In this issue of Blood, Ware and Helms report results from the Stroke With Transfusions Changing To Hydroxyurea (SWiTCH) study designed to determine whether hydroxyurea is as effective as transfusions in preventing recurrent strokes in children with sickle cell anemia. The finding of strokes in 7 of 67children receiving hydroxyurea but none in 66 children who received transfusions led the authors to conclude that hydroxyurea is not effective in mitigating strokes.1
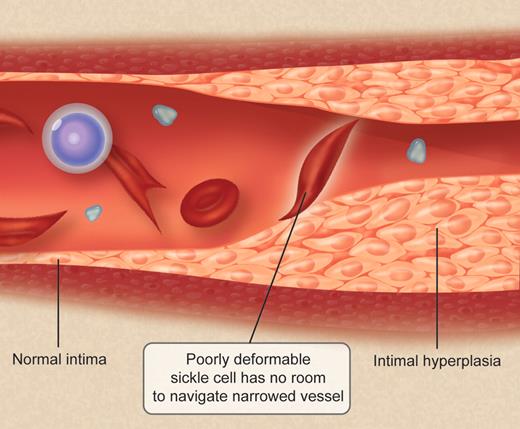
Among patients with sickle cell anemia (SCA), up to 11% will experience a clinical stroke by 20 years of age and 24% by 45 years of age.2 The initiation of the process results from the proliferation of intima, which induces progressive stenosis in the intracranial portions of the internal carotid arteries, with less involvement of middle and anterior cerebral arteries (see figure). Vessel occlusion is amplified by inflammation, excessive adhesion of blood cells to activated endothelium, ischemia-reperfusion injury, hypercoagulable state, and disregulation of vascular tone. Hemorrhages are late-stage events resulting from progressive weakening of the vessel wall, with eventual formation of aneurysm. Hemorrhages may also be secondary to rupture of tortuous collateral vessels (moya-moya syndrome) that develop secondary to stenosis of blood vessels.3
The lumen of cerebral vessels in SCA patients having had a stroke is progressively filled with proliferated intima. Circulating in the narrowed vessels is a challenge for red cells whose deformability is reduced by the sickling. Professional illustration by Debra T. Dartez.
The lumen of cerebral vessels in SCA patients having had a stroke is progressively filled with proliferated intima. Circulating in the narrowed vessels is a challenge for red cells whose deformability is reduced by the sickling. Professional illustration by Debra T. Dartez.
Chronic transfusion is currently the standard of care for the prevention of recurrence of strokes in SCA patients based on long-term observational studies that showed a large decrease in the risk of recurrence.4 There is a high risk of recurrence after discontinuation of transfusion therapy.5 The randomized Stroke Prevention in Sickle Cell Anemia (STOP 1) study demonstrated the efficacy of transfusion in preventing the occurrence of stroke in SCA children with increased blood flow velocity as documented on transcranial doppler (TCD) that is a characteristic feature of a high risk of experiencing a first stroke.6 It should be noted, however, that maintaining hemoglobin S levels below 30% by chronic transfusion does not abolish completely the risk of recurrent strokes.4 Chronic transfusions also do not prevent radiologic progression of vascular lesions.7 Importantly, transfusion therapy induces iron overload, which by itself can provoke organ damage, along with the risk of red-cell alloimmunization. There is, therefore, an urgent need to find an equivalent or a better and more effective therapy to prevent recurrent strokes in SCA patients. Hydroxyurea has been demonstrated to be effective in reducing pain in patients with severe SCA. It improves red-cell hydration and deformability, decreases sickling, reduces inflammation and excessive adherence of blood cells to the damaged endothelium, and improves vascular tone.8 Efficacy of hydroxyurea in preventing strokes was suggested by documentation of a significant decrease in TCD velocities in 21 patients with elevated values; only 1 of these at-risk patients experienced a neurologic event.9 Finally, Ware and Helms had previously reported that in 20 children with strokes on transfusion therapy who had had their transfusions discontinued and switched to hydroxyurea treatment, stroke recurrence rate was only 10%.10 Thus a randomized study comparing the effectiveness of hydroxyurea and of transfusion was needed to establish whether hydroxyurea could be a safe alternative to transfusion in SCA patients having had a stroke.
The SWiTCH study was a noninferiority trial randomizing an alternative treatment (hydroxyurea/phlebotomy) to standard treatment (transfusion/chelation) in 134 children having had a stroke, having been transfused for ≥ 18 months, and with posttransfusional iron overload. The authors developed a composite end point, including both secondary stroke recurrence rate and liver iron content. They accepted an increased stroke rate in the alternative treatment arm because they hypothesized that iron overload would be better managed by phlebotomies than by oral chelation. The study was terminated by the National Heart, Lung, and Blood Institute–appointed Data and Safety Monitoring Board before its completion because the interim analysis indicated that the reduction in liver iron content was not superior on the hydroxyurea/phlebotomy arm, while on the other hand, 7 strokes had been observed in the 67 subjects enrolled in this arm compared with 0 in the 66 subjects on transfusion/chelation. Six strokes were infarctive and 1, fatal, was hemorrhagic. Without superiority in elimination of iron, the increased stroke risk in the hydroxyurea arm was found to be unacceptable.
The use of a composite end point appears to be a limitation of the study complicating effective analysis of the findings. The conclusion of this paper is that transfusion and chelation remain a better way to manage children with SCA, stroke, and iron overload. However, it should be noted that the initial neurologic abnormalities of the children in the present study, which were statistically not different in both arms, were rather severe. In the transfusion/chelation arm and in the hydroxyurea/phlebotomy arm, respectively, 6% and 15% of patients had a previous recurrent stroke, and 8% and 16% had a pre-existing moya-moya vasculopathy. We cannot exclude the possibility that the severe initial vascular lesions may explain the high rate of recurrence in the hydroxyurea arm, and it is unfortunate that the study was not stratified based on the initial severity of vascular damage. It is likely that the improvement of rheology induced by hydroxyurea may not be effective in cases of extreme narrowing of the vessel lumen, or when a moya-moya disease has already developed. The SWiTCH study, however, implies that chronic transfusion is the best option in patients with severe cerebral vasculopathy (previous stroke recurrences, severe stenosis, moya-moya disease). Hydroxyurea in these patients may be a reasonable alternative in those individuals with severe red-cell alloimmunization or living in countries with limited blood supplies. Future studies enrolling patients with less severe damage appear to be warranted. Importantly, a key message is that very early screening of cerebrovascular lesions is extremely important to prevent progressive accumulation of vascular damage.
Conflict-of-interest disclosure: The author is a member of Novartis' speakers' bureau. ■