Abstract
The extra-embryonic yolk sac (YS) is the first hematopoietic site in the mouse embryo and is thought to generate only primitive erythroid and myeloerythroid progenitor cells before definitive HSC emergence within the embryo on E10.5. Here, we have shown the existence of T cell–restricted progenitors in the E9.5 YS that directly engraft in recipient immunodeficient mice. T-cell progenitors were also produced in vitro from both YS and para-aortic splanchnopleura hemogenic endothelial cells, and these T-cell progenitors repopulated the thymus and differentiated into mature T-cell subsets in vivo on transplantation. Our data confirm that the YS produces T-lineage–restricted progenitors that are available to colonize the thymus and provide new insight into the YS as a definitive hematopoietic site in the mouse embryo.
Introduction
Embryonic stem (ES) or induced pluripotent stem (iPS) cells have been intensively studied to understand the mechanisms regulating stem cell self-renewal and cell lineage specification and differentiation. Because ES-cell differentiation into the hematopoietic lineage mirrors the earliest aspects of normal embryonic development,1 it is important to understand the process of developmental hematopoiesis to anticipate the products of ES or iPS differentiation. The first blood progenitor cells appear in the extra-embryonic yolk sac (YS) on E7.0.2 These nucleated red blood cells express embryonic hemoglobin molecules and are called primitive erythroid progenitors. On E8.25, erythroid progenitor cells that express adult-type hemoglobin molecules appear in the YS and are called definitive erythroid progenitors. Likewise, primitive and definitive myeloid cells and megakaryocytes emerge in distinct waves in the YS.3,4 HSCs, which reconstitute all the blood cell lineages that arise in adult mouse BM emerge at E10.5 in the ventral endothelial lining of the aorta in the aorta-gonad-mesonephros (AGM) region,5,6 followed soon after on E11 in the YS, fetal liver, and placenta.7,8 Later in development, HSCs accumulate in the fetal liver before mobilization and emigration into the BM just before birth. In adult mice, medullary HSCs self-renew and provide homeostatic blood cell production throughout life.
T lymphocytes are produced and matured in the thymus, but, because there are no self-renewing stem cells in the thymus, T lymphopoiesis depends on circulating progenitor cells to continuously replenish the organ with precursors. Which BM hematopoietic progenitors colonize the adult thymus has long been controversial, but recent reports suggest that early T-lineage progenitors (lin−CD44+CD25−CD117+IL-7Rαlo−neg) are the most immature T-cell progenitors found in the murine thymus.9
Fetal T lymphopoiesis is also initiated by the colonization of extrathymic progenitor cells into the thymic anlage at E11.10 The site and tissue of T-lymphoid progenitor emergence remains obscure. Interestingly, T, B, and myeloid lineage–committed progenitor cells, as well as multipotent hematopoietic progenitors (lacking long-term stem cell reconstituting ability), have been detected at E10.5 in the AGM region at a time when HSCs are known to emerge.11,12 Kawamoto et al reported that T-, B-, and myeloid-cell potential detectable in the fetal thymus was derived from lineage-restricted progenitors and not from multipotent progenitors.13 They also reported that IL-7Rα+ cells residing in E11 thymic anlage display a more-restricted potential to differentiate into T, natural killer (NK), and dendritic cells, compared with IL-7Rα− cells residing in the fetal liver and blood, which display T, B, and myeloid multilineage potential.14 Furthermore, they reported that the frequency of T-restricted progenitor cells among the lineage−c-kit+IL-7Rα+ cells in E12.5 and E13.5 fetal liver is higher than the frequency of B-restricted progenitor cells, but these T-restricted progenitor cells decreased, whereas B-restricted progenitor cells increased with advancing gestational age, implying that T-progenitor cells emerge earlier than B-progenitor cells.15 Paired immunoglobulin-like receptor–expressing lin−c-kit+IL-7Rα+ cells in E11 fetal liver have been shown to be the precursor of T, NK, and dendritic cells and candidate prethymic T-cell progenitor cells.16 T-committed progenitors have also been detected in E15.5 fetal blood.17 These results suggest that T lymphoid–committed progenitors (but not multipotent progenitors or HSCs) colonize the fetal thymus at E11. The specific site of origin of these T-committed progenitors has remained elusive.
Because only 1 or 2 HSCs exist in the entire embryo at E10.5-E11,7,8 it is difficult to suggest that the circulating E11 T-committed progenitors that are the first cells to seed the thymus are HSC derived. Thus, T-progenitor cells found in the E10.5 AGM and E11 fetal thymus must have emerged somewhere else before this stage. In this regard, T lymphoid potential emergence before HSC detection has been reported in YS and para-aortic splanchnopleura (P-Sp) tissues. Liu and Auerbach18 reported that E8 and E9 YS cells could produce T cells in vitro, but none could be derived from the embryo proper. Nishikawa et al reported that T-cell potential was present in vascular endothelial–cadherin+ (VE-cad+) hemogenic endothelial cells isolated from the YS and P-Sp.19 By contrast, Godin et al20 and Yokota et al21 detected T-cell potential in E9.5 P-Sp tissues but could not detect T-cell potential in the YS. Given the early onset of the embryonic heartbeat and systemic circulation (E8.25), admixture of cells from one tissue to the other has long complicated the search for the site of origin of cells with lymphoid potential.
To solve this problem, we have begun to examine Ncx1 knockout (Ncx1−/−) mouse embryos, which never display a beating heart and, thus, do not possess circulating cells, for evidence of autonomous lymphoid potential in the YS and P-Sp. We previously reported that YS and P-Sp tissues contain innate B-cell progenitors and display concomitant emergence of autonomous B-cell potential in YS and P-Sp tissues.22 This B-cell potential is restricted to producing innate B-1 and marginal zone B cells on transplantation. In this study, we examined Ncx1−/− embryonic tissues cocultured with OP9-delta like-1 (DL1) stromal cells23 to confirm autonomous T-cell potential in the YS and P-Sp. Likewise, we have also found autonomous T-cell potential in precirculation wild-type (WT; < E8.25 4-6 somite stage) YS and P-Sp cells in coculture with these stromal cells. T-progenitor cells derived from YS and P-Sp in vitro were adoptively transferred into the peritoneal cavity and reconstituted the thymus in neonatal NOD/SCID/IL-2γc−/− (NOG) hosts and differentiated into multiple mature T-cell subsets in the spleen and liver. More importantly, some freshly isolated YS cells of WT and Ncx1−/− embryos reconstituted T cells in recipient mice, suggesting that T-restricted progenitors autonomously developed in the YS at a pre-HSC stage. These results imply that the YS produces the first wave of T-cell progenitors at a pre-HSC stage, which are capable of thymic colonization.
Methods
Mice
Ncx1 heterozygous male mice24 on a C57BL/6 background were mated with Ncx1 heterozygous females for generating timed pregnancies. The embryos were harvested, and somite pair (sp) number was counted to properly stage the embryos as previously described.3,22 The anterior embryo was used for genotyping as described.3 Staged 14-19 sp embryos were used as E9.0-aged embryos and 20-24 sp as E9.5-aged embryos. The YS and P-Sp tissues were isolated and digested with 0.125% collagenase (StemCell Technologies) for 5 minutes, followed by incubation in cell dissociation buffer (Invitrogen) to generate a single-cell suspension. NOD/SCID/IL-2γc−/− neonates were used as recipients for adoptive transfer as previously described.22
In vitro cultures
WT or Ncx1−/− YS and P-Sp cells were plated on confluent OP9-DL1 stromal cells (a gift of Dr Juan Carlos Zuniga-Pflucker, University of Toronto)23 in 6-well plates in induction medium (αMEM, 10% FBS, and 5 × 10−5M 2-mercaptoethanol) supplemented with 50 μ/mL IL-7. Suspended cells were collected throughout the coculture period, and the phenotype of the nonadherent cells was analyzed by flow cytometry.
Limiting dilution analysis
VE-cad+ cells sorted from E9.5 YS and P-Sp were plated in 12-well plates confluent with OP9-DL1 at a cell density of 75, 150, and 300 cells/well. Multiple (8-12) wells were plated for each cell density. After 14-21 days of culture, the contents of each well were collected and stained with anti-CD4 and anti-CD8 Abs. Wells containing > 1% of double positive (DP) cells were considered positive. The frequency of DP-producing cells at 37% of negative wells was calculated according to Poisson analysis.
Transplantation assay
Freshly isolated YS and P-Sp cells or T cells derived from CD45.2 WT or Ncx1−/− YS/P-Sp cocultures on OP9-DL1 were suspended in 25 μL of medium and injected into the peritoneal cavity of sublethally (150 cGy) irradiated NOG neonates (CD45.1; 1-3 days old). Two or 5 weeks after transplantation, thymus, spleen, and liver were harvested, and single-cell suspensions from each tissue were made. These cells were stained with various Abs as described in “Cell surface analysis” to detect donor-derived T-cell lineages.
Cell surface analysis
Commercial Abs (eBioscience) were used and include anti–mouse VE-cad (BV14), CD19 (1D3), B220 (RA3-6B2), AA4.1 (AA4.1), CD31(390), c-kit (2B8), IL-7Ra (A7R34), CD45.1 (A20), CD45.2 (104), CD4 (GK1.5), CD8 (53-6.7), TCRβ (H57-597), TCRγδ (eBioGL3), CD3(145-2C11), FoxP3(150D/E4), CD44(IM7), CD25PC61.5), IL-2 (JES6-5H4), and CD65 (H1.2F3). TCRVγ3 (536) was purchased from BD Pharmingen. These Abs were conjugated with FITC, PE, peridinin chlorophyll protein complex cyanine 5.5 (Cy5.5), PE-Cy7, allophycocyanin (APC), or APC-Cy7 in various combinations, and each were used at concentrations that were titrated before use. TCRVβ diversity was analyzed with the use of a TCRVβ screening panel (BD Pharmingen). APC-conjugated anti–α-galactosylceramide (α-Gal) loaded CD1d tetramer complex (PBS-57/CD1d) was provided from the National Institutes of Health Tetramer Core Facility by request. Flow cytometric detection of the cell surface Ags was performed on an LSR II (BD Biosciences) or FACSCalibur instrument (BD Biosciences) with analysis performed with the use of FlowJo 8.8.4 (TreeStar Inc) or CellQuest Pro Version 5.1.1 (BD Biosciences) software.
Anti-CD3 stimulation and proliferation assay
Splenocytes were harvested from mice that received a transplant and stimulated (5 × 106 cells/mL) with plate-bound anti-CD3 (2 μg/mL) and soluble anti-CD28 (1 μg/mL; BD Pharmingen) for 48 hours. To assess cell proliferation, splenocytes were stained with CFSE before stimulation, according to the manufacturer's protocol (Molecular Probes; no. C34554). For intracellular cytokine staining, cells were stimulated for 44 hours and treated with GolgiPlug (BD Pharmingen) for an additional 4 hours. Stimulated cells were stained with anti–mouse CD4, CD8, CD25, CD45.2, and CD45.1 Abs and fixed, and samples for intracellular cytokine staining were permeabilized for staining with anti–mouse IL-2 Ab (BD Pharmingen).
Results
YS and P-Sp tissues display autonomous T-cell potential in vitro
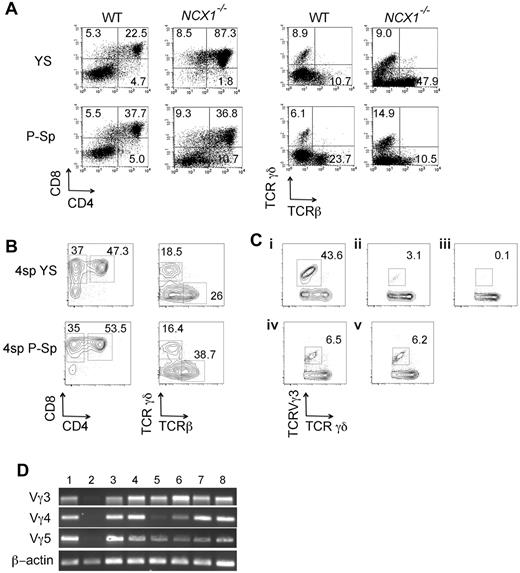
To determine whether the YS and P-Sp can independently generate T cells at a pre-HSC stage, these tissues were dissected from E9.0-E9.5 (14-24 sp) Ncx1−/− and WT embryos and digested into a single-cell suspension. The use of Ncx1−/− embryos lacking circulating blood allowed us to determine the cell autonomous T-cell potential of each tissue. YS and P-Sp cells were separately plated onto a confluent layer of OP9-DL1 stromal cells with added IL-7. After 12-14 days of coculture, large cobblestone area–forming cells beneath the stromal cell layer and a number of floating lymphoid cells were observed. These floating and cobblestone adherent cells were determined to be CD4+CD8+ DP cells, as well as, TCRαβ+ and TCRγδ+ cells and appeared in both Ncx1−/− and WT YS- and P-Sp–derived cocultures (Figure 1A). Interestingly, DP cells emerged in significantly greater numbers in day 11 YS cocultures than in P-Sp cocultures (supplemental Figure 1, supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It is also interesting to mention that CD8 single positive (SP) cells were often detected before DP cells (supplemental Figure 1A), and this kinetic mimics emergence of immature SP cells in the thymus,25 suggesting that this culture system may recapitulate thymic T-cell development. On day18 of coculture, TCRαβ+ cells displayed CD8 SP or DP phenotype, whereas TCRγδ+ cells displayed more restricted double negative (DN) or CD8 SP populations; the latter surface marker expression pattern being similar to that of extrathymic intraepithelial lymphocytes (IELs; supplemental Figure 2). T-cell emergence from precirculation WT (< 4-6 sp) YS and P-Sp tissues was also observed after coculture on the OP9-DL1 stromal cells (Figure 1B). Tissue dissected from the cranial region of 4 sp embryos did not produce any hematopoietic cells when cocultured with OP9-DL1 stromal cells (n = 8; data not shown). Thus, YS as well as P-Sp tissues independently but concomitantly display the potential to generate CD4+CD8+ DP T cells and TCR+ T cells.
YS and P-Sp have autonomous T-cell potential. (A) E9.5 YS and P-Sp cells from WT and Ncx1−/− embryos were plated on OP9-DL1 stromal cells with added IL-7. After 10-14 days of coculture, CD4+CD8+ DP cells as well as TCRβ and TCRγδ+ were produced in each culture. (B) YS and P-Sp cells at 4-sp stage also produced DP as well as CD8+ cells in OP9-DL1 coculture. Representative FACS dot plots are depicted for n = 5 in each YS and P-Sp grouping. (C) TCRVγ3 expression was examined by flow cytometry with (i) E15.5 fetal thymocytes, (ii) cells derived from E14.5 fetal liver lin−c-kit+Sca-1+ (KSL) cells in OP9-DL1 culture, (iii) cells derived from adult BM KSL cells in OP9-DL1 culture, (iv) E9.5 YS-derived cells, and (v) E9.5 P-Sp–derived cells. TCRγδ+ cells were gated for each dot plot analysis. (D) RT-PCR analysis detecting TCR Vγ3, Vγ4, and Vγ5 transcripts. (1) Day 10 neonatal thymocytes, (2) E15.5 thymocytes, and cultured cells in OP9-DL1 derived from (3) 4 sp YS, (4) 4 sp P-Sp, (5) E9.5 YS, (6) E9.5 P-Sp, (7) E14.5 fetal liver KSL, and (8) E15.5 fetal liver common lymphoid progenitor (CLP) cells.
YS and P-Sp have autonomous T-cell potential. (A) E9.5 YS and P-Sp cells from WT and Ncx1−/− embryos were plated on OP9-DL1 stromal cells with added IL-7. After 10-14 days of coculture, CD4+CD8+ DP cells as well as TCRβ and TCRγδ+ were produced in each culture. (B) YS and P-Sp cells at 4-sp stage also produced DP as well as CD8+ cells in OP9-DL1 coculture. Representative FACS dot plots are depicted for n = 5 in each YS and P-Sp grouping. (C) TCRVγ3 expression was examined by flow cytometry with (i) E15.5 fetal thymocytes, (ii) cells derived from E14.5 fetal liver lin−c-kit+Sca-1+ (KSL) cells in OP9-DL1 culture, (iii) cells derived from adult BM KSL cells in OP9-DL1 culture, (iv) E9.5 YS-derived cells, and (v) E9.5 P-Sp–derived cells. TCRγδ+ cells were gated for each dot plot analysis. (D) RT-PCR analysis detecting TCR Vγ3, Vγ4, and Vγ5 transcripts. (1) Day 10 neonatal thymocytes, (2) E15.5 thymocytes, and cultured cells in OP9-DL1 derived from (3) 4 sp YS, (4) 4 sp P-Sp, (5) E9.5 YS, (6) E9.5 P-Sp, (7) E14.5 fetal liver KSL, and (8) E15.5 fetal liver common lymphoid progenitor (CLP) cells.
We also examined TCRVγ3 expression because this is reported to be the first wave of TCR expression found in the fetal thymus.26 YS- and P-Sp–derived TCRγδ+ cells expressed Vγ3 similar to fetal liver–derived T cells (Figure 1C). RT-PCR analysis for Vγ3, Vγ4, and Vγ5 transcripts found that YS-and P-Sp–derived T cells express fetal type Vγ3 and Vγ4 TCR chains, as well as adult type Vγ5 TCR chains,27 similar to the T cells derived from fetal liver KSL and common lymphoid progenitor cells (Figure 1D). These results indicate that YS- and P-Sp–derived TCRγδ+ cells produced not only the first but also later waves of TCRVγ+ cells, similar to fetal liver progenitor cells.
Endothelial cells are the origin of pre-HSC T cells in the mouse embryo
HSCs have been shown to arise from hemogenic endothelial cells in the murine dorsal aorta.28 Several groups have reported that endothelial cells expressing VE-cad or displaying a Tie-2+CD41− phenotype derived from E8.5-E9.5 embryos displayed lymphoid potential.19,21 We isolated VE-cad+CD41− (endothelial) cells and VE-cad-CD41+ (hematopoietic) cells from WT YS and P-Sp cells and plated on OP9-DL1 with added IL-7. Interestingly, CD4+CD8+ DP cells were produced from VE-cad+CD41− endothelial cells but not from VE-cad−CD41+ hematopoietic cells from tissues isolated at 14-24 sp (Figure 2A). After the 26-sp stage of development, DP T cells were produced from both YS and P-Sp VE-cad+CD41− endothelial cells and VE-cad−CD41+ hematopoietic cells (data not shown). This result may reflect the “endothelial-hematopoietic transition” that is known to occur in these tissues during development.22 A limiting dilution assay that used VE-cad+CD41− endothelial cells derived from YS and P-Sp cocultured with OP9-DL1 stromal cells found that as few as 1 in 160 cells produced DP T cells in vitro (Figure 2B).
VE-cad+CD41− hemogenic endothelial cells produce T cells in both YS and P-Sp with similar frequency. (A) VE-cad+CD41− or VE-cad−CD41+ cells were sorted from E9.5 WT YS and P-Sp and were plated on OP9-DL1 stromal cells in a limited dilution manner (75-300 cells/well). After 7-10 days, each well containing cobblestone-forming area and lymphocyte-like cells was analyzed by flow cytometry. (B) CD4+CD8+DP cells were obtained from VE-cad+CD41− cell population, not from VE-cad−CD41+ cells at a frequency of 1 of 162 YS VE-cad+ cells (left panel) and 1 of 165 P-Sp VE-cad+ cells (right panel), respectively.
VE-cad+CD41− hemogenic endothelial cells produce T cells in both YS and P-Sp with similar frequency. (A) VE-cad+CD41− or VE-cad−CD41+ cells were sorted from E9.5 WT YS and P-Sp and were plated on OP9-DL1 stromal cells in a limited dilution manner (75-300 cells/well). After 7-10 days, each well containing cobblestone-forming area and lymphocyte-like cells was analyzed by flow cytometry. (B) CD4+CD8+DP cells were obtained from VE-cad+CD41− cell population, not from VE-cad−CD41+ cells at a frequency of 1 of 162 YS VE-cad+ cells (left panel) and 1 of 165 P-Sp VE-cad+ cells (right panel), respectively.
YS- and P-Sp–derived T cells can transiently reconstitute thymus in vivo
To examine whether T cells generated from YS and P-Sp in vitro can engraft and mature in vivo, we harvested all the cultured cells on day 12 after coculture initiation and injected them into the peritoneal cavity of sublethally irradiated NOG neonates. As a positive control, isolated thymocytes of adult C57BL/6 mice were also injected. Two to 14 weeks after injection, the thymus, spleen, and liver were analyzed for the repopulation by donor cells (Table 1). Although the NOG mice that did not receive a transplant failed to show any evidence of thymic development, the thymus was clearly repopulated with donor T cells in the NOG mice that received a transplant as early as 2 weeks after donor cell injection (Table 1; Figure 3). The recovered donor cell numbers of thymi reconstituted by WT or Ncx1−/− YS/P-Sp–derived T-progenitor cells are depicted in Figure 3A. These thymic cells were 100% donor derived and displayed CD4+ and/or CD8+, with prominent TCRβ+ populations (Figure 3B). Donor T-cell presence in the reconstituted thymus of each host was transient and only persisted for 2-3 weeks, consistent with the known requirement of continuous T-lymphoid progenitor delivery from BM to maintain ongoing lymphopoiesis and the lack of such progenitor cells in the NOG hosts. To examine whether CD4+CD8+ DP and/or DN cells could reconstitute the recipient thymus, DP and DN cells were sorted from YS and P-Sp OP9-DL1 cocultures and injected into NOG neonates. As expected, only the DN population reconstituted the recipient host thymus (supplemental Table 2), although the well-known thymic homing receptor, CCR9,29 was expressed in both DP and DN YS- and P-Sp–derived populations (supplemental Figure 3). A second thymic homing receptor CCR730 was not detected in either population (supplemental Figure 3). Thus, T-cell progenitors derived from YS and P-Sp tissues cocultured on OP9-DL1 stromal cells display thymic-repopulating ability in vivo on transplantation.
YS- and P-Sp–derived T-progenitor cells reconstitute thymus and spleen after injection
| . | No. . | Thymus reconstitution, n/N . | SPL reconstitution, n/N . | CD45.2 in SPL, %, mean ± SD . |
|---|---|---|---|---|
| WT YS | ||||
| 2 wk | 10 | 8/10 | 8/10 | 2.26 ± 1.4 |
| 14 wk | 2 | 0/3 | 2/3 | 15.5 ± 13.0 |
| WT PSP | ||||
| 2 wk | 11 | 9/11 | 9/11 | 2.61 ± 1.5 |
| 14 wk | 3 | 0/3 | 3/3 | 28.9 ± 35.8 |
| NCX1−/− YS | ||||
| 2 wk | 3 | 2/3 | 2/3 | 2 ± 1.1 |
| 4 wk | 1 | 0/1 | 1/1 | 20.9 |
| NCX1−/−PSP | ||||
| 2 wk | 2 | 2/2 | 1/2 | 2.17 |
| 5 wk | 2 | 0/2 | 2/2 | 55.2 ± 4.5 |
| Thymus | ||||
| 2 wk | 1 | 1/1 | 1/1 | 7.58 |
| 4 wk | 1 | 0/1 | 1/1 | 46.3 |
| 32 wk | 1 | 0/1 | 1/1 | 47.5 |
| . | No. . | Thymus reconstitution, n/N . | SPL reconstitution, n/N . | CD45.2 in SPL, %, mean ± SD . |
|---|---|---|---|---|
| WT YS | ||||
| 2 wk | 10 | 8/10 | 8/10 | 2.26 ± 1.4 |
| 14 wk | 2 | 0/3 | 2/3 | 15.5 ± 13.0 |
| WT PSP | ||||
| 2 wk | 11 | 9/11 | 9/11 | 2.61 ± 1.5 |
| 14 wk | 3 | 0/3 | 3/3 | 28.9 ± 35.8 |
| NCX1−/− YS | ||||
| 2 wk | 3 | 2/3 | 2/3 | 2 ± 1.1 |
| 4 wk | 1 | 0/1 | 1/1 | 20.9 |
| NCX1−/−PSP | ||||
| 2 wk | 2 | 2/2 | 1/2 | 2.17 |
| 5 wk | 2 | 0/2 | 2/2 | 55.2 ± 4.5 |
| Thymus | ||||
| 2 wk | 1 | 1/1 | 1/1 | 7.58 |
| 4 wk | 1 | 0/1 | 1/1 | 46.3 |
| 32 wk | 1 | 0/1 | 1/1 | 47.5 |
YS- or P-Sp–derived T-progenitor cells produced in OP9-DL1 coculture were injected into NOG neonates. At different time points after injection, engraftment in the recipient thymus and spleen were analyzed. Donor cell type > 0.1% was considered as reconstituted.
Recipient thymus was transiently reconstituted by YS/P-Sp–derived progenitor cells. (A) YS and P-Sp cells from Ncx1−/− and WT embryos were cocultured with OP9-DL1 for 10-12 days and transplanted into the peritoneal cavity of sublethally irradiated NOG neonates. Two weeks after injection, recipient thymuses were analyzed. Although there was no apparent structural thymus in NOG neonates, mice that received a transplant display an identifiable thymus that contains ∼ 1 × 106 cells. When analyzed at 14 weeks after injection, no thymus was detected in any mice that received a transplant (Table 1). The cell numbers of the thymus from C57BL/6 mice that did not receive a transplant were enumerated as a control. (B) FACS analysis of reconstituted thymus from recipient mice. All the thymocytes were donor derived and consisted of CD4+CD8+ DP cells as well as CD4+ SP and CD8+ SP cells, with TCRβ expression. The number of mice that received a transplant is shown in Table 1.
Recipient thymus was transiently reconstituted by YS/P-Sp–derived progenitor cells. (A) YS and P-Sp cells from Ncx1−/− and WT embryos were cocultured with OP9-DL1 for 10-12 days and transplanted into the peritoneal cavity of sublethally irradiated NOG neonates. Two weeks after injection, recipient thymuses were analyzed. Although there was no apparent structural thymus in NOG neonates, mice that received a transplant display an identifiable thymus that contains ∼ 1 × 106 cells. When analyzed at 14 weeks after injection, no thymus was detected in any mice that received a transplant (Table 1). The cell numbers of the thymus from C57BL/6 mice that did not receive a transplant were enumerated as a control. (B) FACS analysis of reconstituted thymus from recipient mice. All the thymocytes were donor derived and consisted of CD4+CD8+ DP cells as well as CD4+ SP and CD8+ SP cells, with TCRβ expression. The number of mice that received a transplant is shown in Table 1.
YS- and P-Sp–derived T cells differentiate into mature T cells in vivo
Although the host thymic tissue diminished significantly by 4 weeks after donor cell injection, donor-derived cells were dramatically expanded in the host spleen 2-14 weeks after transplantation (Table 1). TCRαβ+CD4+, TCRαβ+CD8+ cells, and CD4+FoxP3+ regulatory T cells were all enumerated in the recipient spleen in animals transplanted with YS- or P-Sp–derived T-progenitor cells (Figure 4A). At 14 weeks after injection, donor CD4+CD62L+CD44dim naive and CD4+CD62L−CD44high memory T-cell populations were also present (Figure 4A). Donor-derived CD3+TCRγδ+ T cells and CD3+α-Gal–loaded-CD1d tetramer+ (αGC/CD1d tet+) NKT cells were also detected in the recipient liver (Figure 4B). Furthermore, donor-derived IEL subsets31 (TCRαβ+ and TCRγδ+CD8+ populations) were also detected (Figure 4C). Of note, the donor-derived CD4/CD8 ratio in the recipient spleen was normal at 2 weeks but declined by 14 weeks after transplantation (supplemental Figure 4), most probably because more CD8+ than CD4+ cells remained in the host reconstituted spleen. This result differs compared with adult thymic cell transplantation, in which reconstituted splenic tissue retains a CD4/CD8 ratio of 1.0 over the short and long term, but it was similar to study results for fetal thymic cell transplantation.32 These results indicated that YS- and P-Sp–derived cells contained early T-lineage–restricted progenitor cells that could engraft the host thymus and differentiate into multiple mature T cells within various lymphoid organs in vivo.
YS- and P-Sp–derived DN T cells can differentiate into distinct T-cell subsets in vivo. Cells from the recipient spleen (A) and liver (B) were analyzed for the presence of mature T-cell populations 2 weeks after transplantation. Memory (CD62LlowCD44high) T cells were analyzed 14 weeks after injection (A right panels, dot plot is CD45.2+CD4+ gated). Regulatory (CD4+FoxP3+) T cells, naive (CD4+CD62LhighCD44low) T cells, and memory (CD4+CD62LlowCD44high) T cells were detected in the recipient spleen (A), and γδT (TCRγδ+CD3+), and α-Gal–loaded CD1d tetramer+ (αGC/CD1d tet+) CD3+ NKT cells were detected in the recipient liver (B). (C) TCRβ+ or TCRγδ+CD8+ intraepithelial T cells were also detected in the intestine of a mouse that received a transplant with P-Sp–derived T cells. ND indicates not determined for YS-derived cells. The representative FACS dot plots are depicted. The number of mice that received a transplant is detailed in Table 1. (D) The TCRVβ repertoires of YS-derived (left) and P-Sp–derived (right) CD3+ T cells engrafted in the recipient spleen are depicted (each n = 3).
YS- and P-Sp–derived DN T cells can differentiate into distinct T-cell subsets in vivo. Cells from the recipient spleen (A) and liver (B) were analyzed for the presence of mature T-cell populations 2 weeks after transplantation. Memory (CD62LlowCD44high) T cells were analyzed 14 weeks after injection (A right panels, dot plot is CD45.2+CD4+ gated). Regulatory (CD4+FoxP3+) T cells, naive (CD4+CD62LhighCD44low) T cells, and memory (CD4+CD62LlowCD44high) T cells were detected in the recipient spleen (A), and γδT (TCRγδ+CD3+), and α-Gal–loaded CD1d tetramer+ (αGC/CD1d tet+) CD3+ NKT cells were detected in the recipient liver (B). (C) TCRβ+ or TCRγδ+CD8+ intraepithelial T cells were also detected in the intestine of a mouse that received a transplant with P-Sp–derived T cells. ND indicates not determined for YS-derived cells. The representative FACS dot plots are depicted. The number of mice that received a transplant is detailed in Table 1. (D) The TCRVβ repertoires of YS-derived (left) and P-Sp–derived (right) CD3+ T cells engrafted in the recipient spleen are depicted (each n = 3).
We also examined the diversity of TCRVβ chains displayed by YS- and P-Sp–derived T cells that had engrafted in recipient splenic tissue (Figure 4D). P-Sp–derived T cells showed a pattern of TCRVβ usage similar to BL/6 adult splenic T cells (Figure 4D right), but YS-derived T cells showed a more-restricted oligoclonal TCRVβ usage pattern (Figure 4D left). Thus, YS- and P-Sp–derived T cells display some molecular differences in T-cell maturation in vivo as reflected in TCRVβ chain usage.
YS- and P-Sp–derived T cells engrafted in the spleen are functional
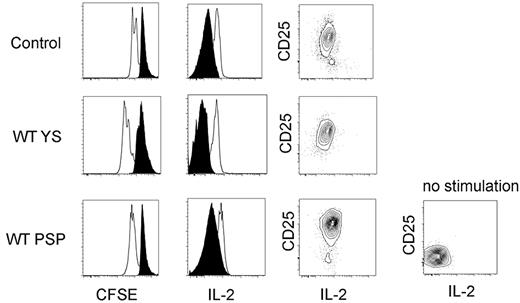
To evaluate the function of donor YS- and P-Sp–derived T cells engrafted in the recipient spleen, we examined proliferation after anti-CD3 stimulation of CFSE-labeled cells (Figure 5 left panel). YS- and P-Sp–derived T cells proliferated similarly to C57BL/6 control spleen cells after anti-CD3 stimulation. CD25 (IL-2Rα chain) expression and intracellular staining of IL-2 were similarly increased after activation (Figure 5 middle and right panels). Thus, both donor YS- and P-Sp–derived CD4+ and CD8+ cells isolated from the recipient spleen were functional and responsive to receptor agonist activation.
YS- and P-Sp–derived T cells engrafted in the recipient spleen are functional. YS- and P-Sp–derived T cells engrafted in the recipient spleen are proliferative on CD3 stimulation and secrete IL-2. Spleen cells from recipient mice 2 weeks after transplantation were plated in RPMI medium with anti-CD3 and anti-CD28 Abs. Donor cell proliferation was analyzed with the use of CFSE staining (left panel: CD45.2+CD4+ gated cells are depicted; CD45.2+CD8+ cells showed a similar proliferation pattern). Nontransplanted BL/6 spleen cells were used as a control. IL-2 secretion was confirmed by intracellular staining (middle panel). CD25 expression was up-regulated in all samples (right panel). Nonstimulated spleen cells were used as a negative control. Filled histogram is at 0 hour, and solid line is at 48 hours after stimulation. YS- and P-Sp–derived spleen cells (n = 5).
YS- and P-Sp–derived T cells engrafted in the recipient spleen are functional. YS- and P-Sp–derived T cells engrafted in the recipient spleen are proliferative on CD3 stimulation and secrete IL-2. Spleen cells from recipient mice 2 weeks after transplantation were plated in RPMI medium with anti-CD3 and anti-CD28 Abs. Donor cell proliferation was analyzed with the use of CFSE staining (left panel: CD45.2+CD4+ gated cells are depicted; CD45.2+CD8+ cells showed a similar proliferation pattern). Nontransplanted BL/6 spleen cells were used as a control. IL-2 secretion was confirmed by intracellular staining (middle panel). CD25 expression was up-regulated in all samples (right panel). Nonstimulated spleen cells were used as a negative control. Filled histogram is at 0 hour, and solid line is at 48 hours after stimulation. YS- and P-Sp–derived spleen cells (n = 5).
E9.5 YS contains T-restricted progenitors
We had previously reported that E9.5 YS and P-Sp contained B-1 progenitor cells by direct transplantation assay.22 In the present studies, 3 NOG recipient mice were engrafted by freshly isolated YS cells (1 WT and 2 Ncx1−/− YS) and displayed only donor T-cell reconstitution that was detectable in the peripheral blood, spleen, and BM as early as 8 weeks (data not shown) and up to 9 months after transplantation (supplemental Table 3; Figure 6A). These donor-derived CD3+ cells in the recipient spleen were TCRβ+ (Figure 6A top right panel). This result indicates that T cell–restricted progenitor cells are fully developed and contained within the E9.5 YS. RT-PCR analysis of whole YS tissue found expression of Delta-1 (thus replicating OP9-DL1 expression), Delta-4, and Notch signaling genes as well as lymphoid transcription factors such as Ikaros and Gata-3 (Figure 6B). These data indicate unequivocally that the YS environment is fully capable of promoting T-cell progenitor development into a thymic-repopulating state in situ and not just a site of T-lymphoid potential that may result from optimized in vitro coculture conditions.
YS contains unipotent T-cell progenitors that can engraft in the NOG neonates. (A) E9.5 YS reconstituted only T cells in the recipient spleen and BM on transplantation. Most of CD3+ cells were TCRβ+. The representative FACS dot plots from a recipient mouse 9 months after transplantation are depicted. The number of mice that received a transplant is shown in supplemental Table 3. (B) RT-PCR was performed with E9.5 WT YS and P-Sp for Notch ligand. The expression of Notch signaling genes (B left panel) and of genes that are important for T lymphoid development (B right panel) are shown.
YS contains unipotent T-cell progenitors that can engraft in the NOG neonates. (A) E9.5 YS reconstituted only T cells in the recipient spleen and BM on transplantation. Most of CD3+ cells were TCRβ+. The representative FACS dot plots from a recipient mouse 9 months after transplantation are depicted. The number of mice that received a transplant is shown in supplemental Table 3. (B) RT-PCR was performed with E9.5 WT YS and P-Sp for Notch ligand. The expression of Notch signaling genes (B left panel) and of genes that are important for T lymphoid development (B right panel) are shown.
Discussion
In this study, using Ncx1−/− embryos that lack systemic blood flow, we have shown 2 important findings that may change the paradigm for understanding the program regulating developmental hematopoiesis in the early mouse embryo. First, E8.25-E9.5 (pre-HSC stage) YS cells as well as P-Sp cells display autonomous T-lymphoid potential derived at high frequency from VE-cad+CD41− hemogenic endothelial cells. Second, and more importantly, T cell–restricted progenitor cells that engraft and repopulate the T-cell lineage in recipient mice functionally emerge in the YS before HSC emergence. These results prove that the YS not only possesses the potential to be a site of T-lymphoid production but actually produces T-lineage–repopulating progenitor cells. The fact that the YS has now been shown to possess autonomous B- and T-cell development in this and prior work,22 as well as to provide all the definitive erythromyeloid progenitor cells that seed the fetal liver,3 clearly resolves the controversy that YS hematopoiesis is multilineage and able to produce definitive myeloid and lymphoid lineages before HSC emergence in the murine embryo.
This raises an intriguing question as to whether these B- and T-progenitor cells are derived directly from hemogenic endothelial cells (HSC independent) or from HSCs that simply cannot be detected by our current transplantation assays (HSC dependent). Because E8.25 YS can give rise to B and T cells in vitro (in coculture with stromal cells lines) 1 day earlier than we can detect neonatal repopulating HSCs,33 and 2 days earlier than adult definitive HSCs emergence,5 one must question whether these lymphoid elements are HSC derived. E9.5 YS contains committed B- and T-progenitor cells that are detectable just one-half day after the first neonatal repopulating HSC detection33 and 1 day earlier than adult repopulating HSC detection in the AGM.5 Considering the time line of stem cell emergence, it is impossible for these extremely rare (1-2) neonatal or adult repopulating HSCs to produce B- or T-restricted progenitors before even being able to be detected by transplantation assays.34 However, Samokhvalov et al have shown that Runx1-expressing cells in the E7.5 YS could be marked and later tracked as B cells in the fetal liver, T cells in the fetal thymus, and HSCs in the adult BM.35 It is intriguing to wonder if the T cells found in the fetal thymus in the studies by Samokhvalov et al could be derivatives of the same YS T-lymphoid progenitor cells that we have detected in the present studies rather than a HSC derivative.
Some additional support exists for YS-derived T-restricted progenitor cell emergence from hemogenic endothelial cells independent from HSCs (or pre-HSCs).36 Murine ES-cell differentiation in vitro mirrors normal murine embryonic development with emergence of definitive progenitor cells from a hemogenic endothelial precursor. Thus, just as the YS developmental hematopoietic progression now is shown from the present work and prior studies, differentiated ES cells first give rise to primitive erythroid cells, followed by definitive erythromyeloid cells, and finally B- and T-lymphoid potential without any obvious HSC activity that can be detected by any currently available transplantation assay.1,37
In some prior work, the YS was reported to lack the potential to form T lymphocytes in vitro, using fetal thymic organ culture or OP9-DL1 cocultures,21,38 whereas another group successfully obtained T lymphocytes from YS tissue with the use of fetal thymic organ culture.18,19 How can these differing study outcomes be rationalized? A detailed examination of these studies and our current results suggest that the method of YS tissue digestion may be a key variable; when mechanically digesting the YS tissue, T-lymphoid potential was not identified, whereas enzymatic digestion of the YS tissue permitted investigators to identify T-lymphoid potential. As previously discussed,36 lymphoid cells are known to be produced from YS hemogenic endothelial cells19,22 that are tightly connected to each other and a basement membrane. Without adequate and optimized collagenase digestion of YS tissue, hemogenic endothelial cells may not be recovered and are lost during sample preparation, extinguishing any opportunity for lymphoid potential detection. Although other more subtle variables may also exist between the present work and prior reports, the present data indicate the presence of T-lineage–repopulating cells residing in freshly dissociated YS tissue.
Some controversy has arisen over the differentiation potential of T-progenitor populations comparing in vitro with in vivo potentials.39 To avoid such controversy, we have transplanted T cells generated in vitro with the use of the OP9-DL1 system, as well as, freshly isolated YS tissue to assay for T cell–repopulating ability. Freshly isolated NCX1−/− and WT YS cells, as well as in vitro–generated YS-derived T cells successfully engrafted and repopulated multiple T-cell lineages in the NOG neonates. This is direct evidence that T-cell progenitors emerge in the YS.
YS/P-Sp–derived donor T-cell progenitors reconstituted the host thymus only for 2 weeks. This observation was compatible with the previous report that the first wave of lymphoid progenitors colonizing the thymus occupied it until the first week after birth (a period of ∼ 2 weeks).40 In addition, 3 months after transplantation, YS- and P-Sp–derived T cells engrafted in the spleen displayed a lower CD4/CD8 ratio than initial levels at 2 weeks (supplemental Figure 4A-B). It seems that, over time after transplantation, donor-derived CD8+ cells persisted longer in the spleen than donor-derived CD4+ cells (supplemental Figure 4C). Usually, in adult thymic or BM cell transplantation, the CD4/CD8 ratio remains ≥ 1.0.32 In contrast, the lower CD4/CD8 ratio shown by YS- and P-Sp–derived T cells is similar to the ratio shown by fetal thymus-derived T-cell transplantation as previously reported.32 Thus, YS- and P-Sp–derived T-cell progenitors behave similar to fetal thymic cells after transplantation.
It is reported that there are 4 waves of T-cell appearance with different TCRVγ usage during fetal thymic development.26 The first CD3+T cells that appear in the E14 thymus express the Vγ3 TCR, but this population is transient and decreases in number by E17. Of interest, Vγ3 TCR+ cells are found only in the skin among peripheral lymphoid organs, expressed by the Thy-1+ dendritic epidermal cells in adult mice and can be produced from fetal liver HSCs in the fetal thymic microenvironment, but not from adult HSCs cultured in fetal or adult thymic environments.41 This first wave of T cells is replaced by TCRγδ-bearing thymocytes, which express Vγ4 and comprise the main population of CD3+ cells at E16-E20 with 2 distinct waves of appearance in the thymus. These Vγ4 TCR+ cells are later distributed in the epithelium of the tongue and of the reproductive organs.42 The fourth wave of TCRαβ-bearing cells appears at ∼ E17 and becomes the main population of CD3+ thymocytes after E19. Interestingly, this TCRαβ complex is detected by the anti-TCRVβ8 Ab,26 which we identified as one of the most frequent Vβ chains used by donor YS-derived T cells found in the recipient spleen (Figure 4C). Because the first T-restricted progenitors found at E11 in the thymic anlage can differentiate into TCRαβ+ cells as well as TCRγδ cells with the use of a variety of Vγ genes (not only Vγ3) in fetal thymic organ culture,14 these cells may produce multiple waves of TCR+ cells. Similarly, our results showing that the YS and P-Sp produce fetal type TCRVγ3 and Vγ4 as well as adult type Vγ527 and αβ cells and that these cells show similar functional behavior to fetal thymocytes on transplantation imply that the YS– and P-Sp–derived T-progenitor cells may contribute to providing some of the initial fetal waves of TCR+ progenitors in the thymus, before derivatives of fetal liver HSCs that appear days later. These results are also congruent with the recent report that fetal and adult T cells display evidence of unique origins, functions, and gene expression patterns during human development.43
Some of the YS- and P-Sp–derived TCRγδ+ subsets appear similar to one of the IEL subsets that develop in an extrathymic environment and can be identified in nude mice or mice that have undergone a thymectomy.31 These TCRγδ+ cells display an unusual Thy-1− surface phenotype and express a CD8 cell surface protein that bears an α chain homo dimer, whereas TCRγδ+ cells in the thymus or peripheral lymphoid organs do not coexpress either CD4 or CD8.31 In contrast, another IEL population expresses TCRαβ and CD8, bearing both α and β chains and is thymus dependent for development. In our YS and P-Sp cocultures with OP9-DL1, we identified TCRγδ+ cells expressing CD8α at a later time course of coculture (supplemental Figure 2) and at least P-Sp–derived T cells repopulated donor-derived IEL in one recipient mouse. Because TCRγδ rearrangement is known to be present in the fetal gut and liver at E11,44 at the same time as T-restricted progenitor emergence in the thymic anlage and near simultaneous fetal liver HSC emergence, one must speculate that these IEL TCRγδ+ cells may be derived from the YS- and/or the P-Sp–derived T-cell progenitors arising from the hemogenic endothelium.
Although we were not able to identify the phenotype (pattern of surface markers) of T-restricted progenitor cells existing in the YS because of its low frequency, the directly engrafting YS-derived T cells were detectable in the recipient mice as long as 9 months after transplantation. This may raise the ensuing question: what is the role of T cells derived from YS and P-Sp before HSC emergence in adult mice? Development of specific marking strategies to determine long-term trafficking of YS and P-Sp T-restricted progenitors will be required to address this point.
In summary, we have identified autonomous T-cell progenitor emergence from hemogenic endothelial cells in the YS and P-Sp and reported that YS-derived T-cell progenitors directly repopulate the thymus of host mice on transplantation. Thus, we propose that YS and/or P-Sp may provide the initial waves of T-cell progenitors that colonize the fetal thymus. These results define the YS as a site of lymphoid, as well as, myeloid lineage development and not simply a site for primitive erythroid development as commonly reported.45 These results have major implications for understanding hematopoietic cell origins from murine and potentially human ES and iPS cells, because the T-lineage cells derived from these pluripotent cells may emerge independent of a HSC precursor.46,47
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Ken Dorshkind and Encarnacion Montecino-Rodriguez for advice on FACS analysis.
This work was supported by the Riley Children's Foundation and the National Institutes of Health (grant R01 AI080759, M.C.Y.; grant R01 AI057459, M.H.K.; and grant P30 DK090948, M.C.Y. and S.J.C.).
National Institutes of Health
Authorship
Contribution: M.Y. designed and performed the experiments, analyzed the data, and wrote the manuscript; P.P. performed the experiments; N.L.G. performed the experiments; S.J.C. contributed vital reagents and edited the manuscript; N.C. provided the intellectual advice; A.A.C. provided the intellectual advice and edited the manuscript; M.H.K. provided intellectual advice and edited the manuscript; and M.C.Y. designed the experiments, provided the intellectual advice, and edited the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mervin C. Yoder, Department of Pediatrics, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St R4-W125, Indianapolis, IN 46202; e-mail: myoder@iupui.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal