In this issue of Blood, Yoshimoto et al show that yolk sac (YS) and para-aortic splanchnopleura (P-Sp) cells, early in development, can generate both adult and fetal type T lymphocytes. The most remarkable conclusion is that T lymphocytes are derived directly from the hemogenic endothelium, before the appearance of hematopoietic stem cells.1
Hematopoietic stem cells (HSCs) are operationally defined by their ability to give rise to all cells of all the hematopoietic lineages. Therefore, it was puzzling that adult BM-derived HSCs did not generate certain B and T lymphocytes subsets, namely B-1 and γδT lymphocytes. B-1 lymphocytes will develop from transplants of fetal liver cells but not from BM HSCs.2 Efficient generation of γδT lymphocytes required both a fetal liver HSC and a fetal host environment.3,4 More sensitive methods for cell isolation and detection later showed that adult HSCs can give rise to low levels of B-1 lymphocytes.5 Similar studies established that only γδT lymphocytes that express the Vγ3 or Vγ4 variable regions of the T-cell receptor needed a fetal HSC. γδT lymphocytes that express other variable regions (Vγ2, Vγ1.1, Vγ5) as well as αβT lymphocytes are generated from adult-type HSCs that first appear around day 16 to 17 of gestation.4 Nevertheless, these findings were consistent with the idea that lymphoid cells develop from 2 types of HSCs. One type of HSC would be active in early development and produce γδT and B-1 lymphocytes. This developmentally immature HSC would be gradually replaced by adult BM HSCs. At the same time, the mature progeny of the fetal HSCs would persist in the adult host and play important roles in adult innate immunity. This model was supported by data that fetal HSCs differ in many respects from adult HSCs. Yet, expression of Lin28b was sufficient to restore the fetal differentiation capacity in adult HSCs,6 pointing to a powerful molecular switch that controls the differentiation capacity of HSCs during development.
During mammalian development, HSCs perform a curious dance through the maturing embryo.7 The first HSCs are found in the YS, then HSCs appear in P-Sp and aorta-gonad-mesonephros region, then in the placenta. Thereafter, HSCs are detected in the fetal liver and spleen, until perinatally HSCs finally emerge in the BM. It is generally believed that the fetal liver does not generate HSCs de novo. Rather, the fetal liver collects and expands HSCs from earlier sites.8 Thus, the fetal liver HSCs that efficiently generate γδT and B-1 lymphocytes should have been produced somewhere else before appearing in the fetal liver. This was shown to be correct for the HSCs that produce B-1 lymphocytes. In a previous report Yoshimoto et al showed that as early as day 9 of development, YS and P-Sp cells give rise to B-1 and marginal zone B lymphocytes but not to B-2 lymphocytes.9 These studies were performed in Ncx1 mice that fail to develop a beating heart. This allowed the authors to exclude circulating cells as sources for B lymphocytes.
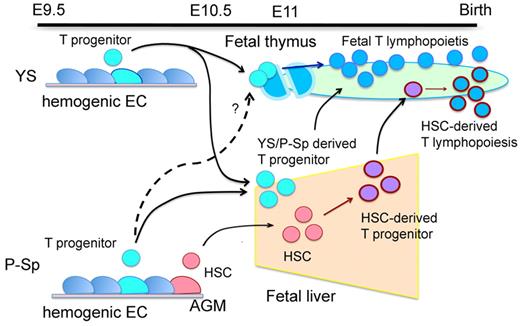
Yoshimoto et al now use the Ncx1 mice to examine the development of T lymphocytes (see figure). In a series of careful experiments they show that YS, as well as P-Sp, cells isolated as early as day 8.25 of development, can give rise to both fetal and adult T lymphocyte subsets on transplantation and in culture.1 The strongest support for this result comes from experiments where YS cells (day 9.5 of development) are directly injected into neonatal hosts. This leads to engraftment of γδT lymphocytes including Vγ3- and Vγ4-expressing γδT lymphocytes, αβT lymphocytes, and natural killer cells. That T lymphocyte precursors are present so early in development is surprising but consistent with the idea that αβT lymphocytes develop from γδT lymphocytes that failed to successfully rearrange their T cell receptors.4 The results by Yoshimoto et al leave us with the puzzling possibility that B but not T lymphocytes require 2 types of precursors/HSCs to generate all cells in the lineage. This would mean that T and B lymphocytes proceed through fundamentally different pathways in development. However, clonal analyses will be necessary to determine whether YS and P-Sp contain 1 precursor/HSC that generates both T lymphocyte lineages or 2 precursor/HSC, 1 for the early γδT lymphocytes and 1 for the adult lineage.
Development of T lymphocytes directly from the hemogenic endothelium during early development. Illustration provided by Mervin C. Yoder (Herman B Wells Center for Pediatric Research).
Development of T lymphocytes directly from the hemogenic endothelium during early development. Illustration provided by Mervin C. Yoder (Herman B Wells Center for Pediatric Research).
Yoshimoto and colleagues show that the nonhematopoietic (VE-cad+CD41−), hemogenic endothelium is the source for the T-cell activity in the YS. The authors argue that the appearance of T lymphocyte precursors precedes the development of HSCs. This argument is based on the high frequency of the T-cell activity in the YS, the low frequency of HSCs in the early embryo, the appearance of transplantable HSCs 0.5 days after the T-precursor activity, and the observation that the VE-cad+CD41−cells seem to repopulate only T lymphocytes in neonatal hosts. The conclusion that T-lymphocyte potential develops independent of HSCs is provocative. There is precedence for the appearance of mature hematopoietic cells before HSC activity both in vivo and in vitro. Yet, alternative interpretations of the findings presented here are possible. For example, it cannot be excluded that the absence of a heartbeat in Ncx1 mice skews the development of the hematopoietic lineages.10 Moreover, the potential for differentiation is not the same as actual differentiation. Whether T lymphocytes are functionally important in the embryo remains to be determined. Yet the conclusion is inescapable that the capacity to generate T lymphocytes develops surprisingly early in development. The data will undoubtedly spark vigorous discussion and new insights into the puzzling early development of HSCs and lymphocytes.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal