by, for example, the induction of the formation of new capillaries (angiogenesis) is the ultimate goal for critical limb ischemia patients. Growth of new blood vessels in the adult occurs through angiogenesis or arteriogenesis (vessel maturation via recruitment of smooth muscle cells) and vasculogenesis (mobilization of BM-derived cells).1,2 In contrast to promising results from animal studies, administration of proangiogenic factors such as fibroblast growth factor 2 (FGF-2, also known as basic FGF) or VEGF-A failed to induce significant improvement in ischemia in several phase 1 clinical trials.3
The plasminogen activation system and matrix metalloproteinases (MMPs), which can cleave growth factors, growth factor receptors, and adhesion molecules and mediate the extracellular matrix degradation that is necessary for cell migration, are widely recognized as being involved in the process of angiogenesis.2,4 Although plasminogen activator inhibitor-1 (PAI-1) is one of the primary regulators of the fibrinolytic system, it also has dramatic effects on cell adhesion, detachment, and migration5 and can inhibit cellular migration by affecting cell adhesion.6,7 PAI-1–deficient (PAI-1−/−) mice showed improved vascular wound healing in models of perivascular electric or transluminal mechanical injury8 due to improved migration of PAI-1−/− smooth muscle cells. The 52-kDa serine protease inhibitor PAI-1 is the major plasma inhibitor of urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) and inhibits plasmin-mediated fibrinolysis.9 Studies in mice have indicated that the PAI-1 mRNA concentration is high in the heart, lung, aorta, and adipose and muscle tissue.10 Plasma and tissue concentrations of PAI-1 increase under pathologic conditions. This increase is mediated by many factors, including reactive oxygen species. PAI-1 is secreted from endothelial cells after ischemia, such as that which occurs in acute myocardial infarction, atherosclerosis, and restenosis.11,12 However, the role of PAI-1 in both promoting and inhibiting vascular remodeling or tissue regeneration and tumor growth or neoangiogenesis is controversial.13–15
The proteases plasmin and MMP-3 cleave and inactivate PAI-1.16,17 The balance between PAI-1 inhibition of plasmin and other proteases and the cleavage of PAI-1 by these proteases, my play a critical role in the modulation of vascular proliferative responses. However, the exact mechanisms by which PAI-1 affects ischemic tissue regeneration and cell migration are not completely understood. In the present study, we demonstrate that, under ischemic conditions, drug-induced PAI-1 inhibition accelerates neoangiogenesis in a model of hind-limb (HL) ischemia. Moreover, PAI-1 inhibition also increases the proangiogenic factors FGF-2 and VEGF-A, the hematopoietic growth factor kit ligand (KitL), and G-CSF. tPA and MMP-9 deficiency and VEGF-A and FGF-2 blockade reversed the PAI-1 inhibitor-mediated improved neovascularization and neutrophil recruitment in the peripheral blood and locally within the ischemic tissue/niche. Remarkably, adoptive transfer of ischemic muscle-derived neutrophils derived from PAI-1 inhibitor-treated mice enhanced revascularization. Pharmacologic PAI-1 blockade under ischemic conditions not only increased the absolute number of granulocyte-1 marker (Gr-1+) myeloid cells, but also enhanced their angiogenic performance.
These data provide a fundamental insight into how PAI-1 conditioning of the ischemic niche induces leukocyte influx and controls angiogenesis and suggest that PAI-1 inhibition using small-molecule inhibitors could be a promising cellular target for the treatment of ischemic diseases.
Methods
Animal studies
MMP-9+/+ and MMP-9−/− mice and tPA+/+ and tPA−/− mice were each used after > 10 back crosses onto a C57BL/6 background. C57BL/6 mice were purchased from SLC. C57BL/6 mice that express GFP under a β-actin promoter were used for transplantation experiments at the age of 6-8 weeks. Animal studies were approved by the animal review board of Juntendo University.
PAI-1 inhibitor
The recently described PAI-1 inhibitor TM5275, 5-chloro-2-((2-(4-(diphenylmethyl) piperazin-1-yl)-2-oxoethoxy acetyl)amino]benzoate, provided by T.M. inhibited PAI-1 activity with a half-maximal inhibition (IC50) value of 6.95μM, as measured by assay of tPA-dependent hydrolysis of a peptide substrate. The IC50 values of TM5007 and PAI-749 are 5.60, and 8.37μM, respectively.18 In vitro, TM5275 (up to 100μM does not interfere with other serpin/serine protease systems such as alpha1-antitrypsin/trypsin and alpha2-antiplasmin/plasmin). Therefore, its PAI-1–inhibitory activity appears to be specific. Preincubation of PAI-1 with TM5275 abolishes detection of the covalent PAI-1–tPA complex by SDS-PAGE.18 Oral administration of 50 mg/kg of TM5275 to mice resulted in a maximum plasma concentration after 1 hour. The highest plasma drug concentration observed was 6.9 mol/L and the terminal phase half-life of the drug was 6.5 hours. No effect of TM5275 on platelet aggregation induced by ADP and collagen was observed 2 hours after oral administration (10 mg/kg).
Study design
The PAI-1 inhibitor was resuspended in 200 μL of 0.5% carboxymethylcellulose (MP Biomedicals) and administered orally (10 mg/kg body weight) daily to mice with or without induction of HL ischemia from days 0-6. Control mice received vehicle (200 μL of 0.5% carboxymethylcellulose). Recombinant tPA (Eizai) resuspended in 150 mL of 0.2% BSA (Sigma-Aldrich) was administered (10 mg/kg body weight) to mice by daily IP injections from days 0-2.19
HL model
Mice were anesthetized with pentobarbital sodium (40 mg/kg body weight) that was given intraperitoneally. Briefly, an incision was made in the skin on the medial aspect of the left thigh. The femoral artery was ligated using 4-0 silk sutures (Ethicon) and cut immediately distal to the inguinal ligament and proximal to the popliteal bifurcation site. Changes in blood flow were recorded at days 0, 1, 4, 7, 14, and 21 after the procedure using a laser Doppler perfusion image analyzer (Moor Instruments). Blood was collected via retroorbital bleeding using heparin-coated and plain capillary tubes and WBCs were counted. Plasma and serum samples were stored at −30°C. Mice were killed 1, 5, 15, and 21 days after resection of the femoral artery.
Isolation of Gr-1 cells from muscle tissue after HL ischemia induction
Muscle-derived Gr-1+ cells were isolated from HL-ischemia–induced C57BL/6 mice treated with or without PAI-1 inhibitor on day 5 using MACS. In brief, muscles were excised and cut into small pieces. After excision, tissue pieces were lysed with a buffer containing 20mM Tris-HCl, 5mM EDTA, 1% collagenase II, 2.4 U/mL of Dispase, 1mM PMSF, and 10 μmol pepstatin for 1.5 hours. Gr-1+ and Gr-1− cells were isolated using the anti–Ly-6G Microbead kit (Miltenyi Biotec). Cell morphology was determined on cytospins after Wright-Giemsa staining.
In vivo blocking experiments
HL-ischemic mice treated with or without the PAI-1 inhibitor (days 0-6) were coinjected intraperitoneally with 10 μg of anti–mouse VEGF-A (AF-493-NA; R&D Systems) or 10 μg of goat specific anti–human FGF-2 (AF-233-NA; R&D Systems) on day 0. Appropriate isotype Ab controls were included.
Transplantation of PAI-1 inhibitor-mobilized Gr-1+ cells
Muscle-derived Gr-1+ cells were isolated from HL-ischemia–induced C57BL/6 donor mice treated with or without PAI-1 inhibitor on day 5 by FACS using a FACSCalibur flow cytometer (BD Biosciences). Gr-1+ cells (5 × 104 cells/injection/d) were injected daily intramuscularly into C57BL/6 recipient mice on days 0 and 1 after HL ischemia induction.
Flow cytometry
PBMCs were stained with the following Abs: CD45-FITC (1:200, clone 30-F11; BD Pharmingen), CD11b-APC (1:200, clone M1/70; BD Pharmingen), and Gr-1-PE (1:200, clone RB6-8C5; BD Pharmingen). Cells were analyzed by FACS.
Histological assessment
Ischemic adductor or, if indicated, hamstring (posterior thigh) muscle tissue samples were snap-frozen in liquid nitrogen. Transverse cuts of the whole leg were prepared. Sections were stained with H&E.
Tissue sections were washed, serum blocked, and stained with the first Ab overnight at 4°C. Muscle sections were stained with the anti-CD31 Ab (clone T-2001; BMA Biomedicals) followed by biotin-conjugated goat anti–rat IgG (Vector Laboratories) and FITC-conjugated streptavidin (Alexa Fluor 488; Molecular Probes). In addition, tissues were stained with the primary anti–mouse FGF-2 (clone D0611; Santa Cruz Biotechnology) and FGF-R1 (clone 755639; Abcam) and VEGF-A Abs, followed by a goat anti–rabbit IgG Ab conjugated with Alexa Fluor 488 (Molecular Probes). Muscle sections were also stained with anti–mouse VWF Ab (Dako), followed by Cy3-conjugated streptavidin (Alexa Fluor 594; Molecular Probes) using the M.O.M kit (Vector Laboratories) according to the manufacturer's instructions. Neutrophils were identified using the Gr-1 Ab (clone RB6-8C5; R&D Systems), followed by biotin-conjugated goat anti–rat IgG (Vector Laboratories) and Cy3-conjugated streptavidin (Alexa Fluor 594; Molecular Probes) Abs. Macrophages were identified using the F4/80 Ab (clone A3-1; AbD Serotec), followed by biotin-conjugated goat anti–rat IgG (Vector Laboratories) and Cy3-conjugated streptavidin (Alexa Fluor 594; Molecular Probes) Abs. In addition, tissues were stained with the primary anti–mouse VEGF-A Ab (clone A-20; Santa Cruz Biotechnology), followed by the goat anti–rabbit IgG Ab conjugated with Alexa Fluor 488 (Molecular Probes). Nuclei were counterstained with DAPI (Molecular Probes).
RT-PCR analysis
Total RNA was extracted from the gastrocnemius muscle of HL-ischemic or untreated mice using RNA TRIzol (Invitrogen) according to the manufacturer's directions. Briefly, 25 mg of muscle was immediately immersed in 1 mL of TRIzol reagent. The muscle was homogenized on ice using a homogenizer. The aqueous and organic phases were separated using 200 μL of chloroform. Total RNA was precipitated using 500 μL of isopropyl alcohol, washed 3 times with 75% ethanol, and redissolved in 24 μL of DEPC-treated H2O. The concentration and purity of the RNA was determined using a UV spectrophotometer by measuring the absorbance at 260 and 280 nm. cDNA was amplified by PCR using the following specific forward and reverse primer pairs: for uPA: (5′-GTCCTCTCTGCAACAGAGTC-3′) and (5′-CTGTGTCTGAGGGTAATGCT-3′); for tPA: (5′-GTACTGCTTTGTGGACT-3′) and (5′-TGCTGTTGGTAAGTTGTCTG-3′); for PAI-1: (5′-AAAGGACTCTATGGGGAGAA-3′) and (5′-TAGGGAGGAGGGAGTTAGAC-3′); and for the b-actin control: (5′-TGACAGGATGCAGAAGGAGA-3′) and (5′-GCTGGAAGGTGGACAGTGAG-3′).
ELISA
Plasma and serum samples from PAI-1 inhibitor-treated and untreated mice with HL-ischemia induction were assayed for murine VEGF-A, MMP-9, KitL, G-CSF, PAI-1, and tPA using ELISA kits (R&D Systems, Cell Sciences, and Molecular Innovations).
Western blotting
Muscle tissue extracts were prepared for Western blotting. Briefly, gastrocnemial muscle tissues were lysed in a buffer containing 20mM Tris-HCl, 5mM EDTA, 1% Triton X-100, 1mM PMSF, and 10μM pepstatin after mashing between 2 glass slides. Whole-muscle lysates were subjected to SDS-PAGE (8%), followed by electroblotting onto a PVDF membrane. Membranes were blocked in 20mM PBS and 0.05% Tween-20 (vol/vol) containing 5% (wt/vol) skim milk powder at room temperature, followed by overnight incubation with the anti–PAI-1 Ab (1:1000; Abcam), and an HRP-conjugated secondary Ab (1:20; Nichirei Biosciences) for 1 hour. Membranes were developed using the ECL Plus system (Amersham Life Sciences).
Reverse fibrin zymography
Reverse fibrin zymography, although similar to fibrin zymography, uses agar gels that contain uPA in addition to fibrinogen and plasminogen to determine unbound/free PAI-1. Protein extracts (50 μg) from normal and ischemic muscle tissues were loaded on an 8% acrylamide gel, and, after SDS-PAGE, the SDS was removed by washing the acrylamide gels with distilled water followed by incubation for 2 hours with a buffer containing 2.5% Triton X-100, 0.05 M/L of Tris-HCl (pH 7.5), and 0.1 M/L of NaCl to renature the enzymes. The gels were then incubated for 12-24 hours at 37°C in buffer containing 0.05 M/L of Tris-HCl (pH 7.5) and 0.01 M/L of CaCl2. After this incubation, the gels were stained for 1 hour with Coomassie blue diluted with 50% methanol and 10% acetic acid and decolorized with buffer containing 25% methanol and 8% acetic acid. No fibrinolysis occurs in the area of the gel where the PAI-1 protein is located, resulting in a visible band. Gels were then photographed.
Fibrin plate assay
Plasma fibrinolytic activity was measured using the modified fibrin plate method. Briefly, blood samples were collected in plain tubes containing 3.2% sodium citrate, mixed at a ratio of 9:1, and centrifuged at 3000g for 20 minutes at 4°C. The euglobulin fraction was prepared by acidification of 1:10 diluted plasma to pH 5.9 with 0.25% (vol/vol) glacial acetic acid at 4°C, which was then centrifuged at 500g at 4°C for 10 minutes. The supernatant was discarded. The euglobulin precipitate was resuspended in an EDTA-gelatin-barbital buffer (pH 7.8), and 30 mL of each sample were placed in identical depressions in a fibrin-agarose plate. Next, 10 mL of a 1.5 mg/mL bovine fibrinogen solution in Barbitol buffer (50mM sodium barbitol, 90mM NaCl, 1.7mM CaCl2, and 0.7mM MgCl2, pH 7.75) and 10 mL of a 1% agarose solution were brought to 45°C in a water bath, and 10 NIH units of thrombin (250 NIH units/mL) were then added into the agarose solution. The fibrinogen and agarose solutions were mixed in a 140-mm Petri dish and kept at room temperature for 2 hours to form fibrin clots. Enzymes were dissolved and diluted to the appropriate concentration in Barbitol buffer. Each enzyme solution (10 mL) was dropped into a hole preformed on the fibrin plate. The plate was incubated at 37°C for 18 hours. The zone of lysis on the fibrin plate (fibrinolytic activity) was measured using Area Manager (Ruka International).
Statistical analyses
All data are presented as means ± SEM. Student t tests were performed. P < .05 was considered significant.
Results
Ischemia is associated with increased expression of PAI-1 in ischemic muscle tissue
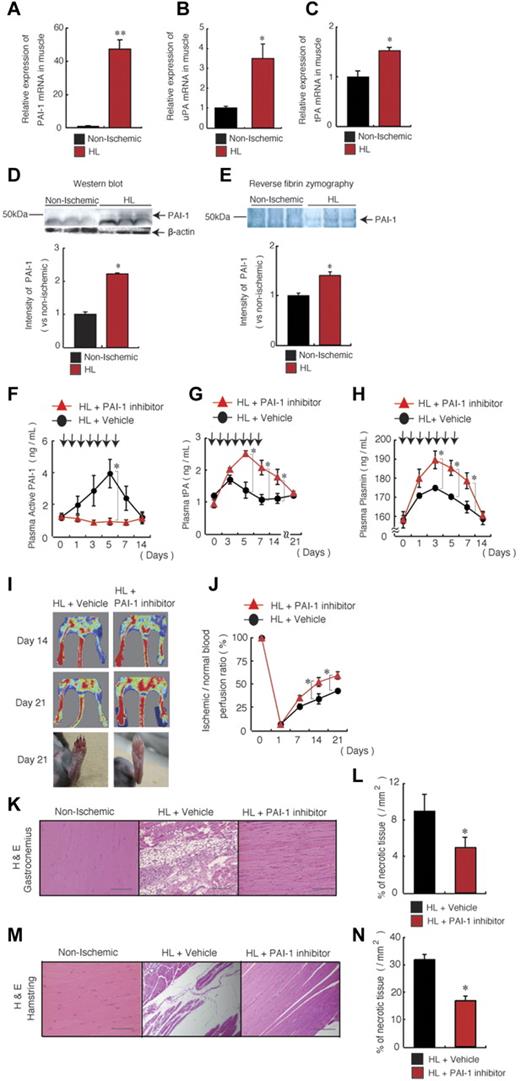
Because oxygen deprivation, such as that which occurs during tissue ischemia, can tip the natural anticoagulant/procoagulant balance,20 in the present study, we investigated whether the fibrinolytic factors are present within ischemic gastrocnemius muscle tissues. An increase in PAI-1, uPA, and tPA mRNA expression, as determined using quantitative PCR (Figure 1A-C), and an increase in PAI-1 protein and activity, as determined by Western blotting and reverse fibrin zymography, respectively (Figure 1D-E), were detected in ischemic muscle tissues from HL ischemia-induced mice compared with nonischemic controls. These results demonstrated that ischemia increased ischemic muscle PAI-1 activity and simultaneously augmented local fibrinolytic activity.
PAI-1 inhibition improves HL-ischemic tissue regeneration. (A-E) C57BL/6 mice were HL treated and gastrocnemius muscles were analyzed on day 1. (A-C) Quantitative RT-PCR analysis of the mRNA expression of PAI-1 (A), uPA (B), and tPA (C) in nonischemic and HL-ischemic muscle tissue using β-actin as an internal control (n = 3/group for all experiments). (D-E) Homogenates of ischemic and nonischemic tissues that were harvested on day 1 after HL-ischemia induction in 3 different C57BL/6 mice were assayed for murine PAI-1 protein by Western blot analysis (D) or for PAI activity by reverse fibrin zymography (E). Densitometric analysis is shown (bottom). (F-N) HL ischemia was induced in C57BL/6 mice, followed by oral administration of the PAI-1 inhibitor or vehicle given daily from days 0-6. Arrows indicate when the PAI inhibitor was administered. Plasma levels of active PAI-1 (F), tPA (G), and plasmin (H) were assayed in PAI-1– or vehicle-treated HL-ischemic mice by ELISA (n = 7/group for PAI-1 and tPA; n = 6/group for plasmin). (I-J) Representative macroscopic images (I) and the limb perfusion ratio (ischemic/nonischemic; J) of ischemic limbs after HL-ischemia induction. Macroscopic evaluation of the limbs on day 21 (I, bottom) shows foot-digit necrosis only in HL + vehicle-treated animals (n = 5/group). (K-N) Muscle sections of a nonischemic limb and of gastrocnemius (K-L) and hamstring muscles (M-N) of the ischemic limbs from treated mice were stained with H&E (after 21 days; scale bars, 200 mm; K,M) and necrotic areas were evaluated (n = 4 for vehicle group; n = 3 for PAI-1 inhibitor group; L,N). Data represent means ± SEM. *P < .05; **P < .001.
PAI-1 inhibition improves HL-ischemic tissue regeneration. (A-E) C57BL/6 mice were HL treated and gastrocnemius muscles were analyzed on day 1. (A-C) Quantitative RT-PCR analysis of the mRNA expression of PAI-1 (A), uPA (B), and tPA (C) in nonischemic and HL-ischemic muscle tissue using β-actin as an internal control (n = 3/group for all experiments). (D-E) Homogenates of ischemic and nonischemic tissues that were harvested on day 1 after HL-ischemia induction in 3 different C57BL/6 mice were assayed for murine PAI-1 protein by Western blot analysis (D) or for PAI activity by reverse fibrin zymography (E). Densitometric analysis is shown (bottom). (F-N) HL ischemia was induced in C57BL/6 mice, followed by oral administration of the PAI-1 inhibitor or vehicle given daily from days 0-6. Arrows indicate when the PAI inhibitor was administered. Plasma levels of active PAI-1 (F), tPA (G), and plasmin (H) were assayed in PAI-1– or vehicle-treated HL-ischemic mice by ELISA (n = 7/group for PAI-1 and tPA; n = 6/group for plasmin). (I-J) Representative macroscopic images (I) and the limb perfusion ratio (ischemic/nonischemic; J) of ischemic limbs after HL-ischemia induction. Macroscopic evaluation of the limbs on day 21 (I, bottom) shows foot-digit necrosis only in HL + vehicle-treated animals (n = 5/group). (K-N) Muscle sections of a nonischemic limb and of gastrocnemius (K-L) and hamstring muscles (M-N) of the ischemic limbs from treated mice were stained with H&E (after 21 days; scale bars, 200 mm; K,M) and necrotic areas were evaluated (n = 4 for vehicle group; n = 3 for PAI-1 inhibitor group; L,N). Data represent means ± SEM. *P < .05; **P < .001.
We next evaluated the effects of administration of the PAI-I inhibitor TM5275 on fibrinolytic factor release into the circulation. The PAI-1 inhibitor TM5275 (hereafter referred to as the PAI-I inhibitor) is an effective novel oral drug that inhibits PAI-1 by preventing binding of PAI-1 to tPA.18 Both active and latent forms of PAI-1 can circulate.20 PAI-1 inhibits tPA and uPA and PAI-1 is usually present in excess over tPA in plasma. In the present study, PAI-1 inhibitor treatment administered daily from days 0-6 blocked the systemic increase in active PAI-1 in plasma (Figure 1F) and augmented plasma tPA (Figure 1G) and plasmin levels during HL-ischemic recovery (Figure 1H). When the PAI inhibitor treatment was suspended, plasma tPA and plasmin levels returned gradually to baseline levels in 14-21 days. These data indicate that PAI inhibition during ischemic recovery creates a fibrinolytic state.
Pharmacologic targeting of PAI-1 promotes ischemic revascularization and tissue regeneration.
We next determined the consequences of PAI-1 inhibition for tissue regeneration after HL ischemia induction by treating ischemia-induced C57BL6/C mice with the PAI-1 inhibitor or vehicle control. Foot digit necrosis was prevented in PAI-1 inhibitor-treated animals (Figure 1I). The ischemic limb of PAI-1 inhibitor-treated mice displayed faster perfusion recovery, as determined by laser Doppler perfusion image analysis, than vehicle-treated controls (Figure 1I-J). Smaller areas of necrosis were detected in histochemically stained ischemic muscle tissue sections of the lower and upper limb (ie, the gastrocnemius and hamstring muscles) from PAI-inhibitor treated than from vehicle-treated mice (Figure 1K-N). These data demonstrate that PAI inhibition accelerates ischemic tissue recovery.
Endogenous tPA and MMP-9 are required for the tissue-regenerative effects observed after PAI inhibition
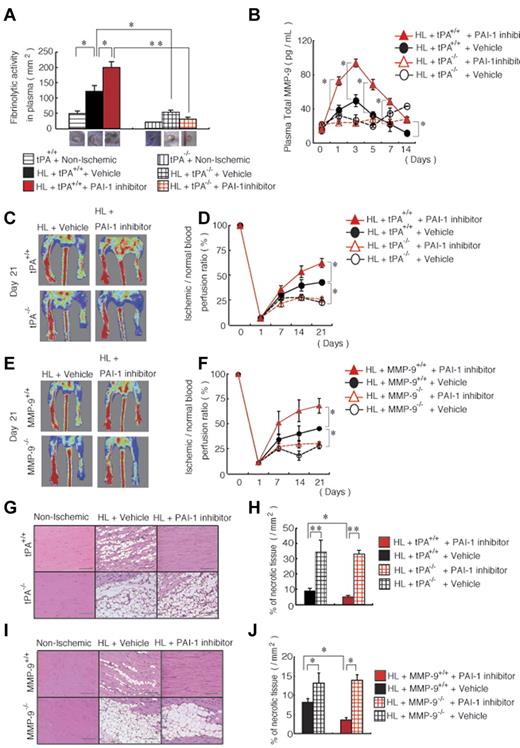
We reported previously that the fibrinolytic factor tPA promotes angiogenesis and tissue regeneration in HL-ischemic tissue, which requires the up-regulation of MMP-9.19 In the present study, we found that PAI-1 inhibitor treatment during ischemic recovery augmented fibrinolytic activity in blood samples from tPA+/+ mice, but not from tPA−/− mice (Figure 2A), and augmented MMP-9 plasma levels during HL-ischemic recovery in tPA+/+ mice compared with vehicle-treated tPA+/+ mice, suggesting that increased tPA and MMP-9 may mediate the observed effects of PAI-I (Figure 2B). No change in MMP-9 plasma levels was observed in HL-ischemia–induced tPA−/− mice treated with vehicle or with the PAI-1 inhibitor (Figure 2B).
Improved ischemic tissue regeneration after PAI-1 inhibitor treatment depends on tPA and MMP-9. (A-J) HL ischemia was induced in tPA+/+, tPA−/−, MMP-9+/+, and MMP-9−/− mice and the mice were then treated with or without PAI-1 inhibitor daily from days 0-6. (A) Fibrinolytic activity in plasma samples of HL-ischemia–induced tPA+/+ and tPA−/− mice was analyzed on day 1 using a fibrin plate assay (n = 3/group). (B) MMP-9 plasma levels were determined in tPA+/+ and tPA−/− mice treated with the PAI-1 inhibitor or with vehicle by ELISA (n = 9 for tPA+/+ mice; n = 3 for tPA−/− mice). (C,E) Representative images of limb perfusion analyzed using a laser Doppler. (D,F) The limb perfusion ratio (ischemic/nonischemic) over time of tPA+/+ and tPA−/− mice (D) and MMP-9+/+ and MMP-9−/− mice (F) treated with the PAI-1 inhibitor or with vehicle (n = 3/group). (G-J) Necrotic areas in sections of H&E-stained muscle sections (G,I) from untreated and PAI-1 inhibitor-treated ischemic limbs (scale bars, 200 mm). (H,J) Necrotic areas in ischemic muscle tissue sections were evaluated after 21 days (n = 3/group). Data represent means ± SEM. *P < .05; **P < .01.
Improved ischemic tissue regeneration after PAI-1 inhibitor treatment depends on tPA and MMP-9. (A-J) HL ischemia was induced in tPA+/+, tPA−/−, MMP-9+/+, and MMP-9−/− mice and the mice were then treated with or without PAI-1 inhibitor daily from days 0-6. (A) Fibrinolytic activity in plasma samples of HL-ischemia–induced tPA+/+ and tPA−/− mice was analyzed on day 1 using a fibrin plate assay (n = 3/group). (B) MMP-9 plasma levels were determined in tPA+/+ and tPA−/− mice treated with the PAI-1 inhibitor or with vehicle by ELISA (n = 9 for tPA+/+ mice; n = 3 for tPA−/− mice). (C,E) Representative images of limb perfusion analyzed using a laser Doppler. (D,F) The limb perfusion ratio (ischemic/nonischemic) over time of tPA+/+ and tPA−/− mice (D) and MMP-9+/+ and MMP-9−/− mice (F) treated with the PAI-1 inhibitor or with vehicle (n = 3/group). (G-J) Necrotic areas in sections of H&E-stained muscle sections (G,I) from untreated and PAI-1 inhibitor-treated ischemic limbs (scale bars, 200 mm). (H,J) Necrotic areas in ischemic muscle tissue sections were evaluated after 21 days (n = 3/group). Data represent means ± SEM. *P < .05; **P < .01.
We next determined whether endogenous tPA and MMP-9 mediate the improved tissue regeneration that is observed after PAI-1 inhibitor treatment. Indeed, PAI-1 inhibitor treatment resulted in faster blood flow recovery in the ischemic limbs of wild-type mice compared with vehicle-treated wild-type mice, but such enhancement was not observed in tPA−/− mice or MMP-9−/− mice (Figure 2C-F). Ischemic tissue sections from PAI-1 inhibitor-treated tPA−/− and MMP-9−/− mice showed vast areas of necrotic tissue compared with PAI-1 inhibitor-treated tPA+/+ and MMP-9+/+ mice (Figure 2G-J). These data indicate that endogenous tPA and MMP-9 are required for the tissue-regeneration-promoting effects of PAI-1 inhibition.
PAI-1 inhibition improves tPA-dependent ischemic revascularization
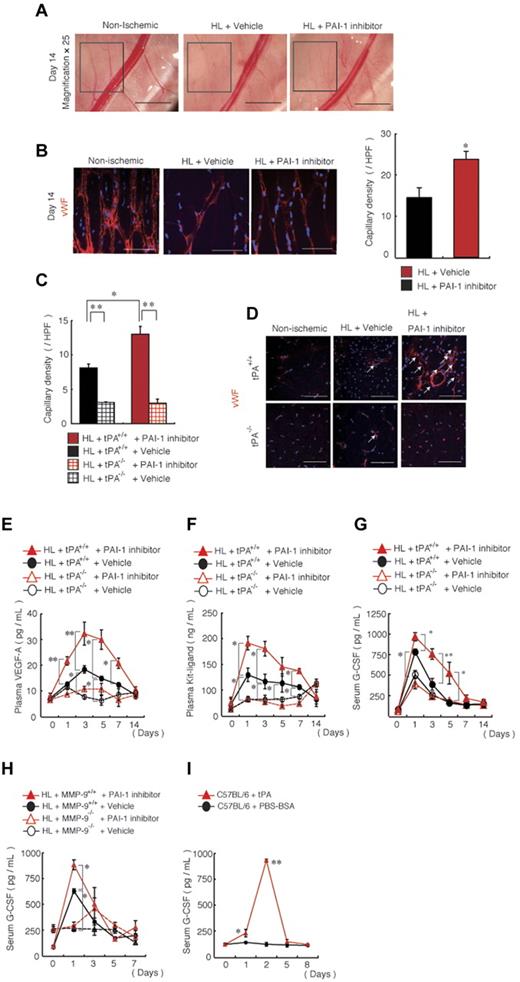
PAI-1 inhibitor treatment resulted in faster collateral vessel growth, which was observed macroscopically (Figure 3A) and microscopically after VWF staining in the ischemic limb of C57BL/6 mice (Figure 3B) and in the ischemic limb of tPA+/+ mice compared with vehicle-treated mice (Figure 3C-D), but did not show such enhancement in the ischemic limb of tPA−/− mice. We have shown previously that tPA treatment increases VEGF-A plasma levels and that tPA administration can promote myeloid-cell expansion by MMP-9–mediated release of KitL from stromal/niche cells.19 Indeed, augmented plasma levels of VEGF-A (Figure 3E) and KitL (Figure 3F) were found in PAI-1 inhibitor-treated HL-ischemic tPA+/+ mice, but not in PAI-1 inhibitor-treated tPA−/− mice. Furthermore, G-CSF, a cytokine known to stimulate BM granulopoiesis, was also increased after PAI-1 inhibitor treatment in HL-ischemic mice in a MMP-9– and tPA-dependent manner (Figure 3G-H). In addition, recombinant tPA administration augmented G-CSF serum levels in normoxic C57BL/6 mice (Figure 3I). These data indicate that PAI-1 inhibition during ischemic recovery augments both angiogenic and hematopoietic factors.
In vivo blockade of PAI-1 augments neoangiogenesis and growth factor release. (A) Macroscopic images of the lower limb region of nonischemic and PAI-1 inhibitor- or vehicle-treated wild-type mice were captured on day 14 after HL ischemia induction (magnification, 25×; scale bars, 2000 mm). The insert box depicts areas of neoangiogenesis. (B-G) HL ischemia was induced in C57BL/6, tPA+/+, and tPA−/− mice, and the mice were then treated with or without PAI-1 inhibitor daily from days 0-6. (B-C) Capillary density was measured in sections of the hamstring (B) and adductor muscles (C) based on immunohistochemical staining of VWF per high power field (HPF). (B,D) Representative images of anti-VWF mAb immunohistochemical staining of ischemic muscle sections from HL-ischemia–induced C57BL/6, tPA+/+, and tPA−/− mice either left untreated or treated with or without the PAI-1 inhibitor (n = 6/group) analyzed on day 14 after the procedure (scale bars, 200 mm). Arrows depict VWF+ capillaries. (E-G) Plasma levels of VEGF-A (E) and KitL (F) and serum levels of G-CSF (G) in HL-ischemia–induced tPA+/+ and tPA−/− mice treated with or without PAI-1 inhibitor were determined by ELISA (for VEGF-A, n = 9 for tPA+/+ mice and n = 3 for tPA−/− mice; for KitL and G-CSF, n = 7 for tPA+/+ mice and n = 3 for tPA−/− mice; for KitL, n = 3 for G-CSF). (H) G-CSF serum levels were analyzed by ELISA in HL-ischemia–induced MMP-9+/+ and MMP-9−/− mice treated with or without PAI-1 inhibitor (H) and in C57BL/6 mice treated with a serpin-resistant tPA mutant (n = 4-5/group). Values represent the means ± SEM. *P < .05; **P < .001.
In vivo blockade of PAI-1 augments neoangiogenesis and growth factor release. (A) Macroscopic images of the lower limb region of nonischemic and PAI-1 inhibitor- or vehicle-treated wild-type mice were captured on day 14 after HL ischemia induction (magnification, 25×; scale bars, 2000 mm). The insert box depicts areas of neoangiogenesis. (B-G) HL ischemia was induced in C57BL/6, tPA+/+, and tPA−/− mice, and the mice were then treated with or without PAI-1 inhibitor daily from days 0-6. (B-C) Capillary density was measured in sections of the hamstring (B) and adductor muscles (C) based on immunohistochemical staining of VWF per high power field (HPF). (B,D) Representative images of anti-VWF mAb immunohistochemical staining of ischemic muscle sections from HL-ischemia–induced C57BL/6, tPA+/+, and tPA−/− mice either left untreated or treated with or without the PAI-1 inhibitor (n = 6/group) analyzed on day 14 after the procedure (scale bars, 200 mm). Arrows depict VWF+ capillaries. (E-G) Plasma levels of VEGF-A (E) and KitL (F) and serum levels of G-CSF (G) in HL-ischemia–induced tPA+/+ and tPA−/− mice treated with or without PAI-1 inhibitor were determined by ELISA (for VEGF-A, n = 9 for tPA+/+ mice and n = 3 for tPA−/− mice; for KitL and G-CSF, n = 7 for tPA+/+ mice and n = 3 for tPA−/− mice; for KitL, n = 3 for G-CSF). (H) G-CSF serum levels were analyzed by ELISA in HL-ischemia–induced MMP-9+/+ and MMP-9−/− mice treated with or without PAI-1 inhibitor (H) and in C57BL/6 mice treated with a serpin-resistant tPA mutant (n = 4-5/group). Values represent the means ± SEM. *P < .05; **P < .001.
PAI-1 inhibition mobilizes neutrophils into the circulation and promotes neutrophil recruitment into ischemic tissues in vivo
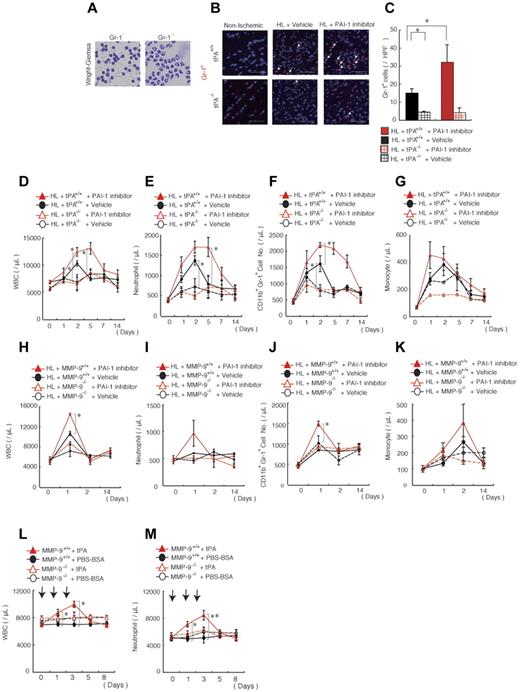
The PAI-1 inhibitor-mediated increase in hematopoietic cytokines prompted us to examine whether the inflammatory response during ischemic recovery might be altered after PAI-1 inhibition. Isolation of leukocytes from ischemic muscle tissues, followed by MACS separation using the anti–Gr-1 Ab, revealed that approximately 40% of infiltrating leukocytes were neutrophils on day 5 of HL ischemia (Figure 4A). PAI-1 inhibitor treatment increased the number of Gr-1+ neutrophils in ischemic sections of tPA+/+ mice, but not in tPA−/− mice, compared with vehicle-treated mice (Figure 4B-C).
Pharmacologic PAI inhibition mobilizes neutrophils into the circulation and improves their tissue infiltration, a process dependent on endogenous tPA and MMP-9. (A) Wright-Giemsa staining of MACS-isolated infiltrating Gr-1+ and Gr-1− cells derived from ischemic tissues of C57BL/6 mice on day 5 after HL induction. (B) Immunofluorescent staining of Gr-1 was performed on nonischemic muscle tissues or on HL-ischemic muscle tissues derived from vehicle- or PAI-1 inhibitor-treated HL-ischemic tPA+/+ and tPA−/− mice 14 days after the HL procedure. PAI-1 inhibitor was administered daily on days 0-6 after the procedure. The arrows indicate Gr-1+ cells (scale bars, 200 mm). Nuclei were counterstained with DAPI (blue). (C) Quantification of Gr-1+ cells in ischemic muscle tissues (n = 3/group). (D-K) The total number of WBCs (D,H) and the number of neutrophils (E,I), CD11b+Gr-1+ cells (F,J), and monocytes (G,K) were determined in the peripheral blood of PAI-1 inhibitor-treated or vehicle-treated tPA+/+ and tPA−/− mice (for B-G, n = 4) and in MMP-9+/+ and MMP-9−/− mice (for H-K, n = 6) by counting (D,E,G,H,I,K) or by FACS analysis (F,J). (L-M) The total number of WBCs (L) and neutrophils (M) were counted in in MMP-9+/+ and MMP-9−/− mice (n = 4). *P < .05; **P < .001 for recombinant tPA-treated versus vehicle-treated C57BL/6 mice. Values represent the means ± SEM. Data are expressed as the absolute number of each cell type per milliliter of blood. *P < .05.
Pharmacologic PAI inhibition mobilizes neutrophils into the circulation and improves their tissue infiltration, a process dependent on endogenous tPA and MMP-9. (A) Wright-Giemsa staining of MACS-isolated infiltrating Gr-1+ and Gr-1− cells derived from ischemic tissues of C57BL/6 mice on day 5 after HL induction. (B) Immunofluorescent staining of Gr-1 was performed on nonischemic muscle tissues or on HL-ischemic muscle tissues derived from vehicle- or PAI-1 inhibitor-treated HL-ischemic tPA+/+ and tPA−/− mice 14 days after the HL procedure. PAI-1 inhibitor was administered daily on days 0-6 after the procedure. The arrows indicate Gr-1+ cells (scale bars, 200 mm). Nuclei were counterstained with DAPI (blue). (C) Quantification of Gr-1+ cells in ischemic muscle tissues (n = 3/group). (D-K) The total number of WBCs (D,H) and the number of neutrophils (E,I), CD11b+Gr-1+ cells (F,J), and monocytes (G,K) were determined in the peripheral blood of PAI-1 inhibitor-treated or vehicle-treated tPA+/+ and tPA−/− mice (for B-G, n = 4) and in MMP-9+/+ and MMP-9−/− mice (for H-K, n = 6) by counting (D,E,G,H,I,K) or by FACS analysis (F,J). (L-M) The total number of WBCs (L) and neutrophils (M) were counted in in MMP-9+/+ and MMP-9−/− mice (n = 4). *P < .05; **P < .001 for recombinant tPA-treated versus vehicle-treated C57BL/6 mice. Values represent the means ± SEM. Data are expressed as the absolute number of each cell type per milliliter of blood. *P < .05.
We next analyzed blood samples to determine whether the augmented neutrophil influx in ischemic tissues was due to an overall increase in circulating blood cells. PAI-1 inhibitor-treated HL-ischemic tPA+/+ mice, but not tPA−/− mice, showed an increase in the number of WBCs, including neutrophils, as determined by cell counting and FACS analysis using Abs against CD11b and Gr-1 (Figure 4D-F), but not monocytes, compared with vehicle-treated animals (Figure 4G). MMP-9 deficiency prevented the leukocyte and neutrophil increase, but not the monocyte increase, caused by PAI-1 inhibitor treatment (Figure 4H-K), indicating that PAI-1 inhibitor-mediated neutrophilia was dependent on endogenous MMP-9. Administration of recombinant tPA induced neutrophilia and, similar to PAI-1 inhibitor administration, this process required endogenous MMP-9 (Figure 4L-M). Therefore, PAI-1 inhibitor treatment not only augmented the absolute number of circulating Gr-1+ cells/neutrophils, but also improved their incorporation into ischemic tissues in a tPA- and MMP-9–dependent manner.
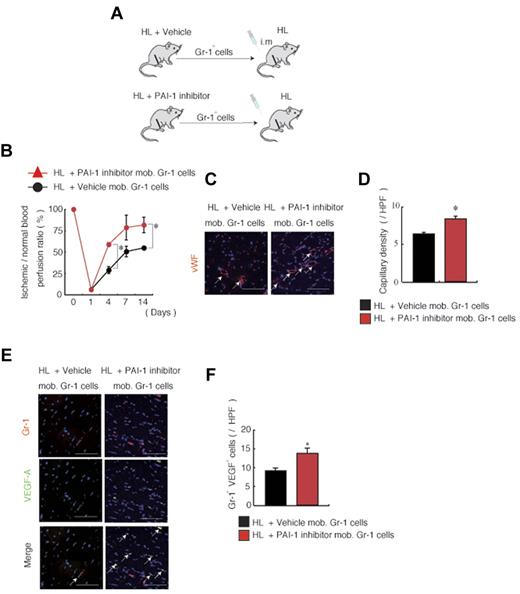
Adoptive transfer of Gr-1+ myeloid cells from PAI-1 inhibitor-treated mice improves revascularization after HL ischemia induction
Our data suggested that neutrophils could be the cellular target for PAI-1 inhibitor-induced improved tissue regeneration. We hypothesized that PAI-1 induction during HL ischemia may alter the ability of neutrophils to stimulate angiogenesis. To test this hypothesis, muscle-derived Gr-1+ cells were obtained from HL-ischemia–induced donor mice that had been treated with or without the PAI-1 inhibitor. These Gr-1+ cells were transplanted into HL recipient mice (Figure 5A) by intramuscular injection. In contrast to cells from vehicle-treated mice, Gr-1+ cells isolated from muscle tissues of PAI-1 inhibitor-treated mice accelerated ischemic reperfusion (Figure 5B) and increased capillary density in ischemic tissues of HL-ischemic recipients (Figure 5C-D). We showed that neutrophils release the proangiogenic factor VEGF-A.21 Consistent with that result, the absolute number of Gr-1+VEGF-1+ cells was higher in ischemic recipient tissues transplanted with Gr-1+ cells from PAI-1–treated mice (Figure 5E-F).
Adoptive transfer of Gr-1+ cells from PAI-1 inhibitor-treated mice improves neoangiogenesis. (A-F) Muscle-derived Gr-1+ cells isolated from HL-ischemia–induced-C57BL/6 donors treated with/without PAI-1 inhibitor were transplanted into HL-ischemia–induced recipients for 3 days (n = 6/group). (A) Experimental scheme of the muscle-derived Gr-1+ cell transplantation assay. (B) Blood flow was determined after transplantation of PAI-1 inhibitor-mobilized versus vehicle-mobilized (mob.) Gr-1+ cells in HL-ischemic C57BL/6 recipients. (C) VWF immunostaining of lower limb ischemic tissue of mice receiving vehicle- or PAI-1 inhibitor-mobilized cell transplantations. Arrows indicate capillaries. Nuclei were counterstained with DAPI (blue staining). Scale bars indicate 200 mm. (D) Capillary density was evaluated per high-power field (HPF). (E) Immunofluorescent staining of Gr-1 and VEGF-A was performed on sections derived from vehicle or PAI-1 inhibitor-mobilized Gr-1 cell-transplanted mice. The arrows indicate transplanted Gr-1+ cells costained with VEGF-A in ischemic tissues. Nuclei were counterstained with DAPI (blue). (F) Quantification of Gr-1+ VEGF-1+ cells under a HPF. Data represent means ± SEM. *P < .05.
Adoptive transfer of Gr-1+ cells from PAI-1 inhibitor-treated mice improves neoangiogenesis. (A-F) Muscle-derived Gr-1+ cells isolated from HL-ischemia–induced-C57BL/6 donors treated with/without PAI-1 inhibitor were transplanted into HL-ischemia–induced recipients for 3 days (n = 6/group). (A) Experimental scheme of the muscle-derived Gr-1+ cell transplantation assay. (B) Blood flow was determined after transplantation of PAI-1 inhibitor-mobilized versus vehicle-mobilized (mob.) Gr-1+ cells in HL-ischemic C57BL/6 recipients. (C) VWF immunostaining of lower limb ischemic tissue of mice receiving vehicle- or PAI-1 inhibitor-mobilized cell transplantations. Arrows indicate capillaries. Nuclei were counterstained with DAPI (blue staining). Scale bars indicate 200 mm. (D) Capillary density was evaluated per high-power field (HPF). (E) Immunofluorescent staining of Gr-1 and VEGF-A was performed on sections derived from vehicle or PAI-1 inhibitor-mobilized Gr-1 cell-transplanted mice. The arrows indicate transplanted Gr-1+ cells costained with VEGF-A in ischemic tissues. Nuclei were counterstained with DAPI (blue). (F) Quantification of Gr-1+ VEGF-1+ cells under a HPF. Data represent means ± SEM. *P < .05.
These data indicate that PAI-1 inhibitor-mediated neoangiogenesis is partially driven by a Gr-1+ muscle-residing cell population.
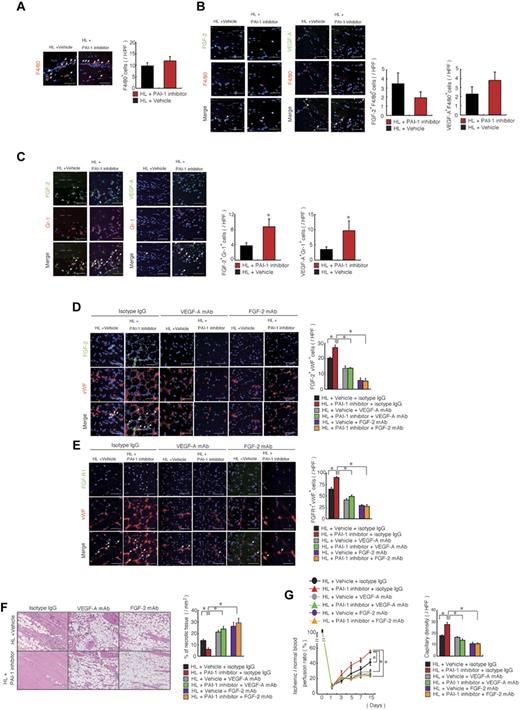
The proangiogenic PAI-1 inhibitor enhances FGF-2 and VEGF-A function/signaling
We reported previously that a serpin-resistant tPA promoted macrophage-mediated angiogenesis.22 To determine whether PAI-1 inhibition accelerates macrophage recruitment into ischemic tissues, we quantified the number of infiltrating F4/80+ cells 3 days after initiation of HL ischemia. PAI-1 inhibition did not increase the recruitment of macrophages in muscle tissues compared with vehicle treatment (Figure 6A).
PAI -inhibition induces angiogenesis during HL-ischemic recovery via FGF-2- and VEGF-A–mediated pathways. (A-C) HL-ischemia–induced C57BL/6 mice were treated with the PAI-1 inhibitor or vehicle. Ischemic sections of PAI-1 inhibitor or vehicle-treated mice 3 days after the HL procedure were costained for F4/80 (A), F4/80 and VEGF-A or F4/80 and FGF-2 (B), or Gr-1 and VEGF-A or Gr-1 and FGF-2 (C). Nuclei were counterstained with DAPI (blue). Left panels are representative immunofluorescent images. Arrows indicate VEGF-A+, FGF-2+, F4/80+, or Gr-1+ cells. Right panel shows the quantification of the indicated cell populations per high-power field (HPF; n = 5/group for each experiment). (D-G) HL-ischemia–induced C57BL/6 mice were treated with the PAI-1 inhibitor and coinjected with neutralizing doses of anti–FGF-2, anti–VEGF-A, or anti-IgG control Abs (n = 4/group). (D-E) Ischemic muscle tissues from Ab-treated animals 14 days after the HL procedure were immunofluorescently costained for FGF-2/VWF and FGF-R1/VWF. Nuclei were counterstained with DAPI (blue staining). Arrows indicate FGF-2+/VWF+ and FGF-R1+/VWF+ cells (scale bars, 200 mm). Right panel shows the indicated cell populations quantified per HPF. (F) Left panel, ischemic muscle tissue sections were stained with H&E (scale bars, 200 mm). Right panel shows the quantification of necrotic areas in ischemic H&E-stained tissue sections. (G) Blood flow was determined at the indicated time points. (H) Ischemic muscle tissue sections stained with Abs against VWF antigen on day 14 were used to determine capillary density. Data represent means ± SEM. *P < .05.
PAI -inhibition induces angiogenesis during HL-ischemic recovery via FGF-2- and VEGF-A–mediated pathways. (A-C) HL-ischemia–induced C57BL/6 mice were treated with the PAI-1 inhibitor or vehicle. Ischemic sections of PAI-1 inhibitor or vehicle-treated mice 3 days after the HL procedure were costained for F4/80 (A), F4/80 and VEGF-A or F4/80 and FGF-2 (B), or Gr-1 and VEGF-A or Gr-1 and FGF-2 (C). Nuclei were counterstained with DAPI (blue). Left panels are representative immunofluorescent images. Arrows indicate VEGF-A+, FGF-2+, F4/80+, or Gr-1+ cells. Right panel shows the quantification of the indicated cell populations per high-power field (HPF; n = 5/group for each experiment). (D-G) HL-ischemia–induced C57BL/6 mice were treated with the PAI-1 inhibitor and coinjected with neutralizing doses of anti–FGF-2, anti–VEGF-A, or anti-IgG control Abs (n = 4/group). (D-E) Ischemic muscle tissues from Ab-treated animals 14 days after the HL procedure were immunofluorescently costained for FGF-2/VWF and FGF-R1/VWF. Nuclei were counterstained with DAPI (blue staining). Arrows indicate FGF-2+/VWF+ and FGF-R1+/VWF+ cells (scale bars, 200 mm). Right panel shows the indicated cell populations quantified per HPF. (F) Left panel, ischemic muscle tissue sections were stained with H&E (scale bars, 200 mm). Right panel shows the quantification of necrotic areas in ischemic H&E-stained tissue sections. (G) Blood flow was determined at the indicated time points. (H) Ischemic muscle tissue sections stained with Abs against VWF antigen on day 14 were used to determine capillary density. Data represent means ± SEM. *P < .05.
To identify the molecular mechanisms underlying the enhanced angiogenesis observed after PAI-1 inhibition, we examined the expression of angiogenesis-related factors in ischemic muscle tissues derived from PAI-1 inhibitor- and vehicle-treated animals. FGF-2 signaling has been associated with neutrophil-mediated angiogenesis23 and PAI-1 activity.22 Immunohistochemical analysis of ischemic muscle tissues demonstrated that the number of F4/80+ cells coexpressing FGF-2 or VEGF-A was not significantly different from vehicle- and PAI-1 inhibitor-treated tissues (Figure 6B). In contrast, the number of ischemic tissue-resident Gr-1+ cells coexpressing both FGF-2 and VEGF-A was higher in sections derived from PAI-1 inhibitor-treated mice (Figure 6C).
FGF-2 can signal through syndecan-4 independently of FGF receptors.22 Therefore, in the present study, we investigated whether expression of FGF-2 or FGFR1 is altered in PAI-1 inhibitor-treated ischemic tissues. Immunohistochemical analysis of ischemic muscle tissue sections revealed that PAI-1 inhibitor treatment augmented FGF-2 and FGF-R1 expression and that this expression colocalized more often to VWF+ cells compared with vehicle-treated controls (Figure 6D-E).
FGF-2–induced angiogenesis requires VEGF signaling.24 Blockade of VEGF-A and FGF-2 signaling with Abs against murine VEGF-A and FGF-2 inhibited the PAI-1 inhibitor-mediated FGF-2 and FGFR-1 increase on VWF+ cells (Figure 6D-E), as well as the PAI-1 inhibitor-mediated ischemic tissue recovery, and reversed the necrosis-reducing effect of PAI-1 inhibitor treatment (Figure 6F-G) and myeloid cell mobilization (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) in an HL-ischemic model.
Although it is clear that FGF-2–induced angiogenesis requires VEGF signaling,24 it was unclear whether VEGF-A and FGF-2 signaling are required for PAI-1 inhibitor-mediated tissue neoangiogenesis. Our present data suggest that this might be the case, because VEGF-A and FGF-2 mAb prevented PAI-1 inhibitor-mediated ischemic tissue recovery and neoangiogenesis in an HL-ischemic model (Figure 6F-G).
The results of the present study indicate that the proangiogenic effects observed after PAI-1 inhibitor treatment are mediated by the 2 potent proangiogeneic factors FGF-2 and VEGF-A, and that neutrophils seem to be a source for these growth factors. In addition, both factors were essential for the PAI-1 inhibitor-mediated recruitment of “angiogenic hematopoietic effector cells” into ischemic tissues.
Discussion
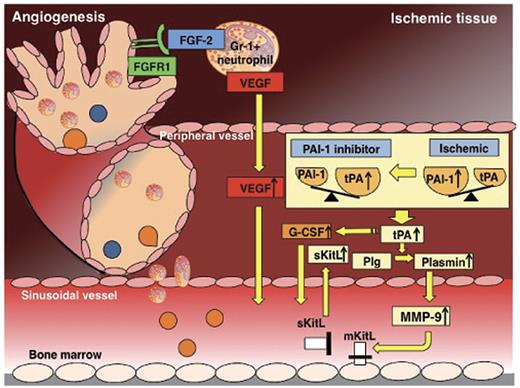
The present study identifies activation of the FGF-2 and VEGF-A pathways as the cause of the proangiogeneic effect of drug-induced PAI-1 deficiency in a murine model of HL ischemia. Our data support a mechanism whereby drug-induced PAI-1 inhibition enhances angiogenesis through up-regulation of endogenous tPA and MMP-9. Both proteases are required for neutrophil mobilization and for the release of proangiogenic cytokines, including FGF-2 and VEGF-A (Figure 7). In addition, muscle-infiltrating CD11b+Gr-1+ neutrophils harvested from PAI-1 inhibitor-treated mice coexpressed FGF-2 and VEGF-A and showed an improved capacity to stimulate angiogenesis on adoptive transfer compared with equal numbers of carrier-treated ischemic tissue-derived neutrophils. Ab-blocking experiments revealed that PAI-inhibitor–induced tissue regeneration required FGF-2 and VEGF-A signaling. These data are important for the design of future cell-based therapies, especially in the light of a recent study demonstrating that BM cells harvested from mice with distant ischemia show a reduced capacity to stimulate angiogenesis on adoptive transfer.25
Schematic diagram showing the various molecules involved in the proangiogenic effect of PAI-1 inhibition. Under ischemic conditions, the local balance between the fibrinolytic factor tPA and one of its endogenous inhibitors, PAI-1, is shifted toward a profibrinolytic state with a local increase in tPA. Ischemia systemically results in a profibrinolytic state, a process dependent on endogenous tPA. Pharmacologic PAI-1 inhibition during ischemic recovery improved tissue regeneration due to an expansion of circulating and tissue-resident Gr-1+ neutrophils coexpressing VEGF-A, FGF-2, and TIMP-1–free MMP-9, and to increased release of the angiogenic factor VEGF-A, the hematopoietic growth factor KitL, and G-CSF. Ab neutralization and genetic-knockout studies indicated that both the improved tissue regeneration and the increase in both circulating and ischemic tissue-resident Gr-1+ neutrophils were dependent on the activation of tPA and MMP-9 and on VEGF-A and FGF-2.
Schematic diagram showing the various molecules involved in the proangiogenic effect of PAI-1 inhibition. Under ischemic conditions, the local balance between the fibrinolytic factor tPA and one of its endogenous inhibitors, PAI-1, is shifted toward a profibrinolytic state with a local increase in tPA. Ischemia systemically results in a profibrinolytic state, a process dependent on endogenous tPA. Pharmacologic PAI-1 inhibition during ischemic recovery improved tissue regeneration due to an expansion of circulating and tissue-resident Gr-1+ neutrophils coexpressing VEGF-A, FGF-2, and TIMP-1–free MMP-9, and to increased release of the angiogenic factor VEGF-A, the hematopoietic growth factor KitL, and G-CSF. Ab neutralization and genetic-knockout studies indicated that both the improved tissue regeneration and the increase in both circulating and ischemic tissue-resident Gr-1+ neutrophils were dependent on the activation of tPA and MMP-9 and on VEGF-A and FGF-2.
FGF family members and its receptors (FGFRs) can promote angiogenesis. In the present study, PAI-1 inhibition-induced augmentation of FGFR1 expression on endothelial cells in ischemic tissues coincided with increased KitL plasma levels. This result is consistent with a previous study showing that FGFR-1–deficient embryoid bodies show decreased expression of KitL.26 KitL can improve tissue recovery in animal models of HL ischemia.27–29 Recombinant tPA therapy augments circulating KitL levels30 and promotes ischemic revascularization.19 Confirming these data, in the present study, we found that drug-induced PAI-1 inhibition raised KitL plasma levels via tPA augmentation. Our data imply a relationship among the fibrinolytic factors PAI-1/tPA, FGFR1 signaling, and KitL production. However, further studies are required to determine how these pathways interact with each other.
PAI-1 in cooperation with integrins, coagulation, fibrinolysis, and endocytosis has been shown to be important for macrophage migration.7 In the present study, we show that pharmacologic blockade of PAI-1 increased MMP-9– and tPA-dependent augmentation of tissue-residing neutrophils, but not F4/80+ macrophages, under ischemic conditions. Therefore, PAI-1 seems to act as a negative regulator of neutrophil recruitment during HL-ischemic recovery. Supporting our observations, Renckens et al demonstrated that PAI-1 gene-deficient mice showed an enhanced early influx of neutrophils to the site of inflammation in a murine model of turpentine-induced tissue injury.31 In this model, no difference was found between PAI-1−/− and PAI-1+/+ mice in factors known to attract neutrophils, including keratinocyte-derived chemokine and macrophage inflammatory protein-2.
Among the factors that can enhance the survival, proliferation, differentiation, and function of neutrophil precursors and mature neutrophils32 is the hematopoietic growth factor G-CSF. In the present study, we demonstrate for the first time that recombinant tPA and endogenous tPA that is enhanced by PAI-1 inhibition promote the release of G-CSF. G-CSF has been shown to improve tissue recovery in animal models of HL21 and myocardial33 and focal cerebral ischemia injuries in both mice and humans34 by modulating various cell types, including endothelial cells and neutrophils.21,32 Various studies have demonstrated the importance of MMP-9 for neutrophil-driven neoangiogenesis in an HL-ischemic model.2,19,21,35 A recent study demonstrated that tissue-infiltrating neutrophil pro-MMP-9 induces angiogenesis catalytically via an FGF-2/FGFR2 pathway.23 Consistent with that study, we have shown previously that pharmacologic PAI-1 inhibition results in the accumulation of FGF-2– and VEGF-A–expressing Gr-1+ neutrophils within ischemic muscle tissues through an effect on endogenous tPA and MMP-9, and in an increase of plasma VEGF-A via up-regulation of endogenous tPA.19 Neutrophils can secrete tissue inhibitors of metalloproteinase (TIMP)–free MMP-9 that can act in concert with, for example, macrophages to liberate proangiogenic growth factors such as VEGF and FGF-2 that are sequestered to the extracellular matrix.
PAI-1 can inhibit cell adhesion and migration by inhibiting the activity of uPA receptor (uPAR)–bound uPA and by preventing integrin association to vitronectin. Studies with uPAR−/− mice have emphasized the critical role of this receptor in leukocyte trafficking.31 Indeed, uPAR−/− mice displayed a profoundly reduced neutrophil recruitment to the peritoneal cavity after IP administration of thioglycollate.36 Our present results are consistent with the findings that neutrophil extravasation into the interstitium after lung ischemia-reperfusion injury after lung transplantation was blocked in tPA-deficient mice.37 At the molecular level, this blockage was associated with reduced expression of platelet endothelial cell adhesion molecule-1 mediated through the tPA/low-density lipoprotein receptor–related protein/NF-κB signaling pathway.
Reichel et al showed that extravasated plasmin(ogen) mediates neutrophil recruitment in vivo via activation of perivascular mast cells and secondary generation of lipid mediators.38
The combined data suggest that strategies aimed at inactivation of PAI-1 (eg, the use of the small-molecule TM5275) could be an immediately clinically applicable therapeutic option for improving angiogenesis in ischemic patients. The results of the present study shed new light on the mechanism by which PAI-1 and tPA enhance neovascularization by modulation of the local and systemic growth factor environment and by alteration of neutrophil migration.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the FACS core facility at the Institute of Medical Science, University of Tokyo (Tokyo, Japan), for their help.
This work was supported by the Japan Society for the Promotion of Science; Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT; to K.H. and B.H.); a Grant-in-Aid for Scientific Research on Priority Areas from MEXT (to K.H.); the Mitsubishi Pharma Research Foundation (to K.H); a Grant-in-Aid for Scientific Research on Innovative Areas from MEXT (B.H.); the SENSHIN Medical Research Foundation (K.H.); Kyowa Hakko Kirin Co Ltd; the Daiichi Sankyo Company; and by the Program for Improvement of the Research Environment for Young Researchers (to B.H.) funded by the Special Coordination Funds for Promoting Science and Technology of MEXT, Japan.
Authorship
Contribution: Y.T., C.N., and K.S.-K. designed and performed the experiments, analyzed the data, and wrote the manuscript; M.O.-K. and M.I. designed and performed the experiments; A.S., I.G., H.K., and Y.S. developed the analytical tools; T.D., T.M., and Y.T. provided reagents; K.O., K.S., and H.N. provided technical support and conceptual advice; and B.H. and K.H. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Hattori, Center for Stem Cell Biology and Regenerative Medicine, Institute of Medical Science, University of Tokyo, 4-6-1, Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: khattori@ims.u-tokyo.ac.jp.
References
Author notes
B.H. and K.H. share senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal